The Effects of Oncological Treatment on Redox Balance in Patients with Uveal Melanoma
Abstract
:1. Introduction
1.1. Epigenetic Differences of Uveal Malignant Melanoma Compared to Cutaneous Melanoma
1.2. Epigenetic Changes in Turn Produce Metabolic Reprogramming of UMM Cells
1.3. Oxidative Stress in Uveal Malignant Melanoma
2. Materials and Methods
2.1. Aim of the Study
2.2. Study Inclusion and Exclusion Criteria
2.3. Laboratory Determinations Methodology
- serum lipid peroxides in micromoles (μmol)/100 mililiters (mL) by measuring the reaction of serum malondialdehyde (MDA) [16,17] as a final product of lipid hydroperoxide degradation [16]. We evaluated by reaction with 2-tiobarbituric acid (TBA) as determined through the Carbonneau method [18]. The chemicals used were: solution of TBA (Sigma) acid 0.7% dissolved in acetic acid 50%; trichloride acetic acid 20% (Sigma); Buffer solution acetic acid—sodium acetate 50 mmols, pH value of 7.
- total serum thiol-albumin groups micromoles (μmol)/100 mililiters (mL) measurement using the Albini method [19] and Ellman reagent substrate (5,5′-dithiobis-(2-nitrobenzoic acid, DTNB) [20] measurement of oxidative protein degradation [20]. The Ellman reactive was prepared by dissolving, at warmth, 4% 5,5′-dithiobis-(2-nitrobenzoic acid (DTNB) in 100 mL of buffer phosphate solution with pH value of 8.0. After reacting plasma serum isolate with DTNB, the resultant compound was measured using spectrophotometry.
- total level of antioxidants in micrograms (μg)/liter (L), inferred via measurement of the ferric reducing ability of serum (FRAS) [21,22]. We prepared an acetate buffer solution 300 millimole (mM) pH 3.6 with 2,4,6-tri(2-pyridyl)-1,3,5-triazine (TPTZ) 10 mM and ferric chloride 20 mM in distilled and pure water obtained using the Millipore system (Milli-Q-Biocel, MilliporeSigma, subsidiary of Merck, Munich, Germany). 1 mM ferrous sulphate was used to prepare standard solutions for the etalon curve. The ferric reducing ability of plasma (FRAP) reactive was prepared from 200 mL of buffer acetate solution (pH 3.6), TPTZ solution, FeCl3 6H2O and distilled and purified water. For 30 μL of serum plasma probe 900 μL of FRAP reagent and 90 μL of water were added. The resulting compound was determined using spectrophotometry.
2.4. Statistics
2.4.1. Reference Value Comparison
2.4.2. Pre/Post-Treatment and 6/12/18/24-Month Follow-Up Comparison
2.4.3. Enucleation Surgery vs. Stereotactic Radiotherapy Comparison
