Validation of a Length-Adjusted Abdominal Arterial Calcium Score Method for Contrast-Enhanced CT Scans
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Inclusion Criteria
2.2. CT Protocol
2.3. Calcium Score Analysis
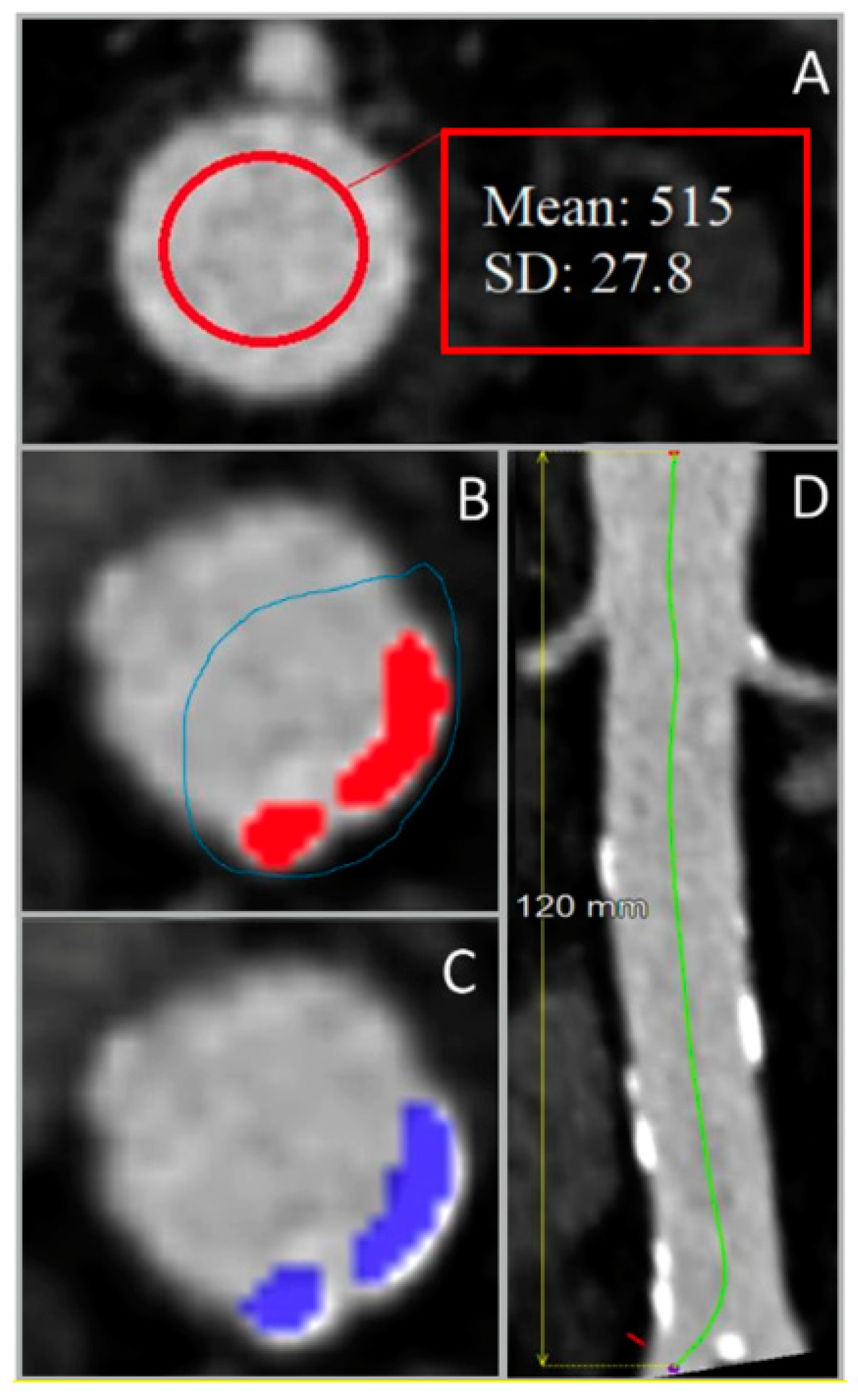
2.3.1. Calcium Segmentation
2.3.2. Interobserver Agreement
2.3.3. Influence of CT Slice Thickness
2.4. Statistical Analysis
3. Results
3.1. Calcium Score Analysis
3.1.1. Noncontrast vs. Contrast-Enhanced CT
3.1.2. Interobserver Agreement
3.1.3. Influence of Slice Thickness
4. Discussion
4.1. Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef]
- Roquer, J.; Ois, A. Atherosclerotic Burden and Mortality. In Handbook of Disease Burdens and Quality of Life Measures; Springer: New York, NY, USA, 2010; pp. 899–918. [Google Scholar]
- Criqui, M.H.; Denenberg, J.O.; McClelland, R.L.; Allison, M.A.; Ix, J.H.; Guerci, A.; Cohoon, K.P.; Srikanthan, P.; Watson, K.E.; Wong, N.D. Abdominal Aortic Calcium, Coronary Artery Calcium, and Cardiovascular Morbidity and Mortality in the Multi-Ethnic Study of Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 1574–1579. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, J.A.; Bhatt, D.L.; Banerjee, S.; Glorioso, T.J.; Josey, K.P.; Swaminathan, R.V.; Maddox, T.M.; Armstrong, E.J.; Duvernoy, C.; Waldo, S.W.; et al. Risk of Obstructive Coronary Artery Disease and Major Adverse Cardiac Events in Patients with Noncoronary Atherosclerosis: Insights from the Veterans Affairs Clinical Assessment, Reporting, and Tracking (CART)Program. Am. Heart J. 2019, 213, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Alluri, K.; Joshi, P.H.; Henry, T.S.; Blumenthal, R.S.; Nasir, K.; Blaha, M.J. Scoring of Coronary Artery Calcium Scans: History, Assumptions, Current Limitations, and Future Directions. Atherosclerosis 2015, 239, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Neves, P.O.; Andrade, J.; Monção, H. Coronary Artery Calcium Score: Current Status. Radiol. Bras. 2017, 50, 182–189. [Google Scholar] [CrossRef]
- Holcombe, S.A.; Horbal, S.R.; Ross, B.E.; Brown, E.; Derstine, B.A.; Wang, S.C. Variation in Aorta Attenuation in Contrast-Enhanced CT and Its Implications for Calcification Thresholds. PLoS ONE 2022, 17, e0277111. [Google Scholar] [CrossRef]
