A New Hybrid Approach Based on Time Frequency Images and Deep Learning Methods for Diagnosis of Migraine Disease and Investigation of Stimulus Effect
Abstract
:1. Introduction
- Akben et al. [6] analyzed the EEG signals obtained from migraine patients and the HC group under flash stimulation in their study and detected MD with an accuracy of 85% using the Support Vector Machine (SVM) classifier.
- Aslan separated the signals into subbands by applying the Tunable Q-Factor Wavelet Transform (TQWT) method to the EEG signals in his studies on the diagnosis of MD. By extracting features from these subbands, the classification process between MD and HC was performed using the Rotation Forest algorithm. As a result of the classification process, an accuracy rate of 89.6% was obtained [10].
- In another study, Aslan applied the Empirical Mode Decomposition (EMD) method to EEG signals and separated the signals into subbands. As a result of the classification process using the features extracted from these subbands, an accuracy rate of 92.7% was obtained using a Random Forest (RF) algorithm [15].
- In another similar study, Subaşı et al., using Discrete Wavelet Transform (DWT) and RF algorithm, distinguished MD from the HC group with an accuracy of 85.95% [16].
- In a study conducted for clinical support purposes, Yin et al. [17] succeeded in distinguishing between tension-type headaches and migraine with 90% accuracy as a result of the system they developed based on the K-Nearest Neighbors (KNN) algorithm.
- In terms of the studies that used DL, Göker [14] created feature vectors by applying the Welch method to EEG signals. She used several ML methods and a Bidirectional Long-Short Term Memory (BiLSTM) model in the classification phase. The most successful performance was achieved using the BiLSTM model, as it succeeded in classifying the MD and HC groups with 95.99% accuracy.
- In this study, a new system based on EEG and DL that can support specialists in the automatic and early diagnosis of MD is proposed.
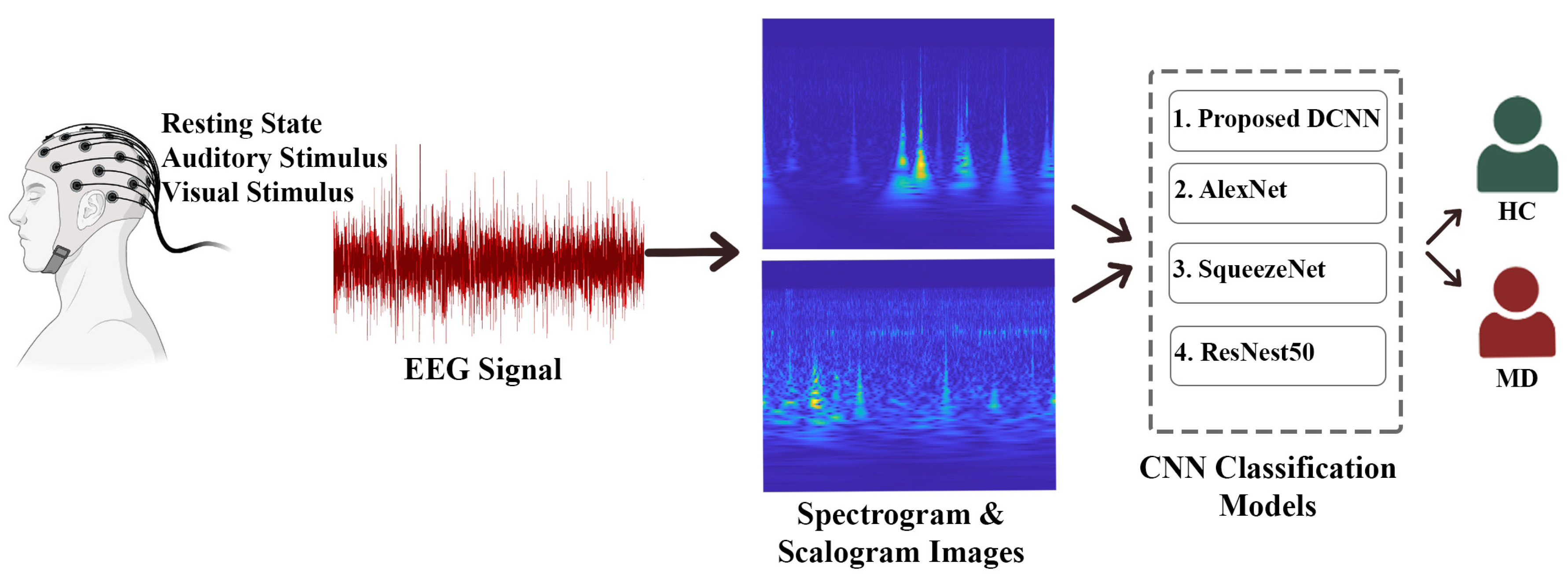
- For this purpose, in this study, recorded EEG signals based on the resting state (R), visual stimulus (V), and auditory stimulus (A) from MD and HC groups were analyzed. As a result of the analyses made possible by signal processing methods and DL models, MD and HC groups were able to be classified. This study aims to be original and to contribute to future studies.
- In this study, 1-D EEG signals obtained from MD and HC groups were preprocessed and noise-free. The noise-free EEG signals were transformed into scalogram-spectrogram images in the time-frequency domain by using the ‘CWT and STFT’ T-F transform methods.
- The classification was carried out by applying the scalogram and spectrogram images of the MD and HC groups to some CNN architectures (AlexNet, ResNet50, SqueezeNet) and to the DCNN model that we created ourselves.