2.4.4. Study Limitations
2.4.5. Local Ethics Committee Approval
3. Results
3.1. Study Population and Follow-Up
3.2. Mean Serum Determinations Results in Dynamic vs. Baseline Reference
3.3. Comparing Serum Determinations of Enucleation Surgery (ES) vs. Stereotactic Radiotherapy (SR) Patients
3.3.1. Antioxidants
3.3.2. Lipid Peroxides
3.3.3. Albumin Thiols
3.3.4. Treatment Dynamics for the First 6 Months
3.3.5. Treatment Dynamics for the Complete 2-Year Study Period
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, V. “Uvea: Neoplasms”. In Robbins and Cotran Pathologic Basis of Disease, Professional Edition (8th ed.); Elsevier: Philadelphia, PA, USA, 2009. [Google Scholar]
- Damato, B. Treatment of primary intraocular melanoma. Expert Rev. Anticancer Ther. 2006, 6, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Reichstein, D.A.; Brock, A.L. Radiation therapy for uveal melanoma: A review of treatment methods available in 2021. Curr. Opin. Ophthalmol. 2021, 32, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Schug, Z.T.; Aplin, A.E. Metabolic Alterations and Therapeutic Opportunities in Rare Forms of Melanoma. Trends Cancer 2021, 7, 671–681. [Google Scholar] [CrossRef]
- Longhitano, L.; Giallongo, S.; Orlando, L.; Broggi, G.; Longo, A.; Russo, A.; Caltabiano, R.; Giallongo, C.; Barbagallo, I.; Di Rosa, M.; et al. Lactate Rewrites the Metabolic Reprogramming of Uveal Melanoma Cells and Induces Quiescence Phenotype. Int. J. Mol. Sci. 2023, 24, 24. [Google Scholar] [CrossRef]
- Mo, Q.; Wan, L.; Schell, M.J.; Jim, H.; Tworoger, S.S.; Peng, G. Integrative Analysis Identifies Multi-Omics Signatures That Drive Molecular Classification of Uveal Melanoma. Cancers 2021, 13, 6168. [Google Scholar] [CrossRef] [PubMed]
- Robertson, A.G.; Shih, J.; Yau, C.; Gibb, E.A.; Oba, J.; Mungall, K.L.; Hess, J.M.; Uzunangelov, V.; Walter, V.; Danilova, L.; et al. Integrative Analysis Identifies Four Molecular and Clinical Subsets in Uveal Melanoma. Cancer Cell 2017, 32, 204–220.e215, Erratum in: Cancer Cell 2018, 33, 151. [Google Scholar] [CrossRef]
- Onken, M.D.; Noda, S.E.; Kaltenbronn, K.M.; Frankfater, C.; Makepeace, C.M.; Fettig, N.; Piggott, K.D.; Custer, P.L.; Ippolito, J.E.; Blumer, K.J. Oncogenic Gq/11 signaling acutely drives and chronically sustains metabolic reprogramming in uveal melanoma. J. Biol. Chem. 2022, 298, 101495. [Google Scholar] [CrossRef]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218, Erratum in: Trends Biochem. Sci. 2016, 41, 287. [Google Scholar] [CrossRef]