- Mylonas, I.; Alam, M.; Amily, N.; Small, G.; Chen, L.; Yam, Y.; Hibbert, B.; Chow, B.J.W. Quantifying Coronary Artery Calcification from a Contrast-Enhanced Cardiac Computed Tomography Angiography Study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 210–215. [Google Scholar] [CrossRef]
- Summers, R.M.; Elton, D.C.; Lee, S.; Zhu, Y.; Liu, J.; Bagheri, M.; Sandfort, V.; Grayson, P.C.; Mehta, N.N.; Pinto, P.A.; et al. Atherosclerotic Plaque Burden on Abdominal CT: Automated Assessment with Deep Learning on Noncontrast and Contrast-Enhanced Scans. Acad. Radiol. 2021, 28, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Elton, D.C.; Lee, S.; Pickhardt, P.J.; Summers, R.M. Image Translation by Latent Union of Subspaces for Cross-Domain Plaque Detection. arXiv 2020, arXiv:2005.11384. [Google Scholar]
- Buijs, R.V.C.; Leemans, E.L.; Greuter, M.; Tielliu, I.F.J.; Zeebregts, C.J.; Willems, T.P. Quantification of Abdominal Aortic Calcification: Inherent Measurement Errors in Current Computed Tomography Imaging. PLoS ONE 2018, 13, e0193419. [Google Scholar] [CrossRef]
- Raggi, P.; Callister, T.Q.; Cooil, B. Calcium Scoring of the Coronary Artery by Electron Beam CT: How to Apply an Individual Attenuation Threshold. Am. J. Roentgenol. 2002, 178, 497–502. [Google Scholar] [CrossRef]
- O’Connor, S.D.; Graffy, P.M.; Zea, R.; Pickhardt, P.J. Does Nonenhanced CT-Based Quantification of Abdominal Aortic Calcification Outperform the Framingham Risk Score in Predicting Cardiovascular Events in Asymptomatic Adults? Radiology 2019, 290, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Benjamens, S.; Rijkse, E.; Te Velde-Keyzer, C.A.; Berger, S.P.; Moers, C.; de Borst, M.H.; Yakar, D.; Slart, R.H.J.A.; Dor, F.J.M.F.; Minnee, R.C.; et al. Aorto-Iliac Artery Calcification Prior to Kidney Transplantation. J. Clin. Med. 2020, 9, 2893. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Ulzheimer, S.; Halliburton, S.S.; Shanneik, K.; White, R.D.; Kalender, W.A. Coronary Artery Calcium: A Multi-Institutional, Multimanufacturer International Standard for Quantification at Cardiac CT. Radiology 2007, 243, 527–538. [Google Scholar] [CrossRef] [PubMed]
- Koo, T.K.; Li, M.Y. A Guideline of Selecting and Reporting Intraclass Correlation Coefficients for Reliability Research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, B.; Kantert, C.; Meyer, T.; Hadamitzky, M.; Martinoff, S.; Schömig, A.; Hausleiter, J. Cardiovascular Risk Assessment Based on the Quantification of Coronary Calcium in Contrast-Enhanced Coronary Computed Tomography Angiography. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 468–475. [Google Scholar] [CrossRef]
