Superficial Endometriosis at Ultrasound Examination—A Diagnostic Criteria Proposal
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Participants
2.2. Protocol
2.3. Procedure
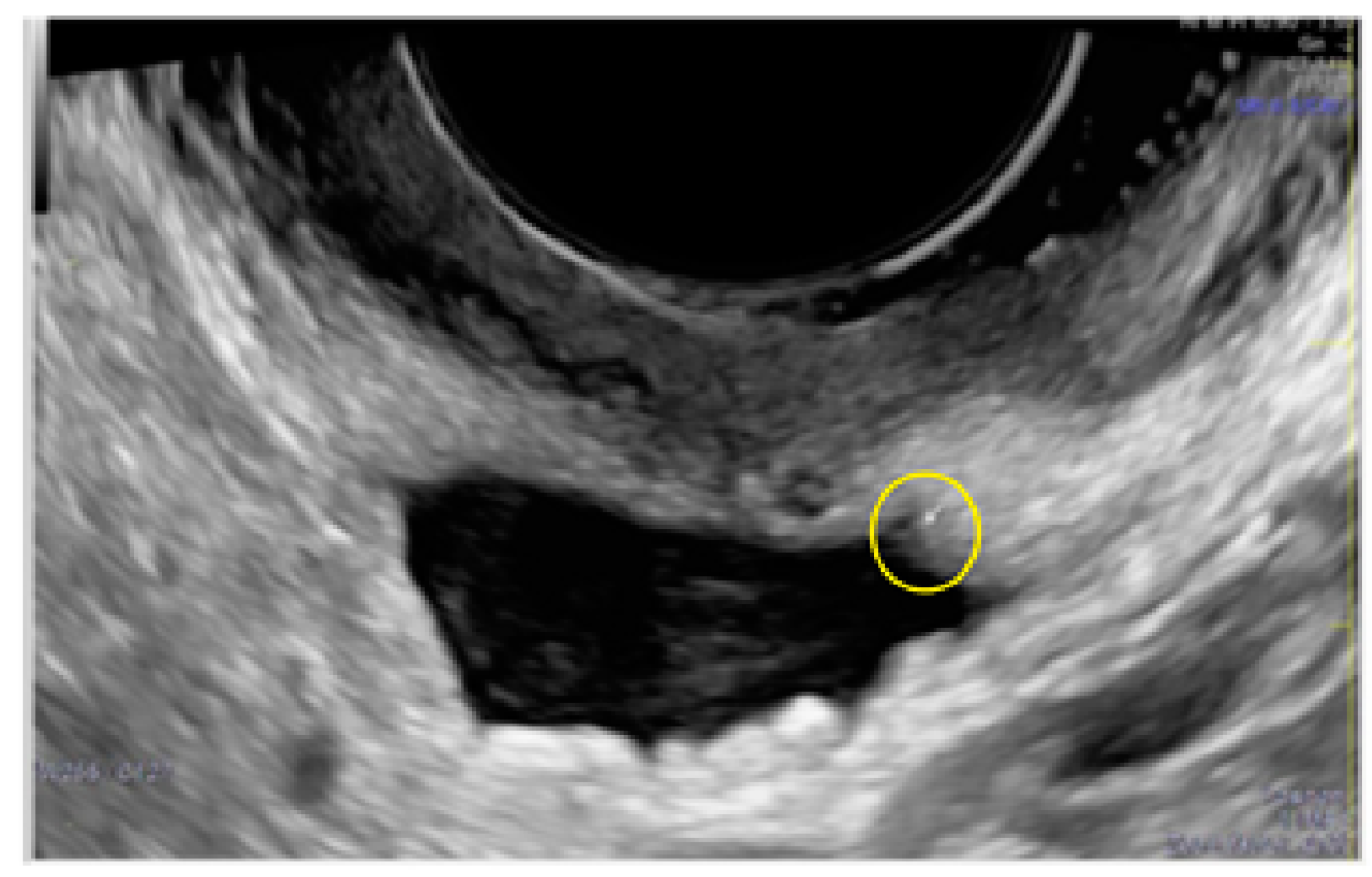
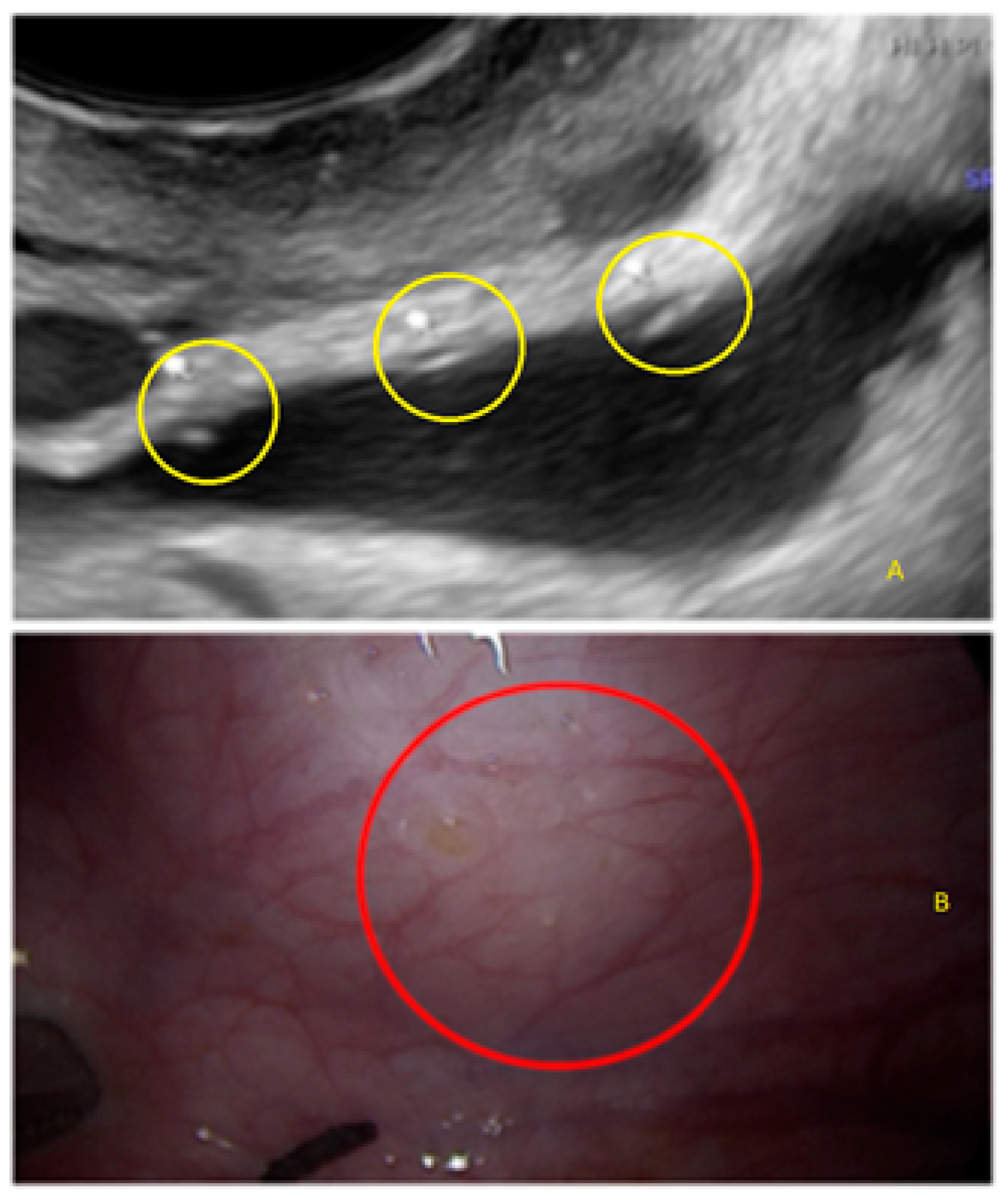