- Chattopadhyay, C.; Oba, J.; Roszik, J.; Marszalek, J.R.; Chen, K.; Qi, Y.; Eterovic, K.; Robertson, A.G.; Burks, J.K.; McCannel, T.A.; et al. Elevated Endogenous SDHA Drives Pathological Metabolism in Highly Metastatic Uveal Melanoma. Investig. Opthalmol. Vis. Sci. 2019, 60, 4187–4195. [Google Scholar] [CrossRef]
- Kapoor, A.; Goldberg, M.S.; Cumberland, L.K.; Ratnakumar, K.; Segura, M.F.; Emanuel, P.O.; Menendez, S.; Vardabasso, C.; Leroy, G.; Vidal, C.I.; et al. The histone variant macroH2A suppresses melanoma progression through regulation of CDK8. Nature 2010, 468, 1105–1109. [Google Scholar] [CrossRef] [PubMed]
- Giallongo, S.; Di Rosa, M.; Caltabiano, R.; Longhitano, L.; Reibaldi, M.; Distefano, A.; Lo Re, O.; Amorini, A.M.; Puzzo, L.; Salvatorelli, L.; et al. Loss of macroH2A1 decreases mitochondrial metabolism and reduces the aggressiveness of uveal melanoma cells. Aging 2020, 12, 9745–9760. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.A.; Maresca, V.; Roccella, M.; Roccella, F.; Sansolini, T.; Grammatico, P.; Balestrazzi, E.; Picardo, M. Antioxidant pattern in uveal melanocytes and melanoma cell cultures. Investig. Opthalmol. Vis. Sci. 1999, 40, 3012–3016. [Google Scholar]
- Luengo, A.; Gui, D.Y.; Vander Heiden, M.G. Targeting Metabolism for Cancer Therapy. Cell Chem. Biol. 2017, 24, 1161–1180. [Google Scholar] [CrossRef]
- Liou, G.Y.; Storz, P. Reactive oxygen species in cancer. Free Radic. Res. 2010, 44, 479–496. [Google Scholar] [CrossRef] [PubMed]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Carbonneau, M.A.; Peuchant, E.; Sess, D.; Canioni, P.; Clerc, M. Free and bound malondialdehyde measured as thiobarbituric acid adduct by HPLC in serum and plasma. Clin. Chem. 1991, 37, 1423–1429. [Google Scholar] [CrossRef]
- Albini, A. Standardization of protein free SH groups in blood plasma. Boll. Soc. Ital. Sperim. 1990, 18, 1829–1898. [Google Scholar]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Cao, G.; Prior, R.L. Comparison of different analytical methods for assessing total antioxidant capacity of human serum. Clin. Chem. 1998, 44, 1309–1315. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Hanikoglu, F.; Hanikoglu, A.; Kucuksayan, E.; Alisik, M.; Gocener, A.A.; Erel, O.; Baykara, M.; Cuoghi, A.; Tomasi, A.; Ozben, T. Dynamic thiol/disulphide homeostasis before and after radical prostatectomy in patients with prostate cancer. Free. Radic. Res. 2016, 50, S79–S84. [Google Scholar] [CrossRef] [PubMed]