- The effect of the stimuli was also examined by performing the classification process in three situations (R-A-V). As a result of the classification process, both the performance comparison of CWT and STFT, which are signal processing methods, and the performance of the DL models used in the study were compared. In this way, the best-performing state, method, and classifier model were determined. Regarding the use of EEG signals, DL, and ML in the diagnosis of MD, as far as we know, this study is the first of its kind compared to similar studies in the literature.
2. Methodology
- In the preprocessing step, the noises in the recorded EEG signals for visual, auditory stimulus, and resting state obtained from the multi-channel were cleaned using a 0.5–40 Hz finite impulse response (FIR) filter.
- Scalogram and spectrogram images were created in the T-F plane of the signals by applying the ‘CWT and STFT’ T-F transform methods for the signal processing of noise-free EEG signals.
- In this study, scalogram and spectrogram images were applied for the first time to CNN architectures (AlexNet, ResNet50, SqueezeNet) and the proposed DCNN model for three states (R-A-V). The classification process analyzed the MD–HC groups for the three situations and the applied methods. Classification performance criteria (Acc., Sens., and Spec.) ratios were obtained and interpreted for all situations and methods applied in the study.
2.1. Participants and Dataset
2.2. Signal Preprocessing and Time-Frequency Transform Techniques
2.2.1. Continuous Wavelet Transform
2.2.2. Short-Time Fourier Transform
2.3. Deep Learning Models
2.3.1. Convolutional Neural Networks and the Proposed DCNN Model
- i.
- ii.
- iii.
2.4. Classification Process and Performance Evaluation Metrics
- The TP is the number of data predicted by the model in the MD class that is actually in the MD class.
- FP is data that does not actually belong to the MD class but that the model mistakenly predicts to belong to the MD class.
- TN is the number of data that is actually in the HC group, correctly predicted by the model as belonging to the HC group.
- FN is the number of data that actually belongs to the MD class but is incorrectly predicted by the model as belonging to the HC group.
3. Experimental Results
4. Discussion
- We think that this study is very comprehensive. In this study, besides the EEG signal and DL model-based automatic diagnosis of MD, the effect of three conditions (R-A-V) was also investigated. In addition, a single T-F method was not used. Their performances were compared by applying CWT and STFT methods, both of which are widely preferred in other studies. In addition to the CNN architectures that are frequently used in studies in the literature, the performances of these classifiers were compared by creating our own DCNN model. To our knowledge, we think that this study is the first in the literature do this.