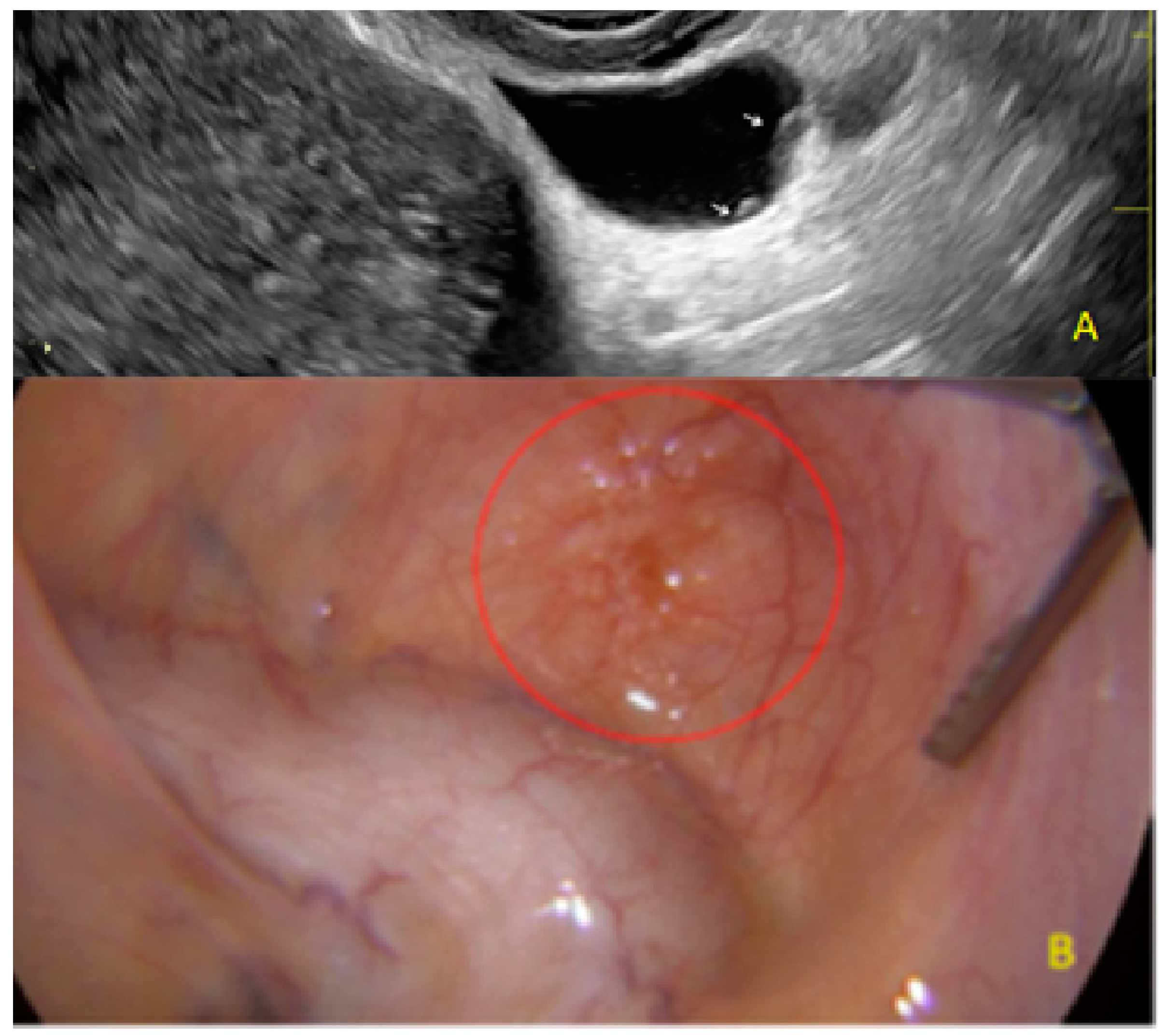
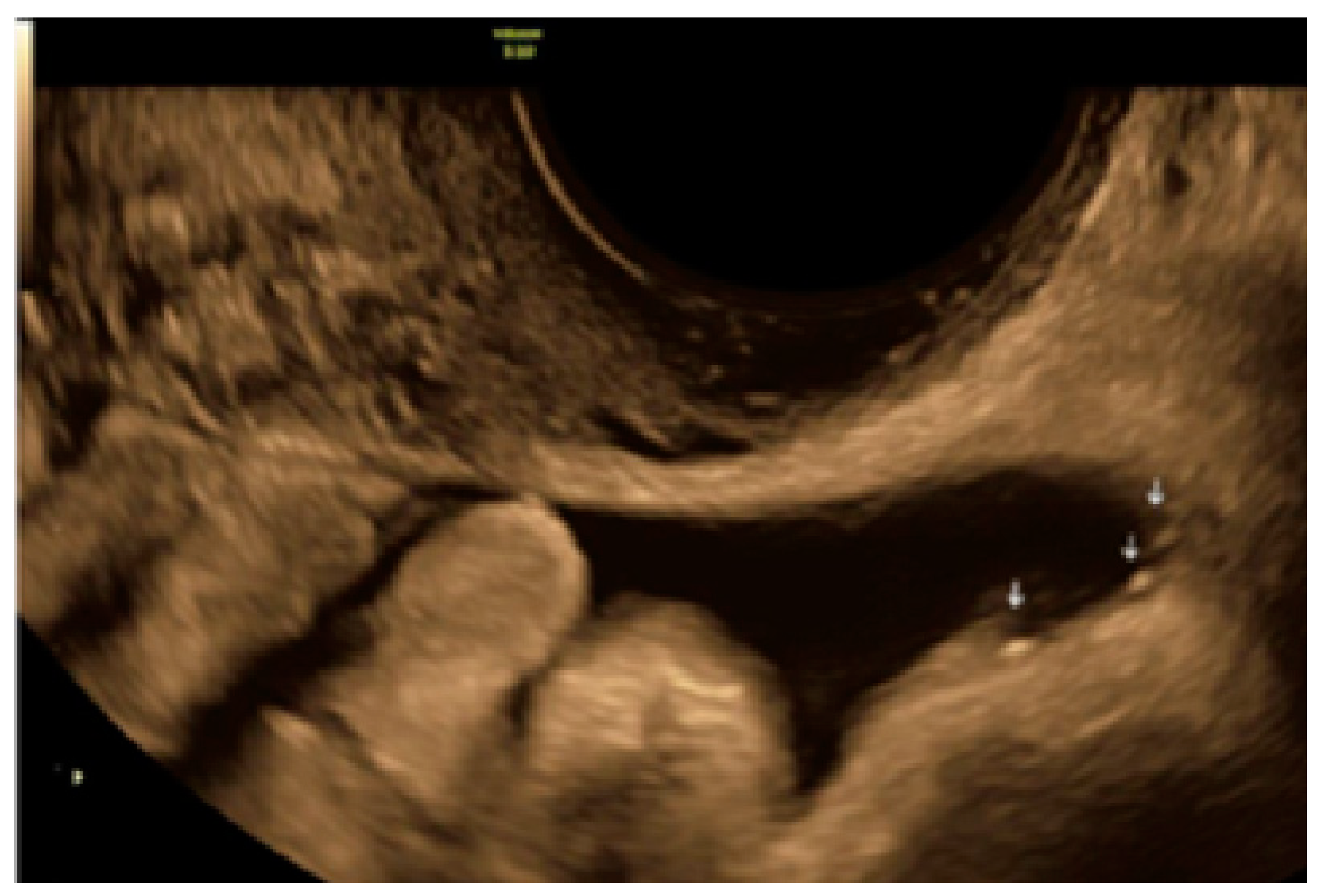
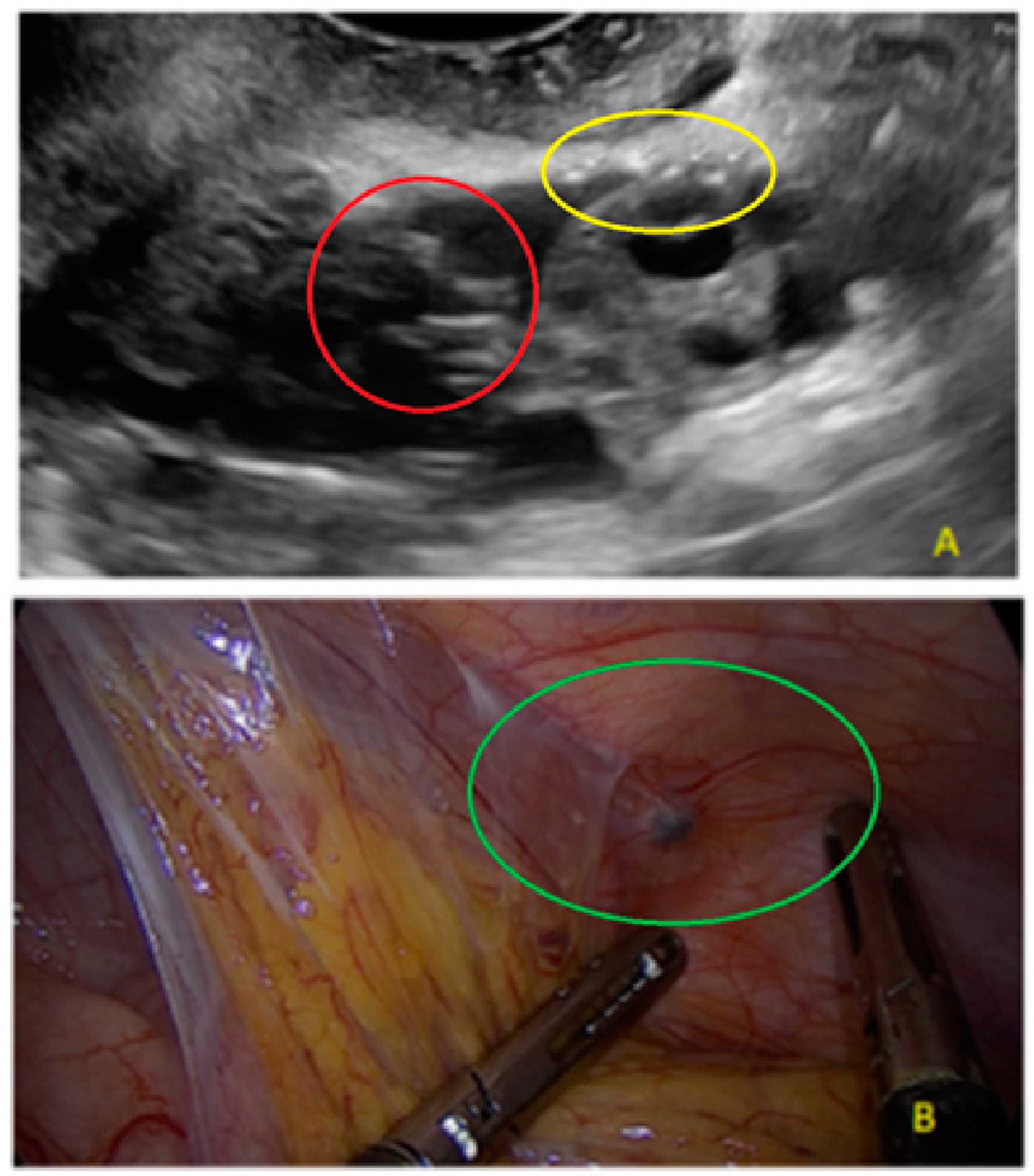
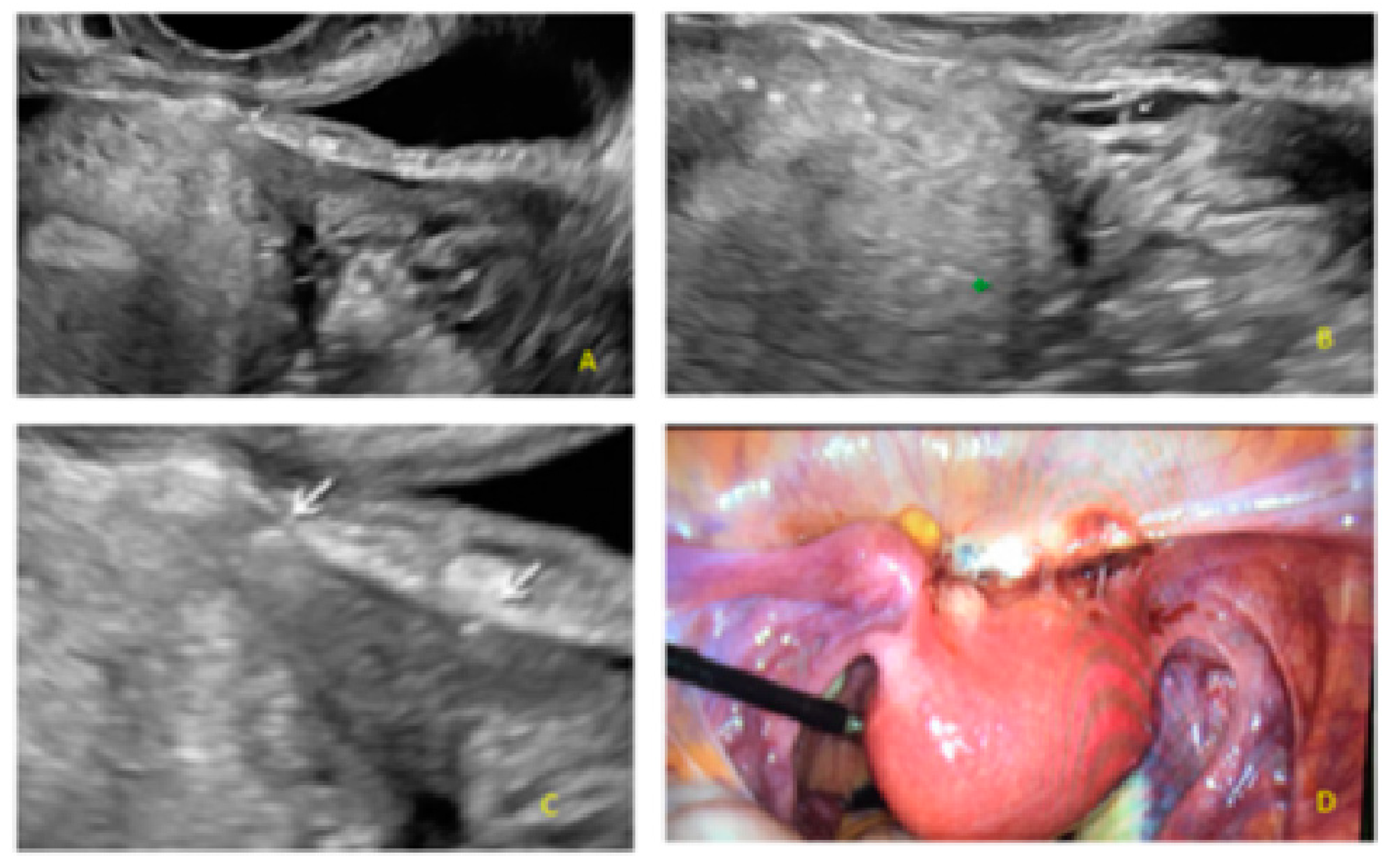
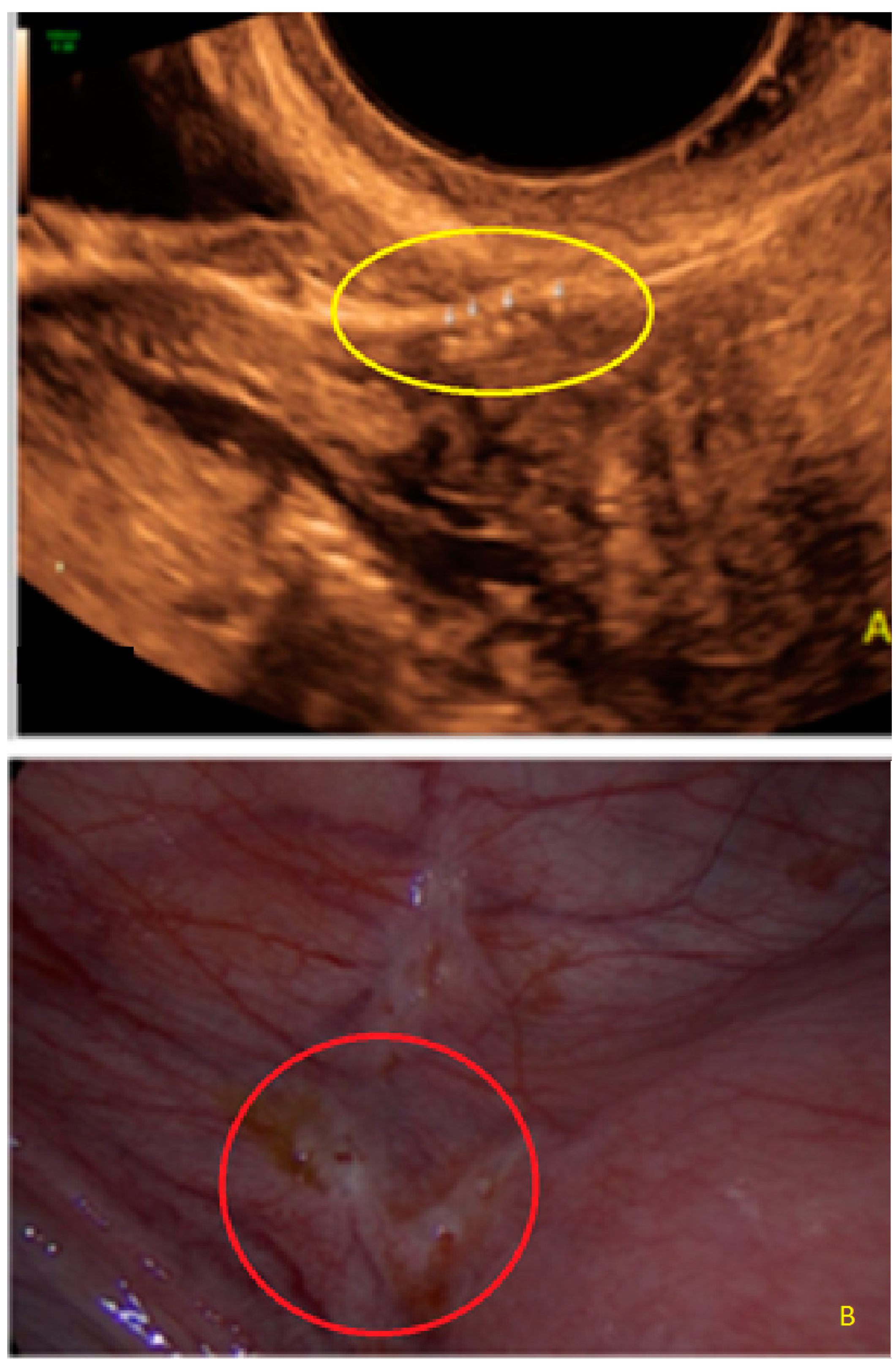
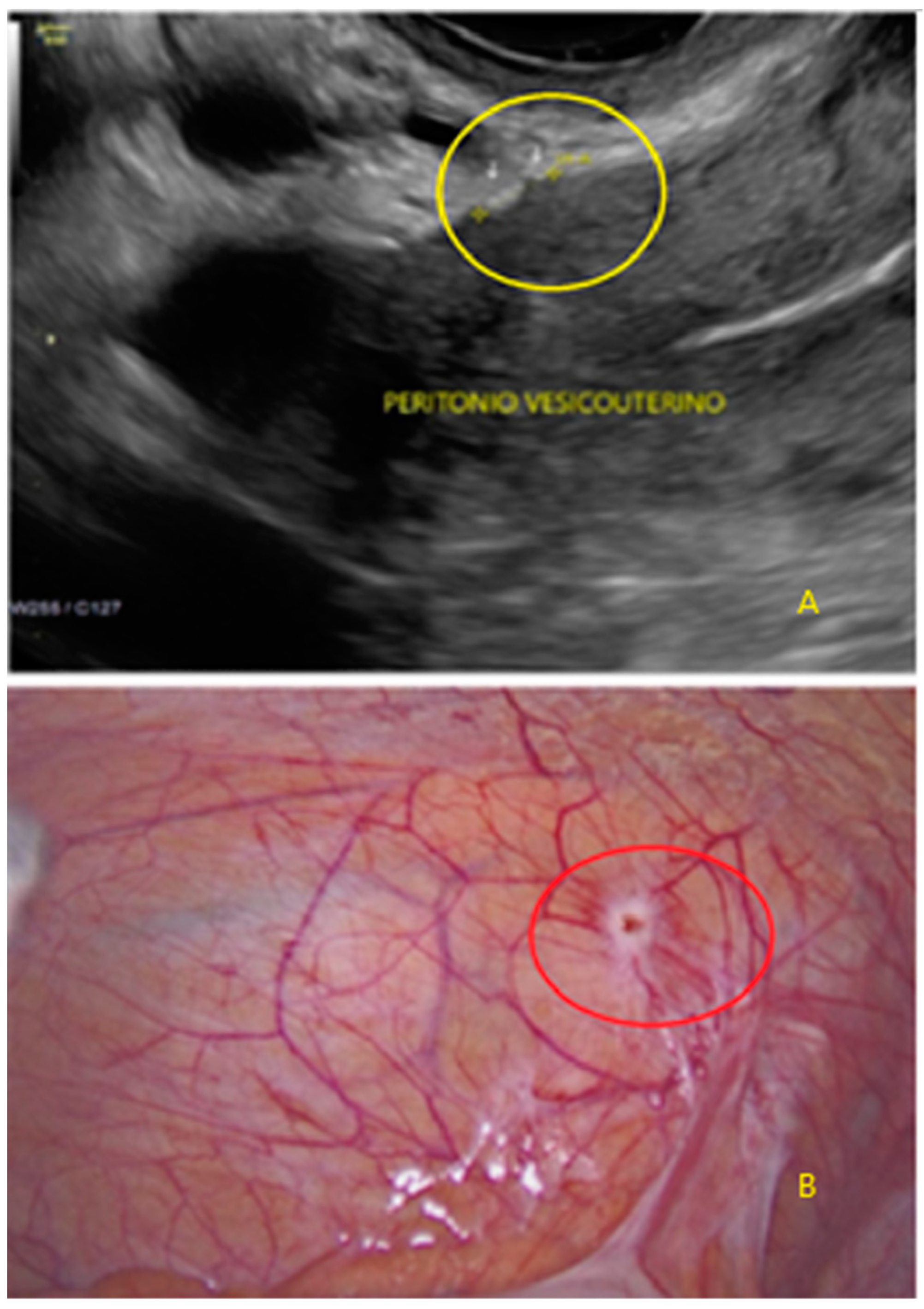