- Nadjiri, J.; Kaissis, G.; Meurer, F.; Weis, F.; Laugwitz, K.L.; Straeter, A.S.; Muenzel, D.; Noël, P.B.; Rummeny, E.J.; Rasper, M. Accuracy of Calcium Scoring Calculated from Contrast-Enhanced Coronary Computed Tomography Angiography Using a Dual-Layer Spectral CT: A Comparison of Calcium Scoring from Real and Virtual Non-Contrast Data. PLoS ONE 2018, 13, e0208588. [Google Scholar] [CrossRef]
- Mühlenbruch, G.; Klotz, E.; Wildberger, J.E.; Koos, R.; Das, M.; Niethammer, M.; Hohl, C.; Honnef, D.; Thomas, C.; Günther, R.W.; et al. The Accuracy of 1- and 3-Mm Slices in Coronary Calcium Scoring Using Multi-Slice CT in Vitro and in Vivo. Eur. Radiol. 2007, 17, 321–329. [Google Scholar] [CrossRef]
- Van Der Bijl, N.; De Bruin, P.W.; Geleijns, J.; Bax, J.J.; Schuijf, J.D.; De Roos, A.; Kroft, L.J.M. Assessment of Coronary Artery Calcium by Using Volumetric 320-Row Multi-Detector Computed Tomography: Comparison of 0.5 Mm with 3.0 Mm Slice Reconstructions. Int. J. Cardiovasc. Imaging 2010, 26, 473–482. [Google Scholar] [CrossRef]
- de Jong, D.J.; van der Star, S.; Bleys, R.L.A.W.; Schilham, A.M.R.; Kuijf, H.J.; de Jong, P.A.; Kok, M. Computed Tomography-Based Calcium Scoring in Cadaver Leg Arteries: Influence of Dose, Reader, and Reconstruction Algorithm. Eur. J. Radiol. 2022, 146, 110080. [Google Scholar] [CrossRef]
- Ghadri, J.R.; Goetti, R.; Fiechter, M.; Pazhenkottil, A.P.; Kest, S.M.; Nkoulou, R.N.; Windler, C.; Buechel, R.R.; Herzog, B.A.; Gaemperli, O.; et al. Inter-Scan Variability of Coronary Artery Calcium Scoring Assessed on 64-Multidetector Computed Tomography vs. Dual-Source Computed Tomography: A Head-to-Head Comparison. Eur. Heart J. 2011, 32, 1865–1874. [Google Scholar] [CrossRef]
- Mao, S.S.; Pal, R.S.; McKay, C.R.; Gao, Y.G.; Gopal, A.; Ahmadi, N.; Child, J.; Carson, S.; Takasu, J.; Sarlak, B.; et al. Comparison of Coronary Artery Calcium Scores between Electron Beam Computed Tomography and 64-Multidetector Computed Tomographic Scanner. J. Comput. Assist. Tomogr. 2009, 33, 175–178. [Google Scholar] [CrossRef] [PubMed]