- Sosa, V.; Moliné, T.; Somoza, R.; Paciucci, R.; Kondoh, H.; Lleonart, M.E. Oxidative stress and cancer: An overview. Ageing Res. Rev. 2013, 12, 376–390. [Google Scholar] [CrossRef] [PubMed]
- Guijarro, M.V.; Leal, J.F.; Blanco-Aparicio, C.; Alonso, S.; Fominaya, J.; Lleonart, M.; Castellvi, J.; Ramon y Cajal, S.; Carnero, A. MAP17 enhances the malignant behavior of tumor cells through ROS increase. Carcinogenesis 2007, 28, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Jensen, S.A.; Sørensen, J.B.; Henriksen, T.; Weimann, A.; Poulsen, H.E. Oxidative damage to guanine nucleosides following combination chemotherapy with 5-fluorouracil and oxaliplatin. Cancer Chemother. Pharmacol. 2012, 69, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, J.; Hu, Y.; Lu, W.; Pelicano, H.; Huang, P. Novel action of paclitaxel against cancer cells: Bystander effect mediated by reactive oxygen species. Cancer Res. 2007, 67, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Liu-Smith, F.; Chiu, C.Y.; Johnson, D.L.; Miller, P.W.; Glazer, E.S.; Wu, Z.; Wilson, M.W. The Sex Differences in Uveal Melanoma: Potential Roles of EIF1AX, Immune Response and Redox Regulation. Curr. Oncol. 2021, 28, 2801–2811. [Google Scholar] [CrossRef]
- Zimmerman, L.E.; McLean, I.W.; Foster, W.D. Does enucleation of the eye containing a malignant melanoma prevent or accelerate the dissemination of tumour cells. Br. J. Ophthalmol. 1978, 62, 420–425. [Google Scholar] [CrossRef]
- Damato, B. Does ocular treatment of uveal melanoma influence survival? Br. J. Cancer 2010, 103, 285–290. [Google Scholar] [CrossRef]
- Manschot, W.A.; van Strik, R. Is irradiation a justifiable treatment of choroidal melanoma? Analysis of published results. Br. J. Ophthalmol. 1987, 71, 348–352. [Google Scholar] [CrossRef]
- Hawkins, B.S.; Collaborative Ocular Melanoma Study Group. The Collaborative Ocular Melanoma Study (COMS) randomized trial of pre-enucleation radiation of large choroidal melanoma: IV. Ten-year mortality findings and prognostic factors. COMS report number 24. Am. J. Ophthalmol. 2004, 138, 936–951. [Google Scholar] [CrossRef] [PubMed]