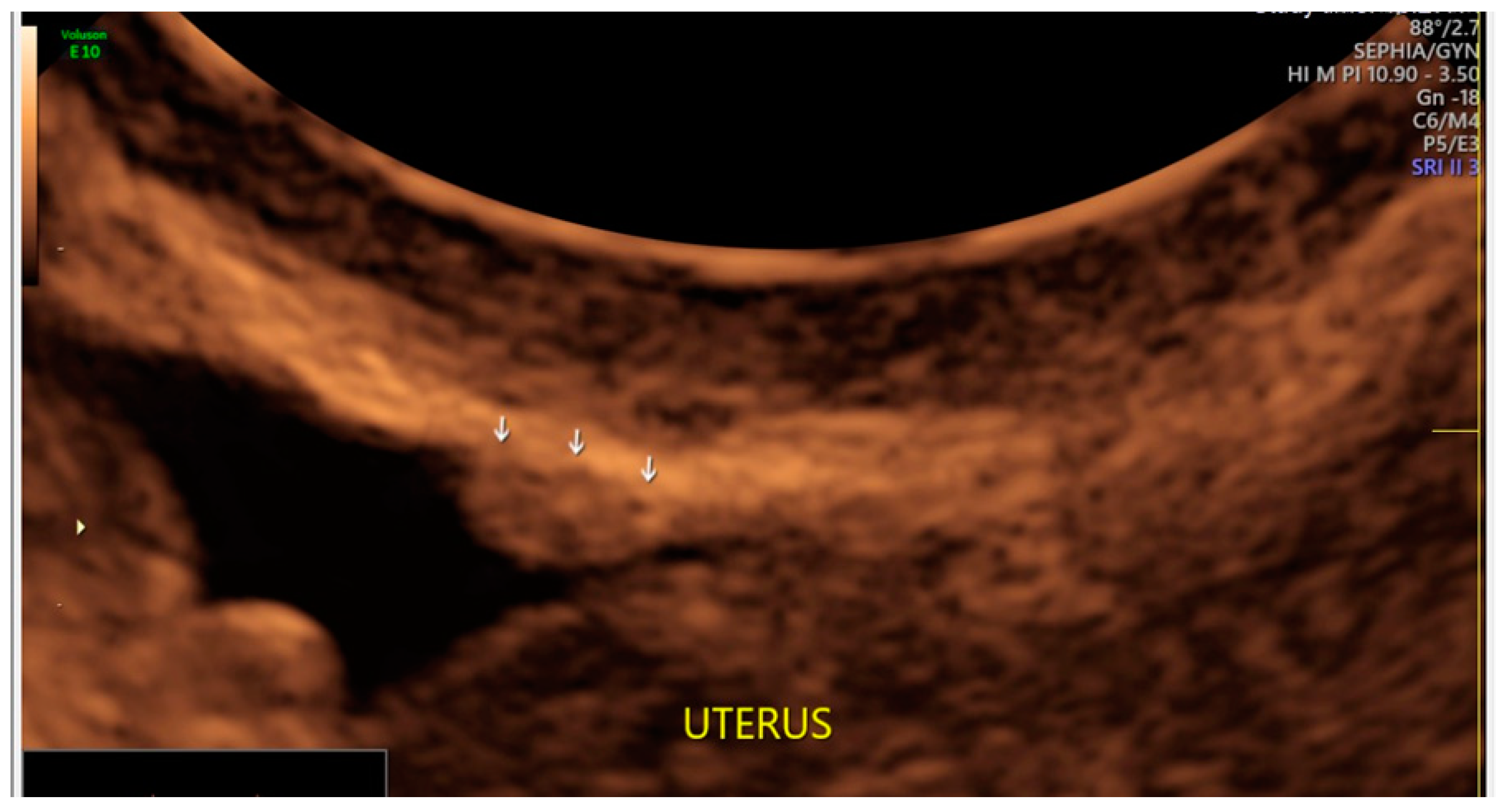
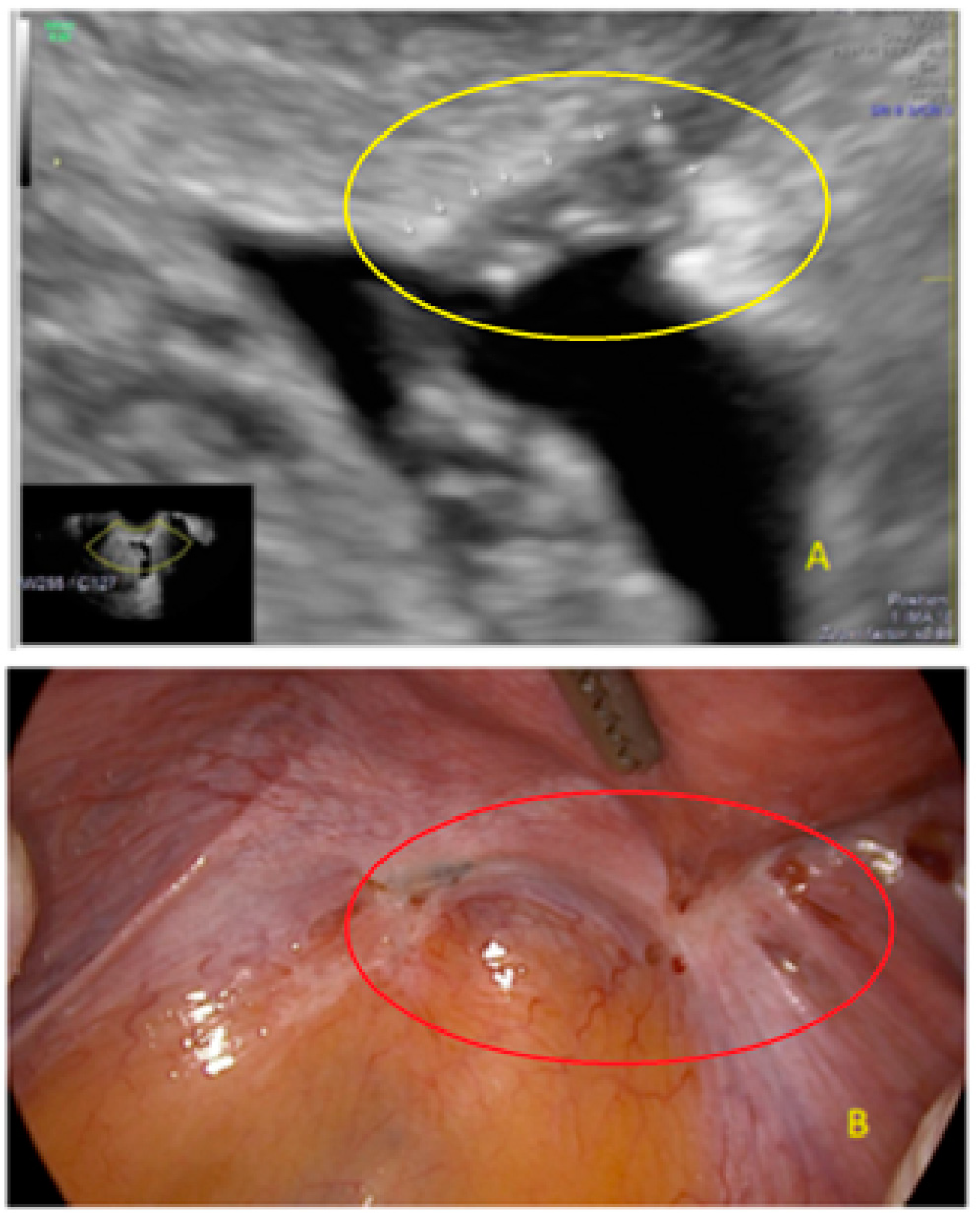
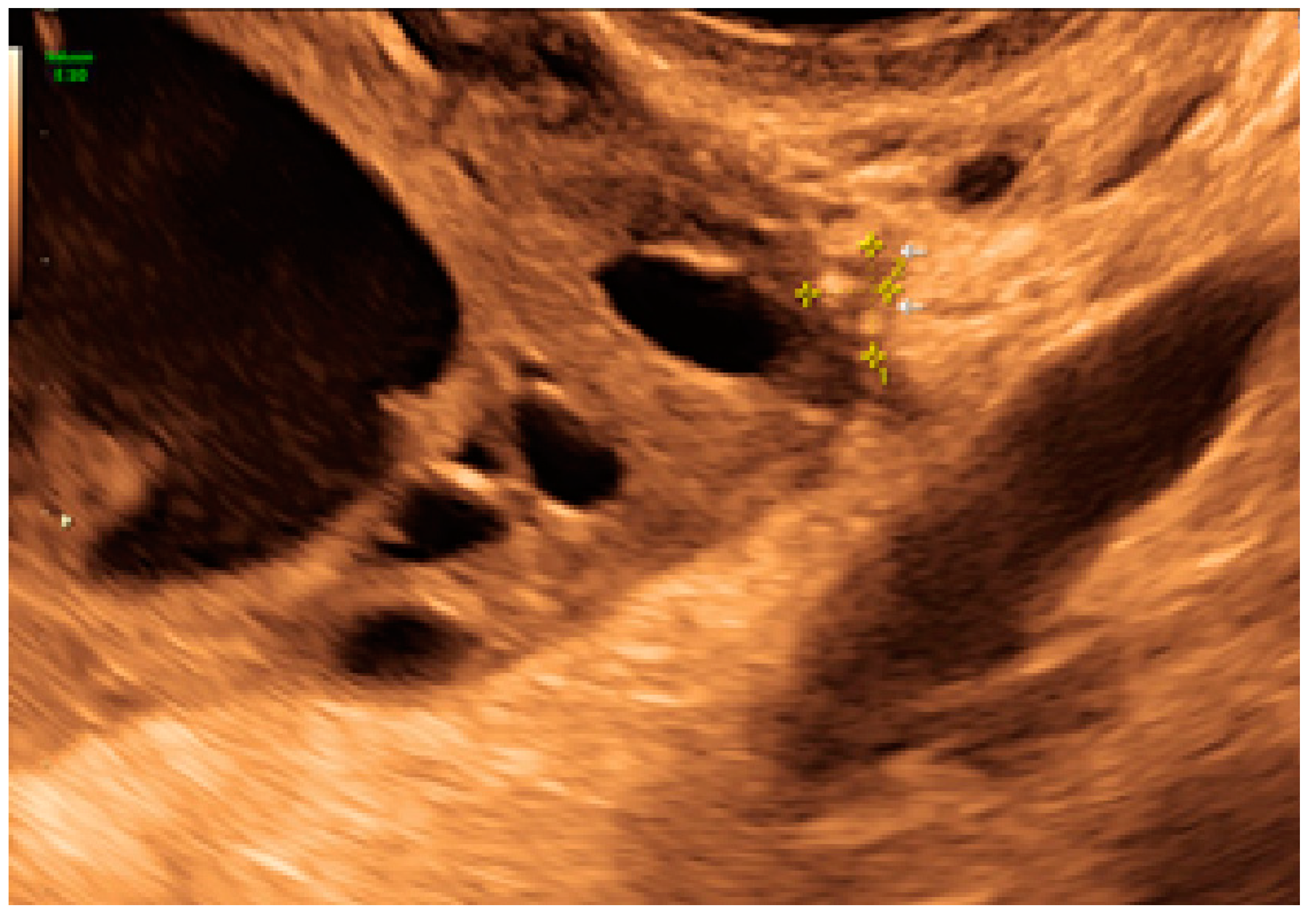
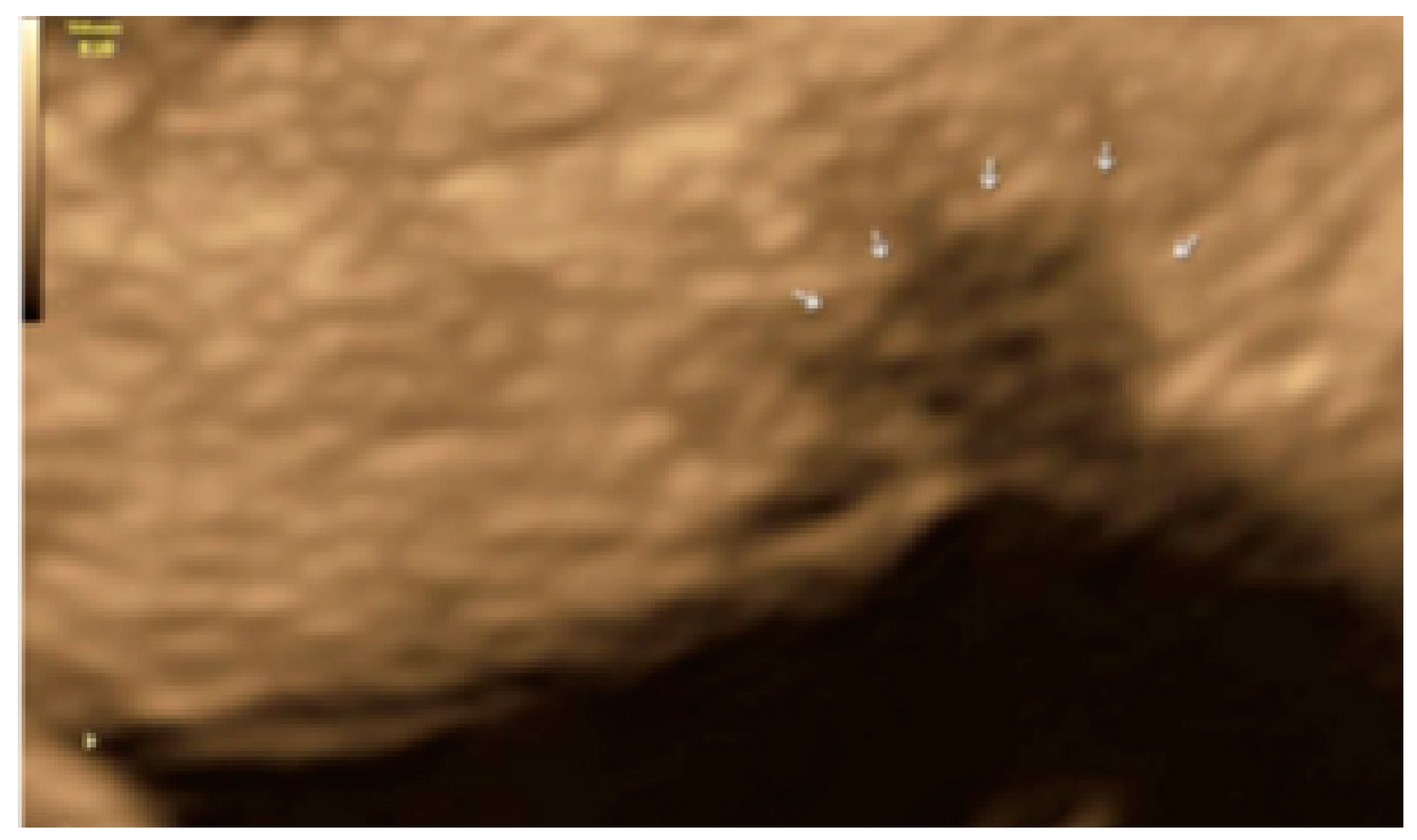
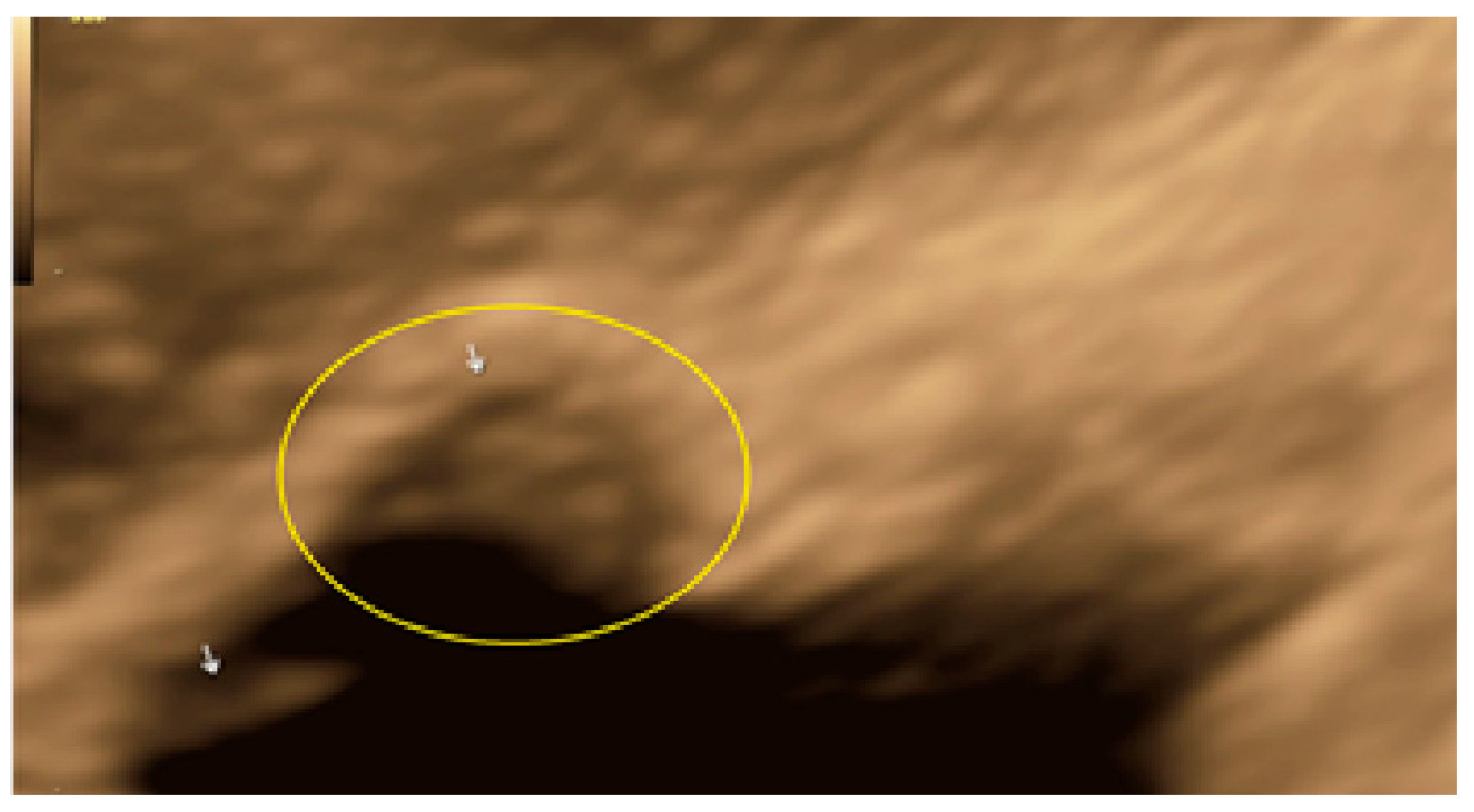
3. Results
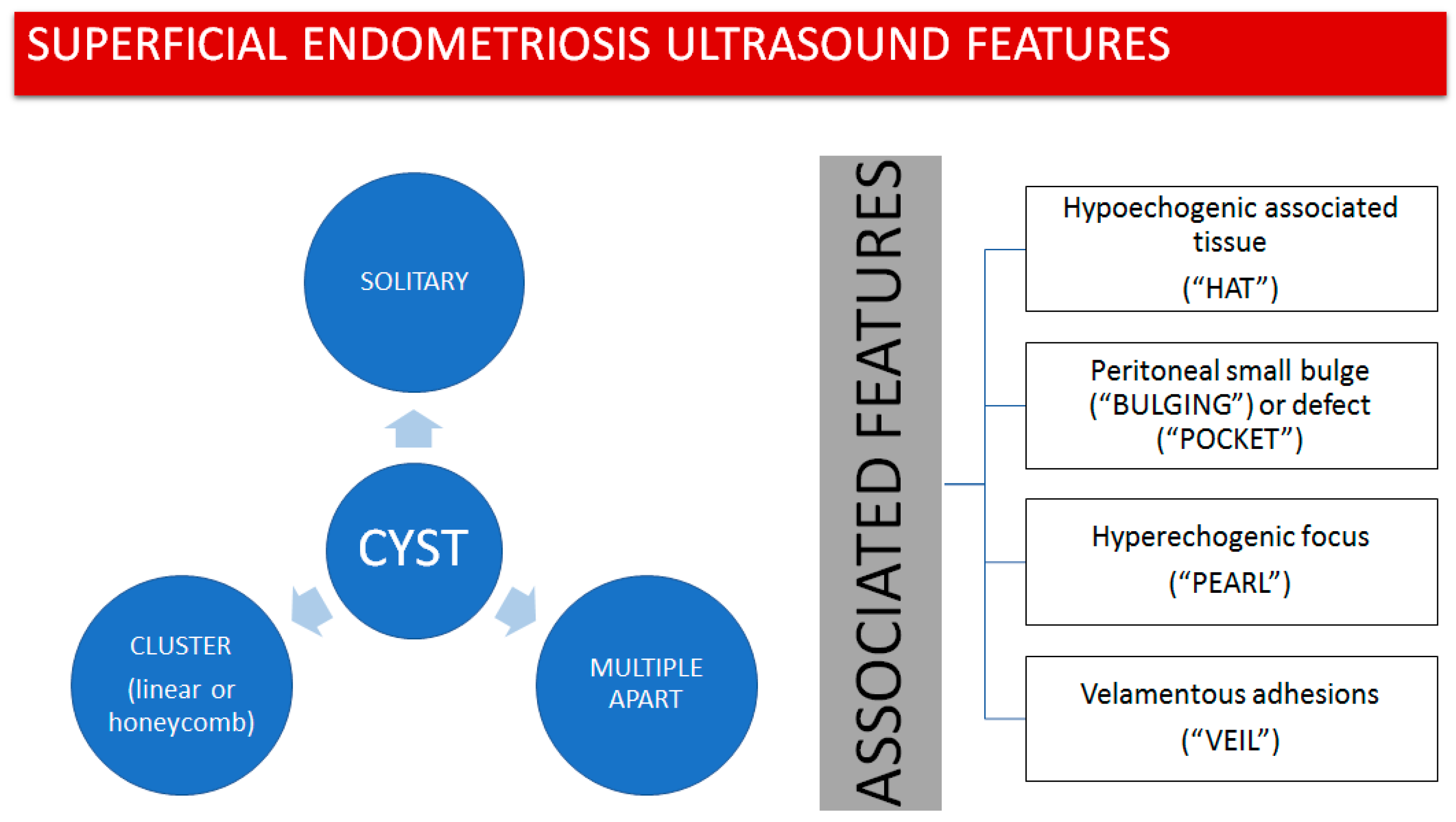
