Radiomics from Mesorectal Blood Vessels and Lymph Nodes: A Novel Prognostic Predictor for Rectal Cancer with Neoadjuvant Therapy
Abstract
:1. Introduction
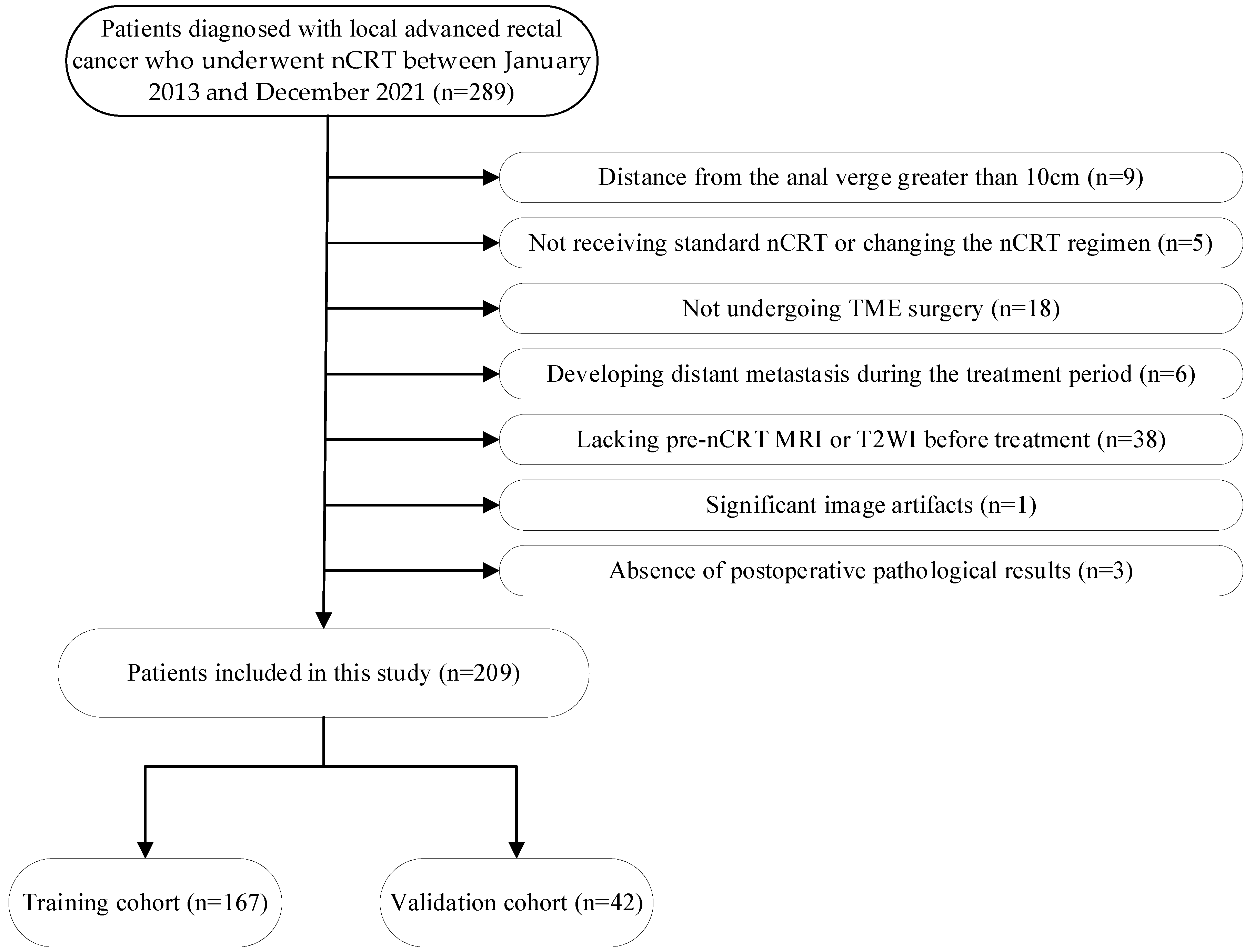
2. Materials and Methods
2.1. Study Patients
2.2. Neoadjuvant Chemoradiotherapy
2.3. Reference Standard
2.4. MRI Protocol
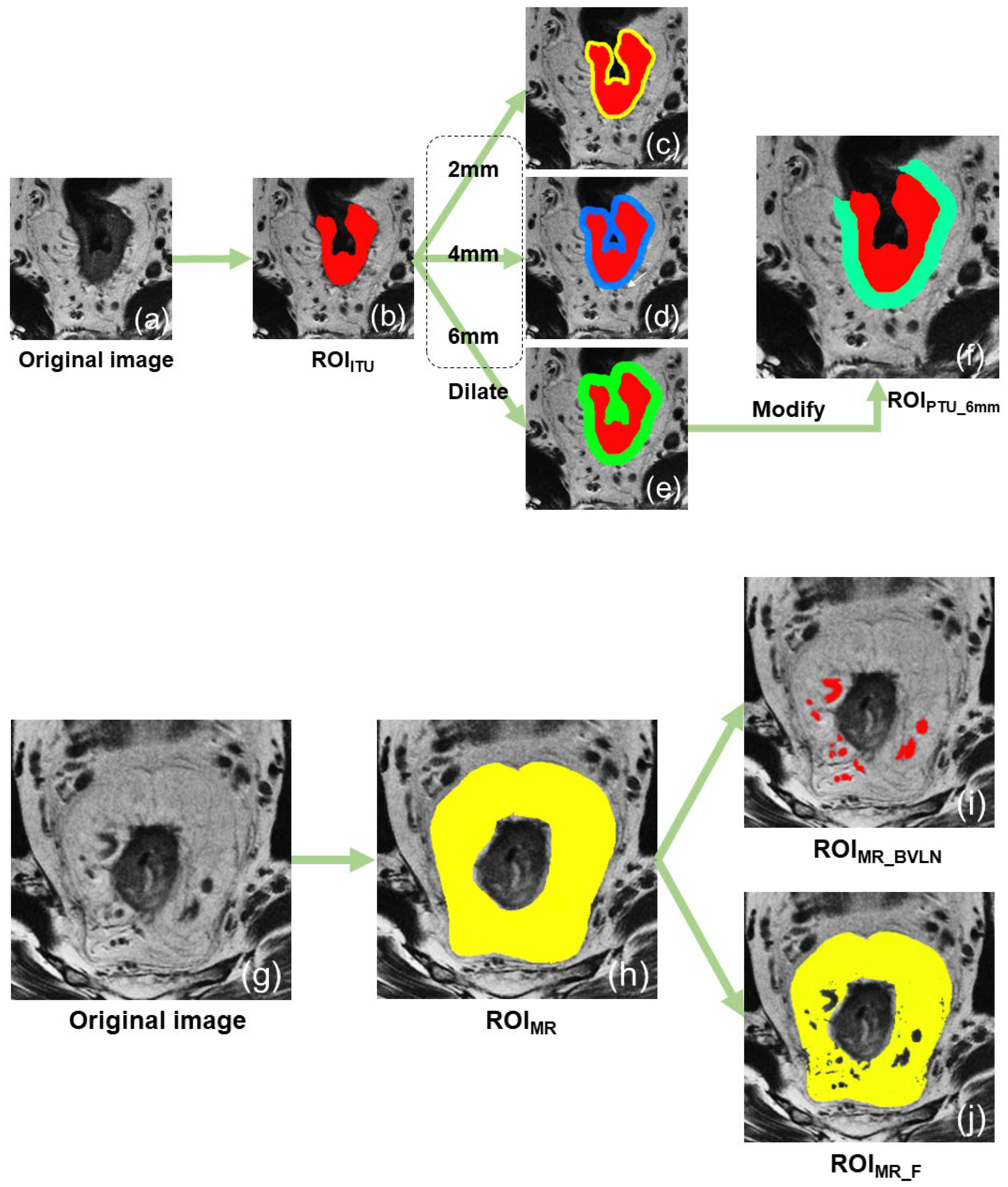
2.5. Image Segmentation
- (1)
- Intratumoral region of interest (ROIITU): First, the location and extent of the lesion was confirmed by combining the DWI and T2WI sequences. Subsequently, the tumor was manually segmented with meticulous attention to detail, ensuring its proper inclusion within the rectal contour and extension beyond the serosa, while simultaneously excluding any fibrous bands or spicules surrounding it.
- (2)
- The 2 mm peritumoral region of interest (ROIPTU_2mm) was generated by applying the “dilation” tool on the uAI platform to the initial ROIITU, thereby expanding its boundaries by 2 mm and retaining the added portion. To ensure that the ROI solely consisted of the rectal wall and mesorectum around the tumor, the areas outside the mesorectal fascia, within the rectal lumen, and inside the tumor were manually excluded.
- (3)
- The 4 mm peritumoral region of interest (ROIPTU_4mm) was segmented using the same method as ROIPTU_2mm, except that the dilation distance was increased to 4 mm.
- (4)
- The 6 mm peritumoral region of interest (ROIPTU_6mm) was segmented using the same method as ROIPTU_2mm, except that the dilation distance was increased to 6 mm.
- (5)
- The mesorectal region of interest (ROIMR) refers to the area within the mesorectal fascia, outside the contours of the rectum and tumor, and below the peritoneal reflection.