- Straatsma, B.R.; Diener-West, M.; Caldwell, R.; Engstrom, R.E. Mortality after deferral of treatment or no treatment for choroidal melanoma. Am. J. Ophthalmol. 2003, 136, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Gragoudas, E.S.; Lane, A.M.; Munzenrider, J.; Egan, K.M.; Li, W. Long-term risk of local failure after proton therapy for choroidal/ciliary body melanoma. Trans. Am. Ophthalmol. Soc. 2002, 100, 43–48. [Google Scholar] [PubMed]
- Diener-West, M.; Earle, J.D.; Fine, S.L.; Hawkins, B.S.; Moy, C.S.; Reynolds, S.M.; Schachat, A.P.; Straatsma, B.R.; Collaborative Ocular Melanoma Study Group. The COMS randomized trial of iodine 125 brachytherapy for choroidal melanoma, III: Initial mortality findings. COMS Report No. 18. Arch. Ophthalmol. 2001, 119, 969–982. [Google Scholar] [CrossRef]
- Honavar, S.G. Is Collaborative Ocular Melanoma Study (COMS) still relevant? Indian J. Ophthalmol. 2018, 66, 1385–1387. [Google Scholar] [CrossRef]
- Kaya, E.; Ozgok, Y.; Zor, M.; Eken, A.; Bedir, S.; Erdem, O.; Ebiloglu, T.; Ergin, G. Oxidative stress parameters in patients with prostate cancer, benign prostatic hyperplasia and asymptomatic inflammatory prostatitis: A prospective controlled study. Adv. Clin. Exp. Med. 2017, 26, 1095–1099. [Google Scholar] [CrossRef]
- Aydin, A.; Arsova-Sarafinovska, Z.; Sayal, A.; Eken, A.; Erdem, O.; Erten, K.; Ozgök, Y.; Dimovski, A. Oxidative stress and antioxidant status in non-metastatic prostate cancer and benign prostatic hyperplasia. Clin. Biochem. 2006, 39, 176–179. [Google Scholar] [CrossRef]
- Mazzuferi, G.; Bacchetti, T.; Islam, M.O.; Ferretti, G. High density lipoproteins and oxidative stress in breast cancer. Lipids Health Dis. 2021, 20, 143. [Google Scholar] [CrossRef]
- Zhang, K.; Ping, L.; Du, T.; Wang, Y.; Sun, Y.; Liang, G.; Wang, X.; Xie, X.; Wei, W.; Xiao, X.; et al. A Novel Systematic Oxidative Stress Score Predicts the Prognosis of Patients with Operable Breast Cancer. Oxidative Med. Cell. Longev. 2021, 2021, 9441896. [Google Scholar] [CrossRef]
- Qian, J.Y.; Hao, Y.; Yu, H.H.; Wu, L.L.; Liu, Z.Y.; Peng, Q.; Li, Z.X.; Li, K.; Liu, Y.; Wang, R.R.; et al. A Novel Systematic Oxidative Stress Score Predicts the Survival of Patients with Early-Stage Lung Adenocarcinoma. Cancers 2023, 15, 1718. [Google Scholar] [CrossRef]
- Yang, Y.; Long, X.; Li, K.; Li, G.; Yu, X.; Wen, P.; Luo, J.; Tian, X.; Zhao, J. Development and validation of an oxidative stress-associated prognostic risk model for melanoma. PeerJ 2021, 9, e11258. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhao, J. Novel oxidative stress-related prognostic biomarkers for melanoma associated with tumor metastasis. Medicine 2021, 100, e24866. [Google Scholar] [CrossRef] [PubMed]