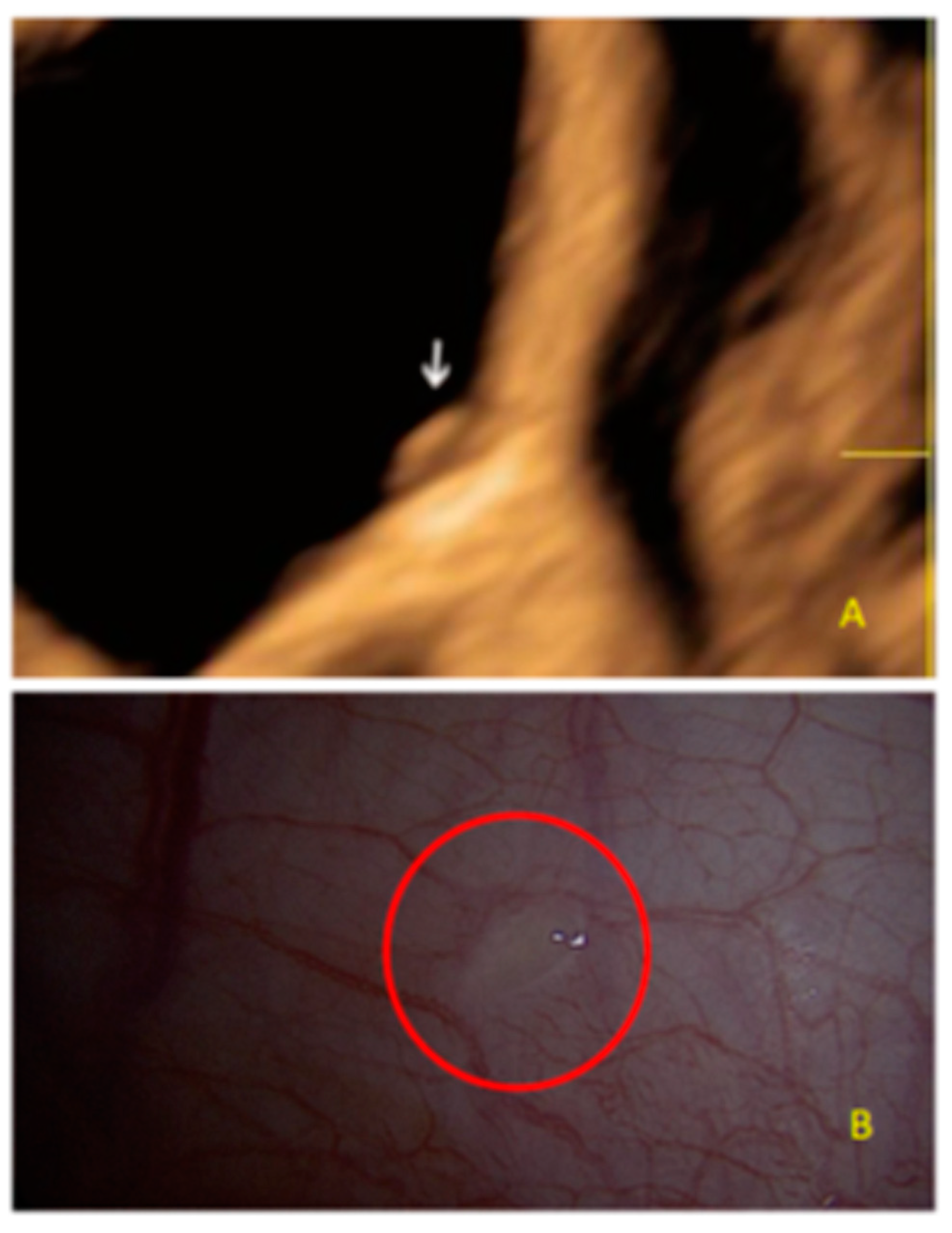
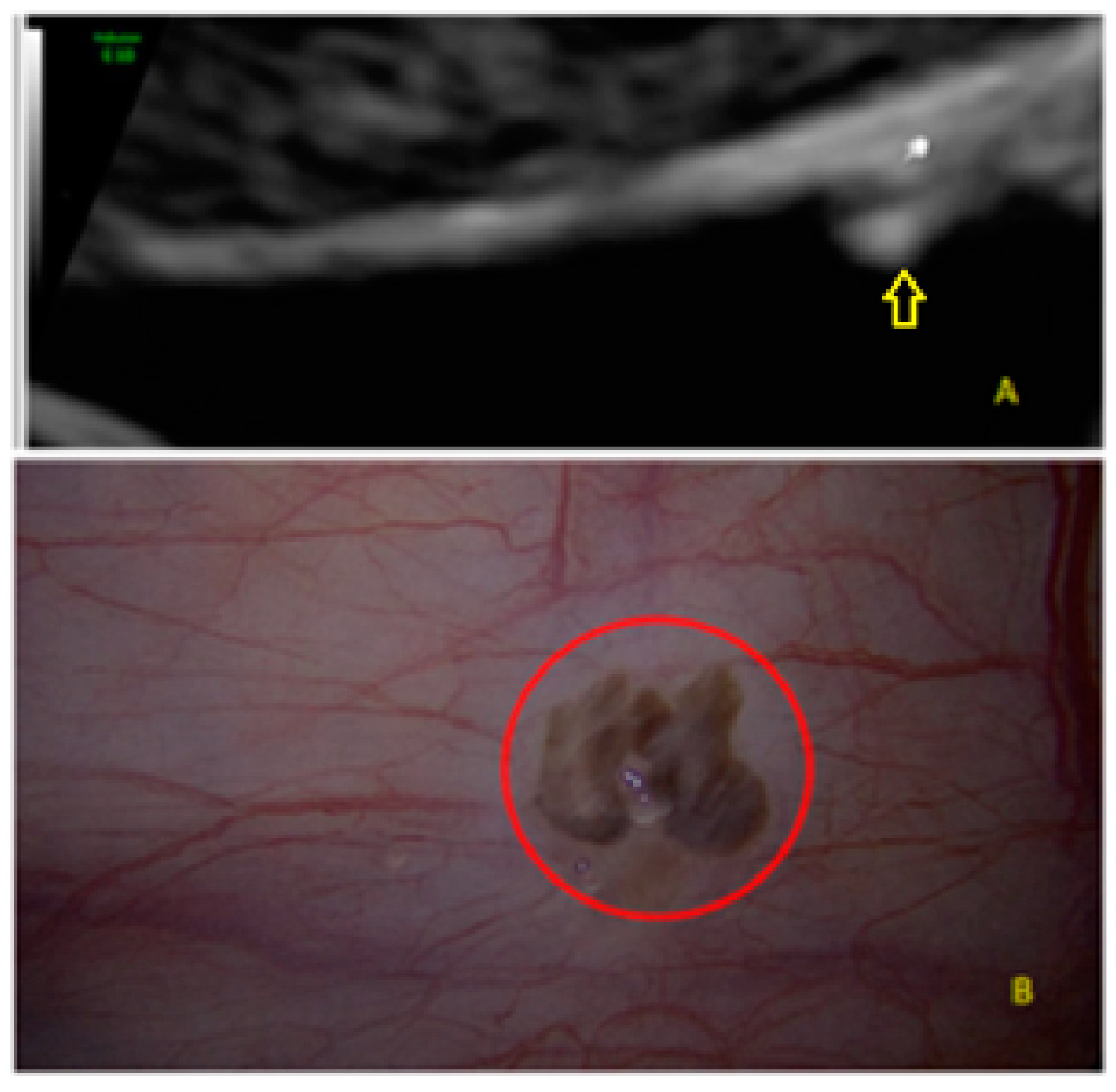
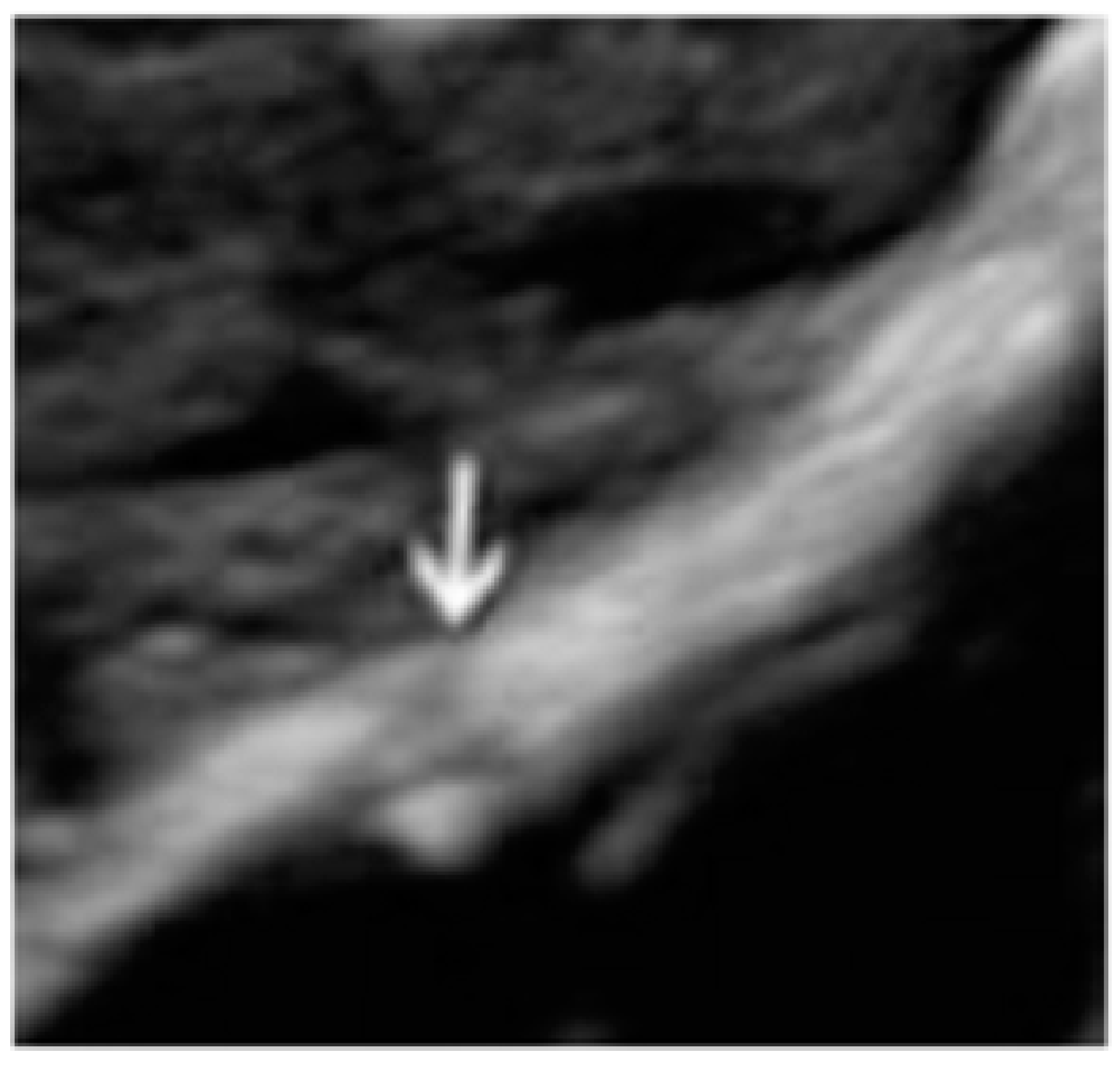
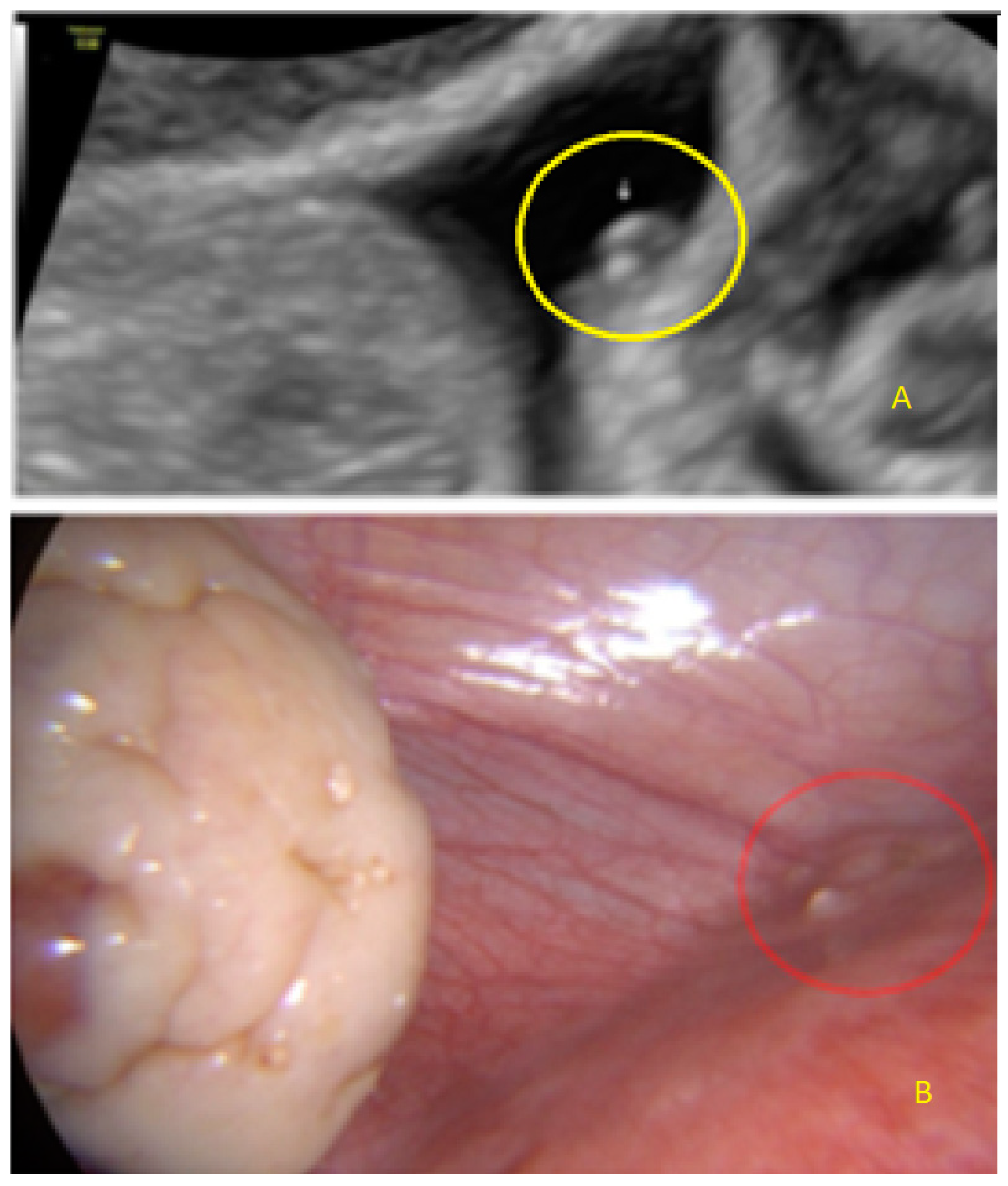
- The presence of hypoechogenic associated tissue (hypoechoic areas surrounding a small cyst area; we called this a “hat”). This tissue does not protrude or invaginate the peritoneal surface.
- The lesion may be convex, protruding from the peritoneal surface into the peritoneal cavity (we called this “bulging”), or it may appear as a concave defect in the peritoneum (we called this a “pocket”).
- The presence of hyperechoic foci (we called this a “pearl”).
- The presence of velamentous (filmy) adhesions associated to the lesion (we called this a “veil”).