- Lira, D.; Padole, A.; Kalra, M.K.; Singh, S. Tube Potential and CT Radiation Dose Optimization. AJR. Am. J. Roentgenol. 2015, 204, W4–W10. [Google Scholar] [CrossRef] [PubMed]
- Mantini, C.; Maffei, E.; Toia, P.; Ricci, F.; Seitun, S.; Clemente, A.; Malagò, R.; Runza, G.; La Grutta, L.; Midiri, M.; et al. Influence of Image Reconstruction Parameters on Cardiovascular Risk Reclassification by Computed Tomography Coronary Artery Calcium Score. Eur. J. Radiol. 2018, 101, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Weininger, M.; Ritz, K.S.; Schoepf, U.J.; Flohr, T.G.; Vliegenthart, R.; Costello, P.; Hahn, D.; Beissert, M. Interplatform Reproducibility of CT Coronary Calcium Scoring Software. Radiology 2012, 265, 70–77. [Google Scholar] [CrossRef]
- Ajlan, M.; Ahmed, A.; Alskaini, A.M.; Abukhaled, N.F.; Alsaileek, A.; Ajlan, A.; Sulaiman, I.F.; Al-Mallah, M.H. The Reproducibility of Coronary Artery Calcium Scoring on Different Software Platforms. Int. J. Cardiol. 2015, 187, 155–156. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Devia-Rodriguez, R.; Derksen, M.; de Groot, K.; Vedder, I.R.; Zeebregts, C.J.; Bokkers, R.P.H.; Pol, R.A.; de Vries, J.-P.P.M.; Schuurmann, R.C.L. Validation of a Length-Adjusted Abdominal Arterial Calcium Score Method for Contrast-Enhanced CT Scans. Diagnostics 2023, 13, 1934. https://doi.org/10.3390/diagnostics13111934
Devia-Rodriguez R, Derksen M, de Groot K, Vedder IR, Zeebregts CJ, Bokkers RPH, Pol RA, de Vries J-PPM, Schuurmann RCL. Validation of a Length-Adjusted Abdominal Arterial Calcium Score Method for Contrast-Enhanced CT Scans. Diagnostics. 2023; 13(11):1934. https://doi.org/10.3390/diagnostics13111934
Chicago/Turabian StyleDevia-Rodriguez, Raul, Maikel Derksen, Kristian de Groot, Issi R. Vedder, Clark J. Zeebregts, Reinoud P. H. Bokkers, Robert A. Pol, Jean-Paul P. M. de Vries, and Richte C. L. Schuurmann. 2023. "Validation of a Length-Adjusted Abdominal Arterial Calcium Score Method for Contrast-Enhanced CT Scans" Diagnostics 13, no. 11: 1934. https://doi.org/10.3390/diagnostics13111934
APA StyleDevia-Rodriguez, R., Derksen, M., de Groot, K., Vedder, I. R., Zeebregts, C. J., Bokkers, R. P. H., Pol, R. A., de Vries, J.-P. P. M., & Schuurmann, R. C. L. (2023). Validation of a Length-Adjusted Abdominal Arterial Calcium Score Method for Contrast-Enhanced CT Scans. Diagnostics, 13(11), 1934. https://doi.org/10.3390/diagnostics13111934