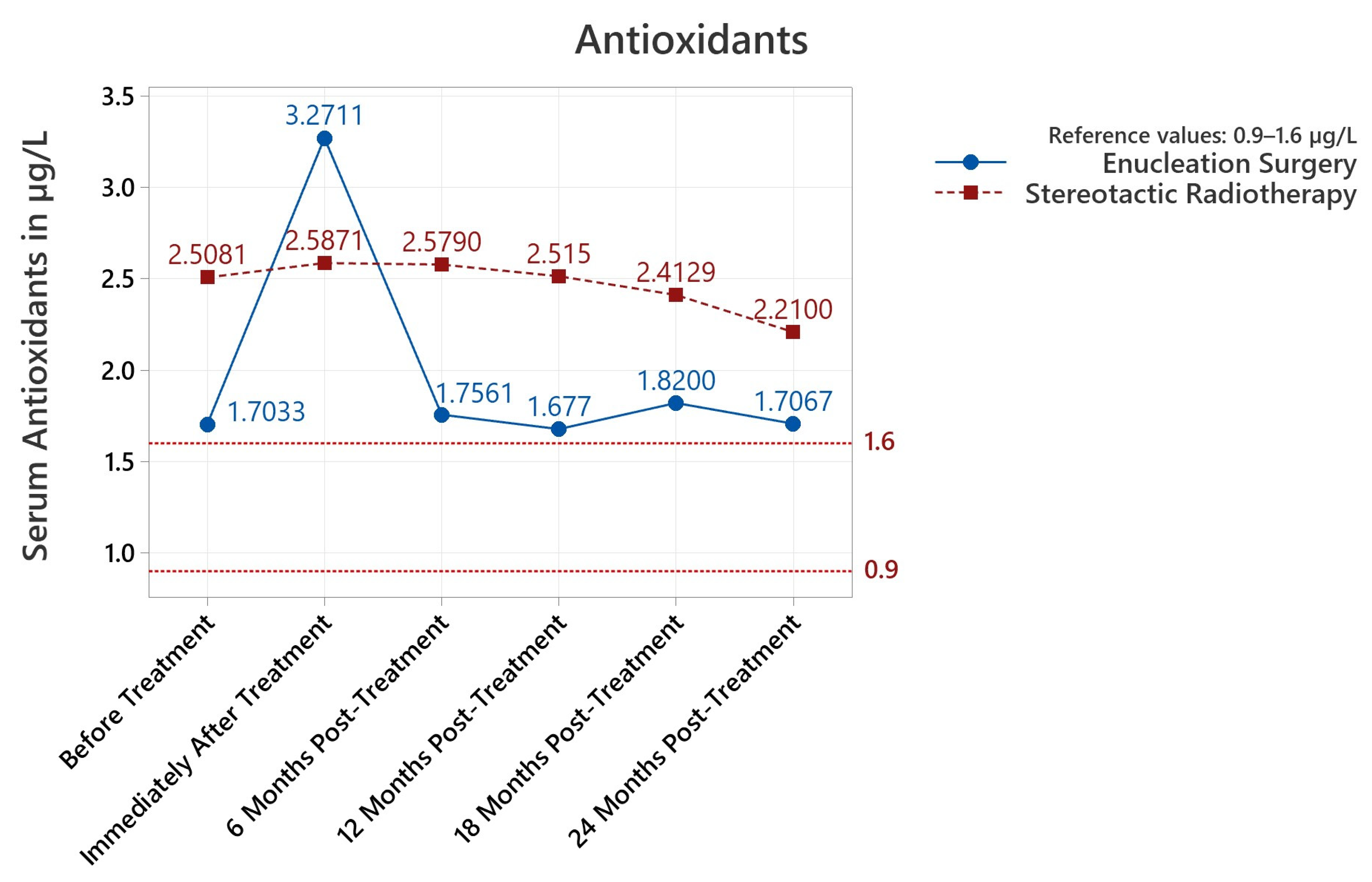

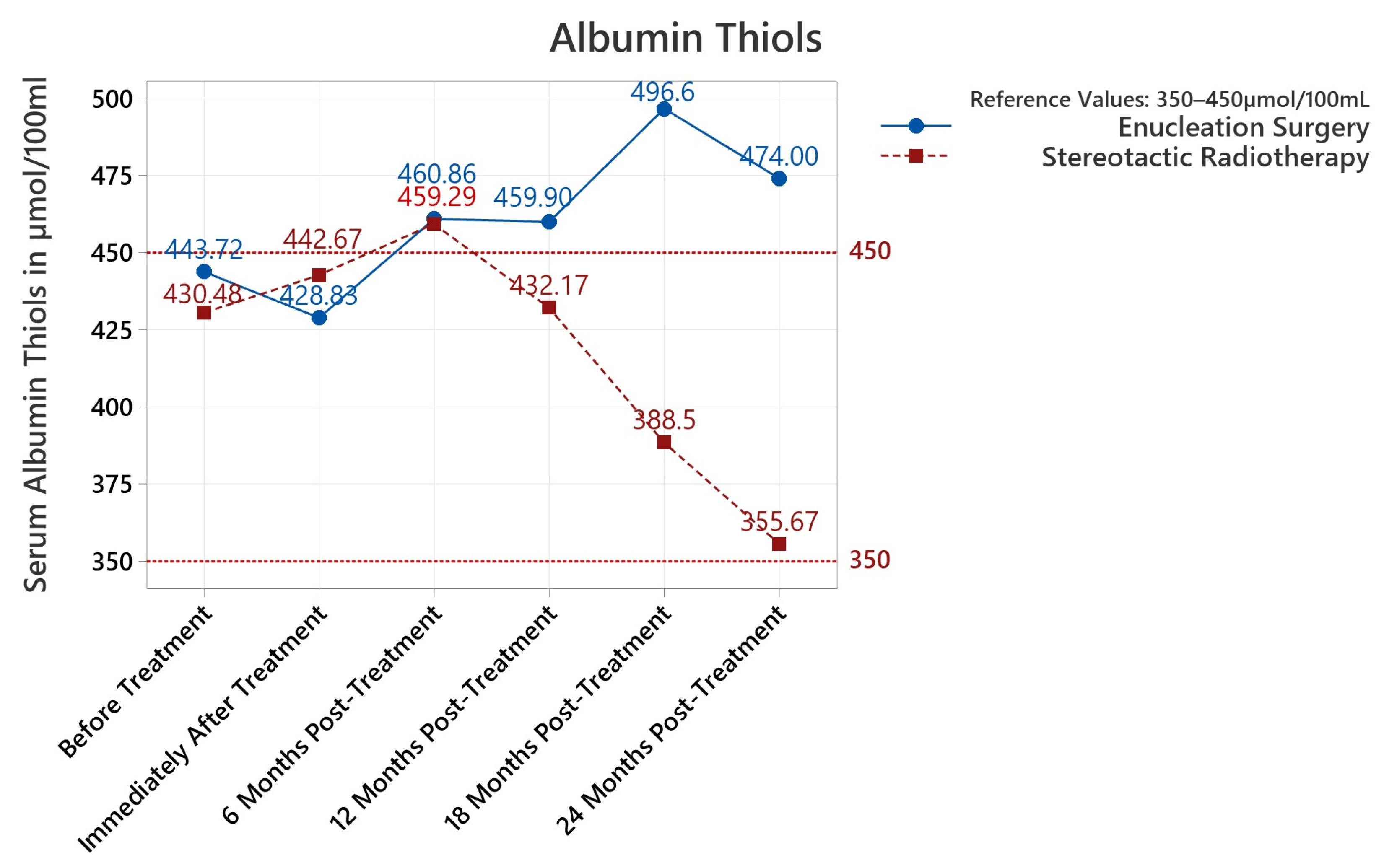
| Antioxidants Reference 0.9–1.6 μg/L | Lipid Peroxides Reference 0–4 μmol/100 mL | Albumin Thiols Reference 350–450 μmol/100 mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Treatment Dynamic | Mean | % inc * | p | Significance | Values over Ref | Mean | % inc * | p | Significance | Values over Ref | Mean | % inc * | p | Significance | Values over Ref |
| Pre-Treat | 2.137 | 133.56% | <0.001 | Yes | 33/39 | 5.132 | 128.30% | <0.001 | Yes | 28/39 | 436.6 | 97.02% | 0.279 | No | 17/39 |
| Post-Treat | 2.903 | 181.43% | 0.049 | Yes | 35/39 | 5.283 | 132.07% | <0.001 | Yes | 33/39 | 436.3 | 96.95% | 0.511 | No | 15/39 |
| 6 Months | 2.199 | 137.45% | <0.001 | Yes | 32/39 | 5.402 | 135.05% | <0.001 | Yes | 33/39 | 460 | 102.22% | 0.624 | No | 21/39 |
| 12 Months | 2.134 | 137.37% | <0.001 | Yes | 18/22 | 5.385 | 134.62% | 0.001 | Yes | 19/22 | 444.8 | 98.84% | 0.734 | No | 9/22 |
| 18 Months | 2.166 | 135.37% | 0.002 | Yes | 10/11 | 4.777 | 119.42% | 0.144 | No | 8/11 | 437.6 | 97.24% | 0.633 | No | 5/11 |
| 24 Months | 1.958 | 122.37% | 0.082 | No | 5/6 | 3.968 | 99.2% | 0.952 | No | 2/6 | 414.8 | 92.17% | 0.290 | No | 2/6 |
| Enucleation vs. Radiotherapy | Antioxidants μg/L | Lipid Peroxides μmol/100 mL | Albumin Thiols μmol/100 mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Dynamic | ES vs. SR 2 Sample t-Test | p | ES vs. SR ANOVA p | ES vs. SR 2 Sample t-Test | p | ES vs. SR ANOVA p | ES vs. SR 2 Sample t-Test | p | ES vs. SR ANOVA p |
| Pre-Treat | +0.805 SR | <0.001 | <0.001 | +1.305 ES | 0.004 | 0.003 | +13.2 SR | 0.602 | 0.595 |
| Post-Treat | +0.68 ES | 0.633 | 0.602 | +1.170 ES | 0.010 | 0.009 | +13.8 SR | 0.757 | 0.744 |
| 6 Months | +0.823 SR | <0.001 | <0.001 | +1.212 ES | 0.005 | 0.005 | +1.6 ES | 0.971 | 0.970 |
| 12 Months | +0.838 SR | <0.001 | <0.001 | +0.485 ES | 0.490 | 0.488 | +27.7 ES | 0.369 | 0.377 |
| 18 Months | +0.593 SR | 0.033 | 0.027 | +0.28 ES | 0.793 | 0.790 | +108.1 ES | 0.020 | 0.022 |
| 24 Months | +0.503 SR | 0.160 | 0.136 | +0.14 ES | 0.909 | 0.904 | +118.3 ES | 0.030 | 0.017 |
| Males vs. Females | Antioxidants μg/L | Lipid Peroxides μmol/100 mL | Albumin Thiols μmol/100 mL | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Treatment Dynamic | M vs. F 2 Sample t-Test | p | M vs. F ANOVA p | M vs. F 2 Sample t-Test | p | M vs. F ANOVA p | M vs. F 2 Sample t-Test | p | M vs. F ANOVA p |