- Although EEG- and DL-based studies have been conducted on the diagnosis of MD [14,35], this study is the first of its kind. From our research, it became clear that there are few studies on the diagnosis of migraine disease, with DL-based studies being especially lacking. Due to this, we think that this study is important in terms of filling this gap in the literature.
- We consider it an advantage that the dataset used in this study is new and has not been used much.
- It is known that the CWT method gives more detailed features than other T-F methods and is preferred in other studies [35]. Upon examining the results obtained in this study, the CWT method was found to be more successful, which is in alignment with the existing literature.
- It has been seen that the DCNN model proposed in the study gives close results or is partially more successful with the CNN architectures that are widely preferred in the literature. We think that the proposed model can be evaluated in future studies on different migraine data or on the diagnosis of neurological diseases.
- As far as we know, this study is the first study regarding EEG signals and DL-based diagnosis of MD based on the resting state and visual and auditory stimuli. According to the results obtained in this study (Table 1, Table 2 and Table 3), we think that the proposed system has potential in the diagnosis of MD.
- Studies on MD diagnosis using EEG signals and DL models are very scarce. For this reason, there have not been many studies in which we can compare the results obtained in this study.
- EEG-, ML-, and DL-based studies on the diagnosis of MD are scarce, and there is no such study on the stimulus effect as far as we know. For this reason, although the results we obtained in this study are promising, there is no study in which we can compare the stimulus effect.
- We think that the number of data used in the study was sufficient. However, more data could have improved our results.
- The method used in the study and the proposed model could not be tested because there was no other migraine data. The performance of the proposed method and model can be compared by using different migraine data in the future.
5. Conclusions and Future Work
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levy, D. Migraine pain and nociceptor activation—Where do we stand? Headache J. Head Face Pain 2010, 50, 909–916. [Google Scholar] [CrossRef]
- Hussein, R.; Palangi, H.; Ward, R.; Wang, Z.J. Epileptic seizure detection: A deep learning approach. arXiv 2018, arXiv:1803.09848. [Google Scholar]
- Goadsby, P.J.; Holland, P.R.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of migraine: A disorder of sensory processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, Á.; Vécsei, L. Neurogenic inflammation: The participant in migraine and recent advancements in translational research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Upadhyay, R.; Manglick, A.; Reddy, D.K.; Padhy, P.K.; Kankar, P.K. Channel optimization and nonlinear feature extraction for Electroencephalogram signals classification. Comput. Electr. Eng. 2015, 45, 222–234. [Google Scholar] [CrossRef]
- Akben, S.B.; Tuncel, D.; Alkan, A. Classification of multi-channel EEG signals for migraine detection. Biomed. Res. 2016, 27, 743–748. [Google Scholar]