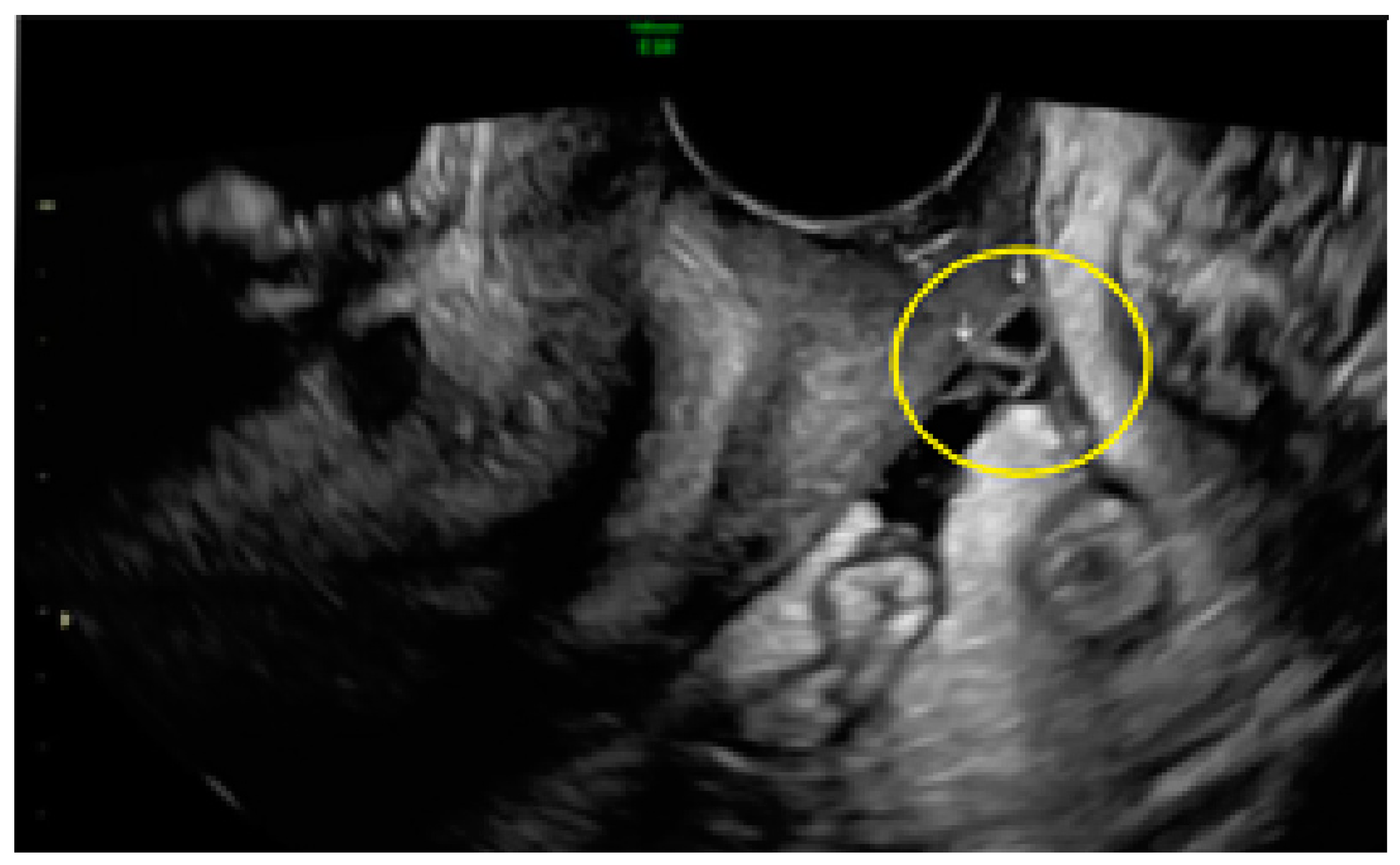
3.1. Cystic Solitary Lesion
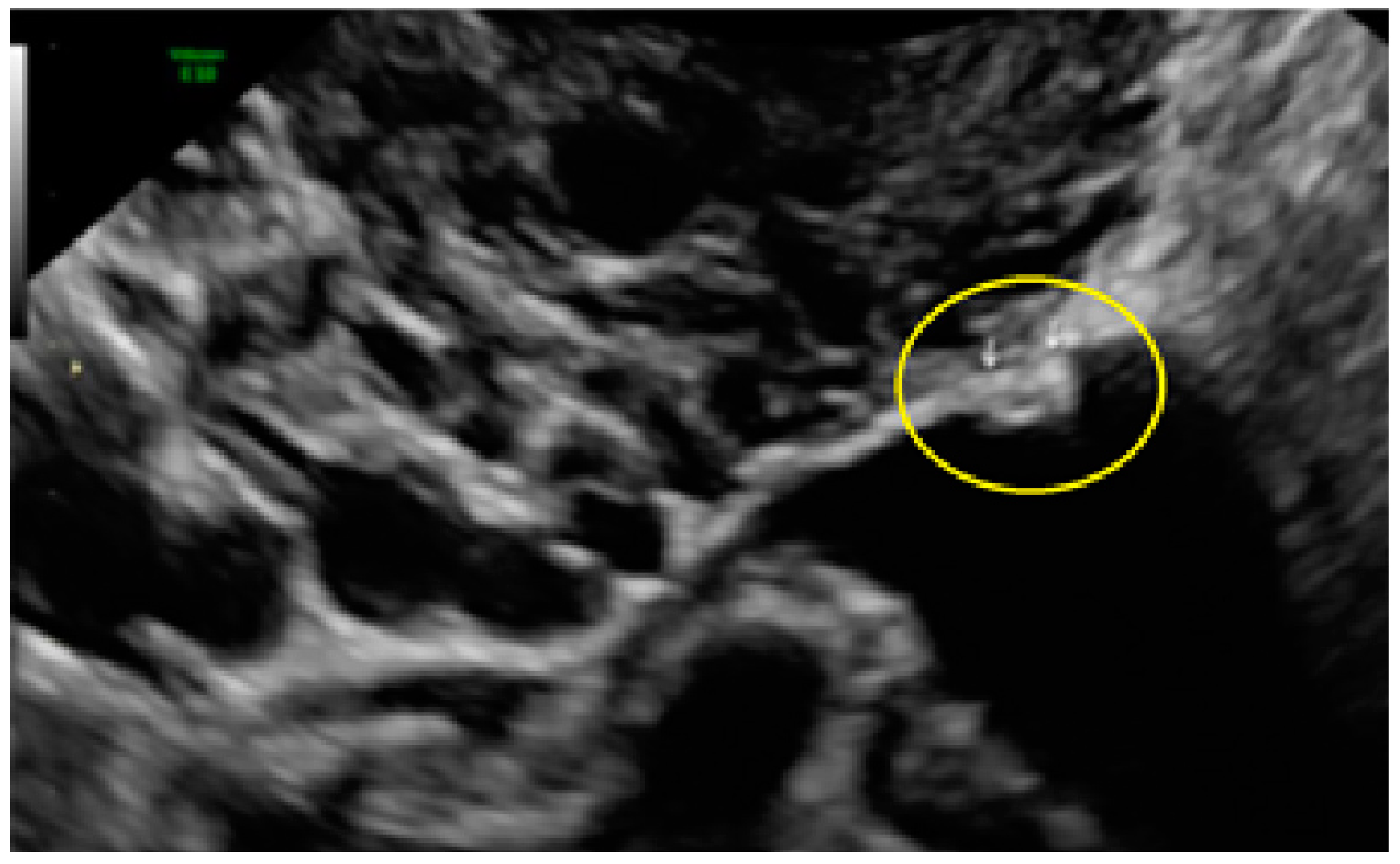
3.2. Cystic Multiple Separate Lesions
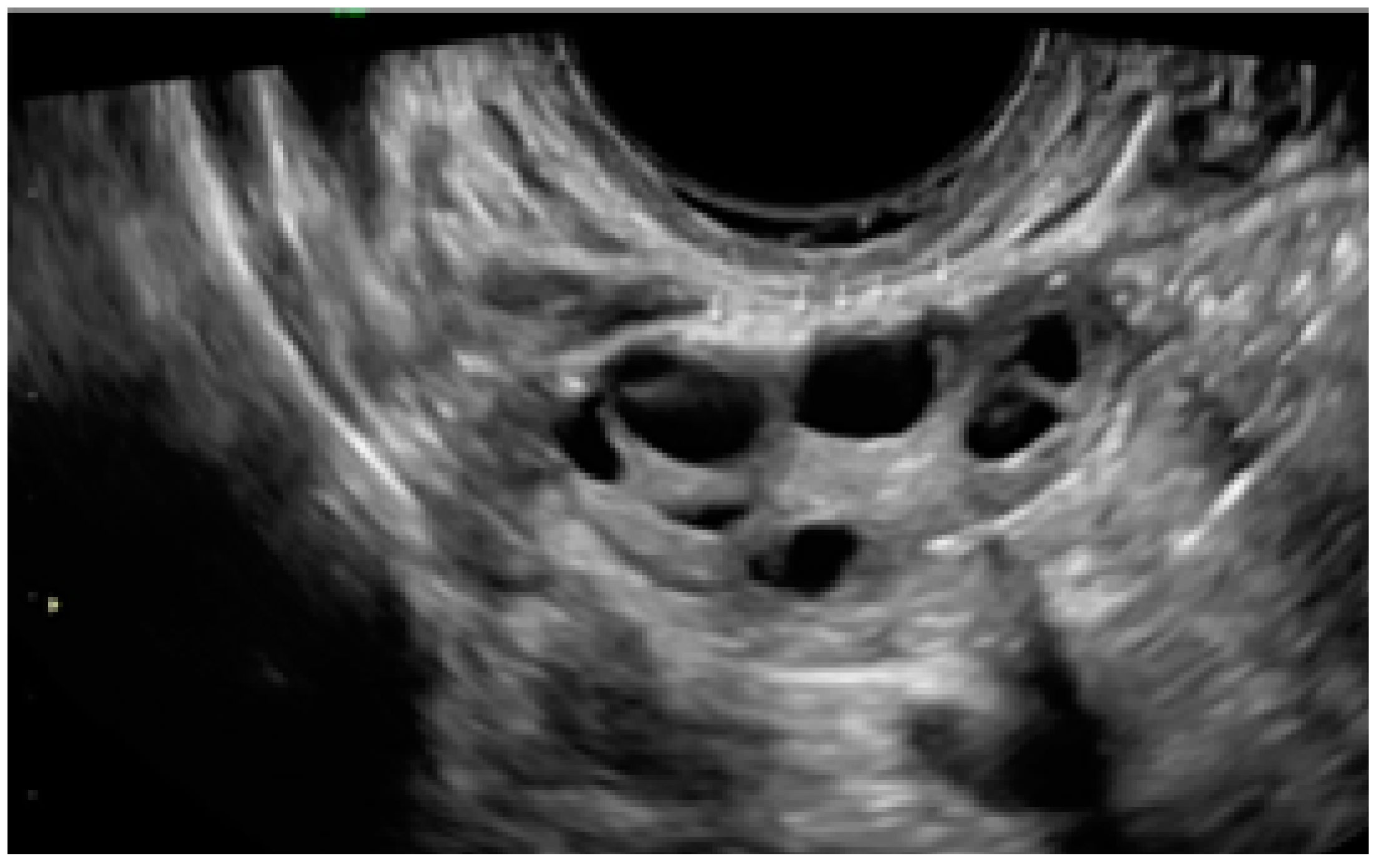
3.3. Cystic Lesions Arranged in a Cluster
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zondervan, K.T.; Becker, C.M.; Missmer, S.A. Endometriosis. N. Engl. J. Med. 2020, 26, 1244–1256. [Google Scholar] [CrossRef] [PubMed]
- Nisolle, M.; Donnez, J. Peritoneal endometriosis, ovarian endometriosis, and adenomyotic nodules of the rectovaginal septum are three different entities. Fertil. Steril. 1997, 68, 585–596. [Google Scholar] [CrossRef] [PubMed]
- International Working Group of AAGL, ESGE, ESHRE and WES; Tomassetti, C.; Johnson, N.P.; Petrozza, J.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Lee, T.T.M.; Missmer, S.; Vermeulen, N.; et al. An international terminology for endometriosis. Hum. Reprod. Open 2021, 2021, hoab029. [Google Scholar]
- Zondervan, K.T.; Becker, C.M.; Koga, K.; Missmer, S.A.; Taylor, R.N.; Viganò, P. Endometriosis. Nat. Rev. Dis. Prim. 2018, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Kitajima, M.; Hiraki, K.; Fujishita, A.; Nakashima, M.; Masuzaki, H. Visible and occult microscopic lesions of endometriosis. Gynecol. Min. Invasive Ther. 2014, 3, 109–114. [Google Scholar] [CrossRef]
- Colgrave, E.M.; Bittinger, S.; Healey, M.; Dior, U.P.; Rogers, P.A.W.; Keast, J.R.; Girling, J.E.; Holdsworth-Carson, S.J. Superficial peritoneal endometriotic lesions are histologically diverse and rarely demonstrate menstrual cycle synchronicity with matched eutopic endometrium. Hum. Reprod. 2020, 35, 2701–2714. [Google Scholar] [CrossRef]
- Amro, B.; Ramirez Aristondo, M.E.; Alsuwaidi, S.; Almaamari, B.; Hakim, Z.; Tahlak, M.; Wattiez, A.; Koninckx, P.R. New Understanding of Diagnosis, Treatment and Prevention of Endometriosis. Int. J. Environ. Res. Public Health 2022, 19, 6725. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Tahlak, M.; Keckstein, J.; Wattiez, A.; Martin, D.C. The epidemiology of endometriosis is poorly known as the pathophysiology and diagnosis are unclear. Best Pract. Res. Clin. Obstet. Gynaecol. 2021, 71, 14–26. [Google Scholar] [CrossRef]
- Horne, A.W.; Daniels, J.; Hummelshoj, L.; Cox, E.; Cooper, K.G. Surgical removal of superficial peritoneal endometriosis for managing women with chronic pelvic pain: Time for a rethink? BJOG 2019, 126, 1414–1416. [Google Scholar] [CrossRef]
- Reis, F.M.; Santulli, P.; Marcellin, L.; Borghese, B.; Lafay-Pillet, M.C.; Chapron, C. Superficial Peritoneal Endometriosis: Clinical Characteristics of 203 Confirmed Cases and 1292 Endometriosis-Free Controls. Reprod. Sci. 2020, 27, 309–315. [Google Scholar] [CrossRef]
- Hirsch, M.; Dhillon-Smith, R.; Cutner, A.S.; Yap, M.; Creighton, S.M. The Prevalence of Endometriosis in Adolescents with Pelvic Pain: A Systematic Review. J. Pediatr. Adolesc. Gynecol. 2020, 33, 623–630. [Google Scholar] [CrossRef]
- Becker, C.M.; Bokor, A.; Heikinheimo, O.; Horne, A.; Jansen, F.; Kiesel, L.; King, K.; Kvaskoff, M.; Nap, A.; Petersen, K.; et al. ESHRE guideline: Endometriosis. Hum. Reprod. Open. 2022, 2022, hoac009. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Shaikh, R.; Farquhar, C.; Jordan, V.; Scheffers, C.S.; Mol, B.W.; Johnson, N.; Hull, M.L. Blood biomarkers for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD012179. [Google Scholar] [CrossRef]
- Nisenblat, V.; Bossuyt, P.M.; Farquhar, C.; Johnson, N.; Hull, M.L. Imaging modalities for the non-invasive diagnosis of endometriosis. Cochrane Database Syst. Rev. 2016, 2, CD009591. [Google Scholar] [CrossRef] [PubMed]
- Guerriero, S.; Saba, L.; Pascual, M.A.; Ajossa, S.; Rodriguez, I.; Mais, V.; Alcazar, J.L. Transvaginal ultrasound vs magnetic resonance imaging for diagnosing deep infiltrating endometriosis: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2018, 51, 586–595. [Google Scholar] [CrossRef]
- Noventa, M.; Scioscia, M.; Schincariol, M.; Cavallin, F.; Pontrelli, G.; Virgilio, B.; Vitale, S.G.; Laganà, A.S.; Dessole, F.; Cosmi, E.; et al. Imaging Modalities for Diagnosis of Deep Pelvic Endometriosis: Comparison between Trans-Vaginal Sonography, Rectal Endoscopy Sonography and Magnetic Resonance Imaging. A Head-to-Head Meta-Analysis. Diagnostics 2019, 9, 225. [Google Scholar] [CrossRef] [PubMed]
- Chowdary, P.; Stone, K.; Ma, T.; Readman, E.; McIlwaine, K.; Druitt, M.; Ellett, L.; Cameron, M.; Maher, P. Multicentre retrospective study to assess diagnostic accuracy of ultrasound for superficial endometriosis-Are we any closer? Aust. N. Z. J. Obstet. Gynaecol. 2019, 59, 279–284. [Google Scholar] [CrossRef]
- Leonardi, M.; Robledo, K.P.; Espada, M.; Vanza, K.; Condous, G. SonoPODography: A new diagnostic technique for visualizing superficial endometriosis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 254, 124–131. [Google Scholar] [CrossRef]