| Pre-Treat | +0.045 M | 0.821 | 0.827 | +0.995 M | 0.029 | 0.029 | +17.7 F | 0.479 | 0.479 |
| Post-Treat | +1.19 M | 0.307 | 0.364 | +0.695 M | 0.148 | 0.134 | +20 F | 0.620 | 0.637 |
| 6 Months | +0.095 F | 0.611 | 0.619 | +0.860 M | 0.062 | 0.054 | +7.3 M | 0.850 | 0.861 |
| 12 Months | +0.037 M | 0.885 | 0.884 | +0.716 M | 0.302 | 0.300 | +8.6 M | 0.785 | 0.784 |
| 18 Months | +0.007 M | 0.981 | 0.983 | +2.015 M | 0.035 | 0.031 | +71.2 M | 0.223 | 0.168 |
| 24 Months | +0.342 F | 0.601 | 0.386 | +0.528 M | 0.586 | 0.671 | +46.0 M | 0.645 | 0.528 |
| Antioxidants μg/L | Lipid Peroxides μmol/100 mL | Albumin Thiols μmol/100 mL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre-Treatment Versus | Paired S t-Test M. Diff | p | Significance | ANOVA p | Variance Diff | Paired S t-Test M. Diff | p | Significance | ANOVA p | Variance Diff | Paired S t-Test M. Diff | p | Significance | ANOVA p | Variance Diff |
| Post-Treat | −0.766 | 0.238 | No | 0.242 | No | −0.1505 | 0.120 | No | 0.644 | No | 0.3 | 0.982 | No | 0.990 | No |
| 6 Months | −0.0625 | 0.239 | No | 0.650 | No | −0.2703 | 0.001 | Yes | 0.400 | No | −23.4 | 0.180 | No | 0.326 | No |
| 12 Months | −0.0123 | 0.836 | No | 0.987 | No | −0.318 | 0.029 | Yes | 0.527 | No | −23.7 | 0.093 | No | 0.682 | No |
| 18 Months | 0.0958 | 0.205 | No | 0.883 | No | −0.045 | 0.805 | No | 0.484 | No | −27.3 | 0.217 | No | 0.969 | No |
| 24 Months | 0.243 | 0.086 | No | 0.506 | No | 0.182 | 0.307 | No | 0.067 | No | 24.7 | 0.057 | No | 0.517 | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Păsărică, M.A.; Curcă, P.F.; Burcea, M.; Schmitzer, S.; Dragosloveanu, C.D.M.; Grigorescu, A.C. The Effects of Oncological Treatment on Redox Balance in Patients with Uveal Melanoma. Diagnostics 2023, 13, 1907. https://doi.org/10.3390/diagnostics13111907
Păsărică MA, Curcă PF, Burcea M, Schmitzer S, Dragosloveanu CDM, Grigorescu AC. The Effects of Oncological Treatment on Redox Balance in Patients with Uveal Melanoma. Diagnostics. 2023; 13(11):1907. https://doi.org/10.3390/diagnostics13111907
Chicago/Turabian StylePăsărică, Mihai Adrian, Paul Filip Curcă, Marian Burcea, Speranța Schmitzer, Christiana Diana Maria Dragosloveanu, and Alexandru Călin Grigorescu. 2023. "The Effects of Oncological Treatment on Redox Balance in Patients with Uveal Melanoma" Diagnostics 13, no. 11: 1907. https://doi.org/10.3390/diagnostics13111907