- Ndiku, A.C.; Ghedira-Chkir, R.; Khalifa, A.B.; Dogui, M. Electroencephalography signal classification for automatic interpretation of electroencephalogram based on Artificial Intelligence. In Proceedings of the 2022 8th International Conference on Control, Decision and Information Technologies (CoDIT), Istanbul, Turkey, 17–20 May 2022. [Google Scholar]
- Yao, S.; Zhu, J.; Li, S.; Zhang, R.; Zhao, J.; Yang, X.; Wang, Y. Bibliometric analysis ofquan-titative electroencephalogram (QEEG) research in neuropsychiatric disorders from 2000 to 2021. Front. Psychiatry 2022, 13, 830819. [Google Scholar] [CrossRef]
- Burgos, N.; Bottani, S.; Faouzi, J.; Thibeau-Sutre, E.; Colliot, O. Deep learning for brain dis-orders: From data processing to disease treatment. Brief. Bioinform. 2021, 22, 1560–1576. [Google Scholar] [CrossRef]
- Aslan, Z. Migraine detection from EEG signals using tunable Q-factor wavelet transform and ensemble learning techniques. Phys. Eng. Sci. Med. 2021, 44, 1201–1212. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Y.; Chen, Q.; Zhai, Q.; Liu, N.; Huang, Y.; Sun, T.; Lin, Y.; He, L.; Hou, Y.; et al. Application of EEG in migraine. Front. Hum. Neurosci. 2023, 17, 1082317. [Google Scholar] [CrossRef]
- Akhter, M.P.; Jiangbin, Z.; Naqvi, I.R.; Abdelmajeed, M.; Fayyaz, M. Exploring deep learn-ing approaches for Urdu text classification in product manufacturing. Enterp. Inf. Syst. 2022, 16, 223–248. [Google Scholar] [CrossRef]
- Nafea, M.S.; Ismail, Z.H. Supervised Machine Learning and Deep Learning Techniques for Epileptic Seizure Recognition Using EEG Signals—A Systematic Literature Review. Bioengineering 2022, 9, 781. [Google Scholar] [CrossRef]
- Göker, H. Automatic detection of migraine disease from EEG signals using bidirectional long-short term memory deep learning model. Signal Image Video Process. 2023, 17, 1255–1263. [Google Scholar] [CrossRef]
- Aslan, Z. An Empirical Mode Decomposition approach for automated diagnosis of mi-graine. Biomed. Signal Process. Control 2022, 73, 103413. [Google Scholar] [CrossRef]
- Subasi, A.; Ahmed, A.; Aličković, E.; Hassan, A.R. Effect of photic stimulation for migraine detection using random forest and discrete wavelet transform. Biomed. Signal Process. Control 2019, 49, 231–239. [Google Scholar] [CrossRef]
- Yin, Z.; Dong, Z.; Lu, X.; Yu, S.; Chen, X.; Duan, H. A clinical decision support system for the diagnosis of probable migraine and probable tension-type headache based on case-based reasoning. J. Headache Pain 2015, 16, 29. [Google Scholar] [CrossRef]
- Chamanzar, A.; Behrmann, M.; Grover, P. Ultra High-Density EEG Recording of Interictal Migraine and Controls: Sensory and Rest. 2020. Available online: https://kilthub.cmu.edu/articles/dataset/Ultra_highdensity_EEG_recording_of_interictal_migraine_and_controls_sensory_and_rest/12636731 (accessed on 13 May 2022).
- Chamanzar, A.; Haigh, S.M.; Grover, P.; Behrmann, M. Abnormalities in cortical pattern of coherence in migraine detected using ultra high-density EEG. Brain Commun. 2021, 3, fcab061. [Google Scholar] [CrossRef]
- Latifoğlu, F. A novel singular spectrum analysis-based multi-objective approach for optimal FIR filter design using artificial bee colony algorithm. Neural Comput. Appl. 2020, 32, 13323–13341. [Google Scholar] [CrossRef]