- Guerriero, S.; Condous, G.; van den Bosch, T.; Valentin, L.; Leone, F.P.G.; Van Schoubroeck, D.; Exacoustos, C.; Installé, A.J.F.; Martins, W.P.; Abrao, M.S.; et al. Systematic approach to sonographic evaluation of the pelvis in women with suspected endometriosis, including terms, definitions and measurements: A consensus opinion from the International Deep Endometriosis Analysis (IDEA) group. Ultrasound Obstet. Gynecol. 2016, 48, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Revised American Society for Reproductive Medicine. Classification of endometriosis: 1996. Fertil. Steril. 1997, 67, 817–821. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Martin, D. Treatment of deeply infiltrating endometriosis. Curr. Opin. Obstet. Gynecol. 1994, 6, 231–241. [Google Scholar] [CrossRef]
- Kennedy, S.; Bergqvist, A.; Chapron, C.; D’Hooghe, T.; Dunselman, G.; Greb, R.; Hummelshoj, L.; Prentice, A.; Saridogan, E. ESHRE guideline for the diagnosis and treatment of endometriosis. Hum. Reprod. 2005, 20, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Stratton, P.; Winkel, C.A.; Sinaii, N.; Merino, M.J.; Zimmer, C.; Nieman, L.K. Location, color, size, depth, and volume may predict endometriosis in lesions resected at surgery. Fertil. Steril. 2002, 78, 743–749. [Google Scholar] [CrossRef]
- Martin, D.C. Endometriosis: Correlation between histologic and visual findings at laparoscopy. Am. J. Obstet. Gynecol. 2003, 188, 1663. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.J.; Hentz, J.G.; Magtibay, P.M.; Cornella, J.L.; Magrina, J.F. Endometriosis: Correlation between histologic and visual findings at laparoscopy. Am. J. Obstet. Gynecol. 2001, 184, 1407–1411. [Google Scholar] [CrossRef]
- Stripling, M.C.; Martin, D.C.; Chatman, D.L.; Zwaag, R.V.; Poston, W.M. Subtle appearance of pelvic endometriosis. Fertil. Steril. 1988, 49, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Wykes, C.B.; Clark, T.J.; Khan, K.S. Accuracy of laparoscopy in the diagnosis of endometriosis: A systematic quantitative review. Br. J. Obstet. Gynaecol. 2004, 111, 1204–1212. [Google Scholar] [CrossRef]
- Redwine, D.B. Age-related evolution in color appearance of endometriosis. Fertil. Steril. 1987, 48, 1062–1063. [Google Scholar] [CrossRef]
- Ghai, V.; Jan, H.; Shakir, F.; Haines, P.; Kent, A. Diagnostic delay for superficial and deep endometriosis in the United Kingdom. J. Obstet. Gynaecol. 2020, 40, 83–89. [Google Scholar] [CrossRef]
- Redwine, D.B. Peritoneal pockets and endometriosis. Confirmation of an important relationship, with further observations. J. Reprod. Med. 1989, 34, 270–272. [Google Scholar]
- Reid, S.; Leonardi, M.; Lu, C.; Condous, G. The association between ultrasound-based ‘soft markers’ and endometriosis type/location: A prospective observational study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 171–178. [Google Scholar] [CrossRef]
- Yong, P.J.; Sutton, C.; Suen, M.; Williams, C. Endovaginal ultrasound-assisted pain mapping in endometriosis and chronic pelvic pain. J. Obstet. Gynaecol. 2013, 33, 715–719. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.J.; Rombauts, L.; Ades, A.; Leong, K.; Paul, E.; Piessens, S. Poor sensitivity of transvaginal ultrasound markers in diagnosis of superficial endometriosis of the uterosacral ligaments. J. Endometr. Pelvic Pain Disord. 2018, 10, 10–17. [Google Scholar] [CrossRef]
- Guerriero, S.; Ajossa, S. Transvaginal ultrasonography in superficial endometriosis. Aust. N. Z. J. Obstet. Gynaecol. 2020, 60, E5. [Google Scholar] [CrossRef]
- Chen-Dixon, K.; Uzuner, C.; Mak, J.; Condous, G. Effectiveness of ultrasound for endometriosis diagnosis. Curr. Opin. Obstet. Gynecol. 2022, 34, 324–331. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Espada, M.; Lu, C.; Stamatopoulos, N.; Condous, G. A Novel Ultrasound Technique Called Saline Infusion SonoPODography to Visualize and Understand the Pouch of Douglas and Posterior Compartment Contents: A Feasibility Study. J. Ultrasound Med. 2019, 38, 3301–3309. [Google Scholar] [CrossRef] [PubMed]
- Bazot, M.; Daraï, E. Sonography and MR imaging for the assessment of deep pelvic endometriosis. J. Minim. Invasive Gynecol. 2005, 12, 178–185. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pedrassani, M.; Guerriero, S.; Pascual, M.Á.; Ajossa, S.; Graupera, B.; Pagliuca, M.; Podgaec, S.; Camargos, E.; Vieira de Oliveira, Y.; Alcázar, J.L. Superficial Endometriosis at Ultrasound Examination—A Diagnostic Criteria Proposal. Diagnostics 2023, 13, 1876. https://doi.org/10.3390/diagnostics13111876
Pedrassani M, Guerriero S, Pascual MÁ, Ajossa S, Graupera B, Pagliuca M, Podgaec S, Camargos E, Vieira de Oliveira Y, Alcázar JL. Superficial Endometriosis at Ultrasound Examination—A Diagnostic Criteria Proposal. Diagnostics. 2023; 13(11):1876. https://doi.org/10.3390/diagnostics13111876
Chicago/Turabian StylePedrassani, Marcelo, Stefano Guerriero, María Ángela Pascual, Silvia Ajossa, Betlem Graupera, Mariachiara Pagliuca, Sérgio Podgaec, Esdras Camargos, Ygor Vieira de Oliveira, and Juan Luis Alcázar. 2023. "Superficial Endometriosis at Ultrasound Examination—A Diagnostic Criteria Proposal" Diagnostics 13, no. 11: 1876. https://doi.org/10.3390/diagnostics13111876
APA StylePedrassani, M., Guerriero, S., Pascual, M. Á., Ajossa, S., Graupera, B., Pagliuca, M., Podgaec, S., Camargos, E., Vieira de Oliveira, Y., & Alcázar, J. L. (2023). Superficial Endometriosis at Ultrasound Examination—A Diagnostic Criteria Proposal. Diagnostics, 13(11), 1876. https://doi.org/10.3390/diagnostics13111876