- Craik, A.; He, Y.; Contreras-Vidal, J.L. Deep learning for electroencephalogram (EEG) clas-sification tasks: A review. J. Neural Eng. 2019, 16, 031001. [Google Scholar] [CrossRef]
- Kuo, C.E.; Chen, G.T.; Liao, P.Y. An EEG spectrogram-based automatic sleep stage scoring method via data augmentation, ensemble convolution neural network, and expert knowledge. Biomed. Signal Process. Control 2021, 70, 102981. [Google Scholar] [CrossRef]
- Addison, P.S. Introduction to redundancy rules: The continuous wavelet transform comes of age. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2018, 376, 20170258. [Google Scholar] [CrossRef]
- Kumar, A.; Gandhi, C.P.; Zhou, Y.; Vashishtha, G.; Kumar, R.; Xiang, J. Improved CNN for the diagnosis of engine defects of 2-wheeler vehicle using wavelet synchro-squeezed transform (WSST). Knowl.-Based Syst. 2020, 208, 106453. [Google Scholar] [CrossRef]
- Kwok, H.K.; Jones, D.L. Improved instantaneous frequency estimation using an adaptive short-time Fourier transform. IEEE Trans. Signal Process. 2000, 48, 2964–2972. [Google Scholar] [CrossRef]
- Aslan, Z.; Akin, M. A deep learning approach in automated detection of schizophrenia us-ing scalogram images of EEG signals. Phys. Eng. Sci. Med. 2022, 45, 83–96. [Google Scholar] [CrossRef]
- Chaudhary, S.; Taran, S.; Bajaj, V.; Sengur, A. Convolutional neural network based ap-proach towards motor imagery tasks EEG signals classification. IEEE Sens. J. 2019, 19, 4494–4500. [Google Scholar] [CrossRef]
- Alom, M.Z.; Taha, T.M.; Yakopcic, C.; Westberg, S.; Sidike, P.; Nasrin, M.S.; Van Esesn, B.C.; Awwal, A.A.S.; Asari, V.K. The history began from alexnet: A comprehensive survey on deep learning approaches. arXiv 2018, arXiv:1803.01164. [Google Scholar]
- Byeon, Y.H.; Pan, S.B.; Kwak, K.C. Intelligent deep models based on scalograms of electro-cardiogram signals for biometrics. Sensors 2019, 19, 935. [Google Scholar] [CrossRef]
- Sengur, A.; Akbulut, Y.; Guo, Y.; Bajaj, V. Classification of amyotrophic lateral sclerosis disease based on convolutional neural network and reinforcement sample learning algorithm. Health Inf. Sci. Syst. 2017, 5, 9. [Google Scholar] [CrossRef]
- Moghaddari, M.; Lighvan, M.Z.; Danishvar, S. Diagnose ADHD disorder in children using convolutional neural network based on continuous mental task EEG. Comput. Methods Programs Biomed. 2020, 197, 105738. [Google Scholar] [CrossRef]
- Caliskan, A.; Rencuzogullari, S. Transfer learning to detect neonatal seizure from electro-encephalography signals. Neural Comput. Appl. 2021, 33, 12087–12101. [Google Scholar] [CrossRef]
- Elhassouny, A.; Smarandache, F. Trends in deep convolutional neural Networks architectures: A review. In Proceedings of the 2019 International Conference of Computer Science and Renewable Energies (ICCSRE 2019), Agadir, Morocco, 22–24 July 2019; pp. 1–8. [Google Scholar]
- Khare, S.K.; Bajaj, V.; Acharya, U.R. SPWVD-CNN for automated detection of schizophrenia patients using EEG signals. IEEE Trans. Instrum. Meas. 2021, 70, 1–9. [Google Scholar] [CrossRef]
- Aslan, Z. Deep Convolutional Neural Network-Based Framework in the Automatic Diagnosis of Migraine. Circuits Syst. Signal Process. 2022, 42, 3054–3071. [Google Scholar] [CrossRef]
- Jindal, K.; Upadhyay, R.; Singh, H.S.; Vijay, M.; Sharma, A.; Gupta, K.; Gupta, J.; Dube, A. Migraine disease diagnosis from EEG signals using Non-linear Feature Extraction Technique. In Proceedings of the 2018 IEEE International Conference on Computational Intelligence and Computing Research, Madurai, India, 13–15 December 2018; pp. 1–4. [Google Scholar]
- Gautam, R.; Sharma, M. Prevalence and diagnosis of neurological disorders using different deep learning techniques: A meta-analysis. J. Med. Syst. 2020, 44, 49. [Google Scholar] [CrossRef]





| CWT | STFT | |||||
|---|---|---|---|---|---|---|
| Classification Model | Acc. | Sens. | Spec. | Acc. | Sens. | Spec. |
| DCNN | 99.46 | 99.90 | 99.08 | 99.32 | 99.60 | 99.06 |
| AlexNet | 99.74 | 99.90 | 99.52 | 99.02 | 99.18 | 98.88 |
| SqueezeNet | 99.12 | 99.18 | 99.08 | 98.78 | 98.56 | 98.96 |
| ResNet50 | 98.86 | 98.64 | 99.06 | 98.58 | 97.82 | 99.26 |
| CWT | STFT | |||||
|---|---|---|---|---|---|---|
| Classification Model | Acc. | Sens. | Spec. | Acc. | Sens. | Spec. |
| DCNN | 99.44 | 99.04 | 99.74 | 99.02 | 98.80 | 99.22 |
| AlexNet | 99.28 | 98.80 | 99.66 | 98.92 | 97.96 | 99.66 |
| SqueezeNet | 98.40 | 96.64 | 99.66 | 98.14 | 97.20 | 98.88 |
| ResNet50 | 98.98 | 98.30 | 99.48 | 98.36 | 97.24 | 99.24 |
| CWT | STFT | |||||
|---|---|---|---|---|---|---|
| Classification Model | Acc. | Sens. | Spec. | Acc. | Sens. | Spec. |
| DCNN | 98.96 | 98.24 | 99.50 | 98.90 | 97.82 | 99.74 |
| AlexNet | 98.94 | 99.38 | 98.62 | 98.80 | 98.26 | 99.22 |
| SqueezeNet | 98.32 | 97.72 | 98.86 | 97.90 | 97.22 | 98.52 |
| ResNet50 | 98.54 | 98.30 | 98.70 | 98.18 | 97.80 | 98.50 |
| Study | Data Type | Dataset | Best Methods | Best Classifier | Accuracy (%) |
|---|---|---|---|---|---|
| Akben et al. [6] (2016) | EEG (V) | 30 MD-30 HC | AR Burg | SVM | 85 |
| Aslan [10] (2021) | EEG (R) | 18 MD-21 HC | TQWT | Rotation Forest | 89.6 |
| Göker [14] (2022) | EEG (R) | 18 MD-21 HC | Welch | BiLSTM | 95.99 |
| Aslan [15] (2022) | EEG (R) | 18 MD-21 HC | EMD | RF | 92.47 |
| Subaşı et al. [16] (2019) | EEG (R) | 15 MD-15 HC | DWT | RF | 85.95 |
| Jindal et al. [36] (2018) | EEG (R) | 13 MD-13 HC | Permutation Entropy Fractal Dimensions | RF | 88 |
| Proposed study | EEG (R) EEG (A) EEG (V) | 18 MD-21 HC | CWT | AlexNet Proposed DCNN Proposed DCNN | 99.74 99.44 98.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orhanbulucu, F.; Latifoğlu, F.; Baydemir, R. A New Hybrid Approach Based on Time Frequency Images and Deep Learning Methods for Diagnosis of Migraine Disease and Investigation of Stimulus Effect. Diagnostics 2023, 13, 1887. https://doi.org/10.3390/diagnostics13111887
Orhanbulucu F, Latifoğlu F, Baydemir R. A New Hybrid Approach Based on Time Frequency Images and Deep Learning Methods for Diagnosis of Migraine Disease and Investigation of Stimulus Effect. Diagnostics. 2023; 13(11):1887. https://doi.org/10.3390/diagnostics13111887
Chicago/Turabian StyleOrhanbulucu, Fırat, Fatma Latifoğlu, and Recep Baydemir. 2023. "A New Hybrid Approach Based on Time Frequency Images and Deep Learning Methods for Diagnosis of Migraine Disease and Investigation of Stimulus Effect" Diagnostics 13, no. 11: 1887. https://doi.org/10.3390/diagnostics13111887