- (6)
- The mesorectal fat region of interest (ROIMR_F) was created using the “threshold separation” tool on the uAI platform. The signal intensity threshold was adjusted to select only the fat signals (high signals) within the ROIMR, and a manual correction was carried out to remove the non–fat contents.
- (7)
- The mesorectal blood vessels + lymph nodes region of interest (ROIMR_BVLN) was created by adjusting the signal intensity threshold to select the middle to low signals within the ROIMR, which were mostly composed of the blood vessels and lymph nodes. A manual correction of the ROI was then performed to ensure that it only included blood vessels and lymph nodes.
2.6. Clinical and Follow–Up Information
2.7. Radiomics Feature Extraction and Selection
2.8. Model Construction
2.9. Statistical Analysis
3. Results
3.1. Clinical Features
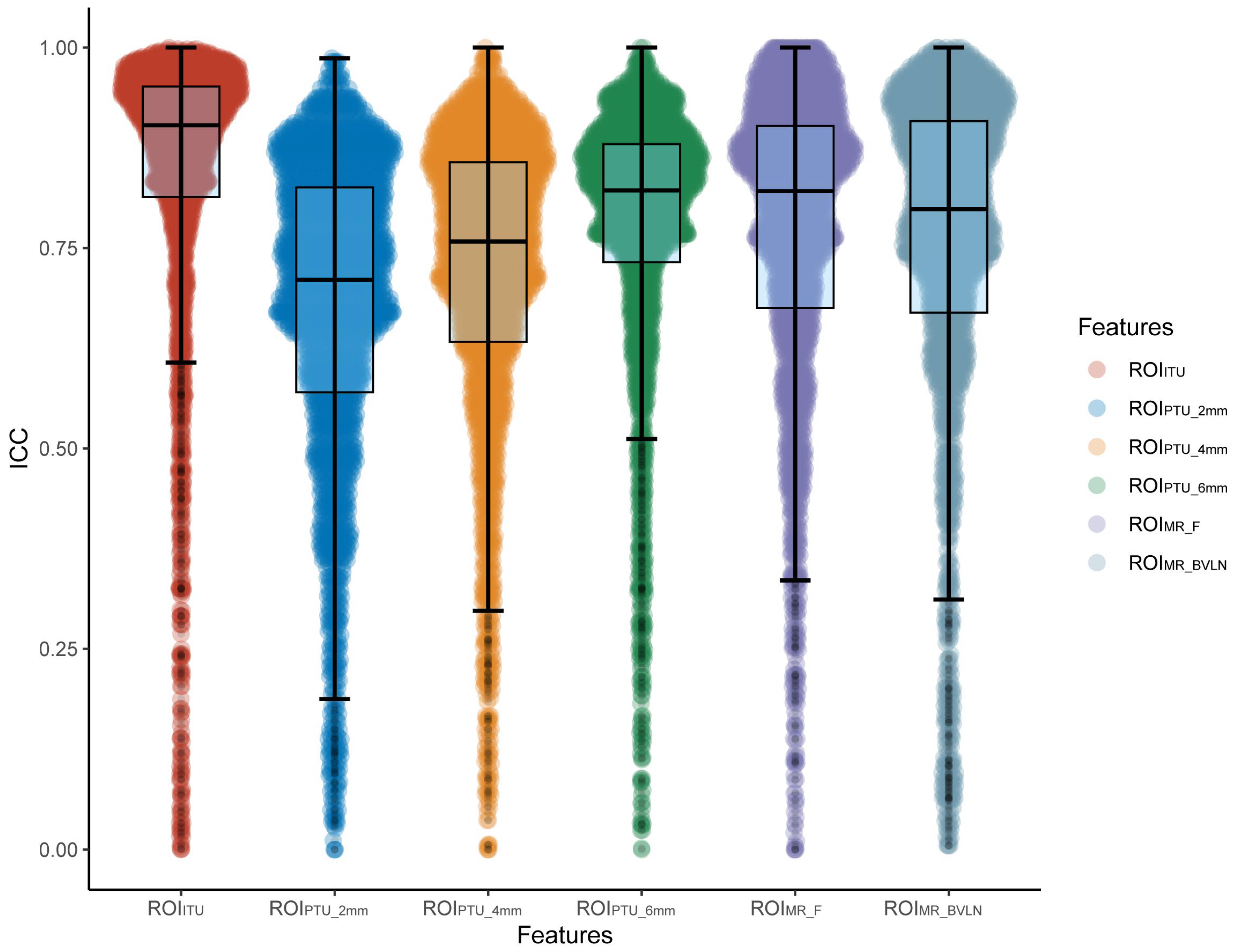
3.2. Feature Screening
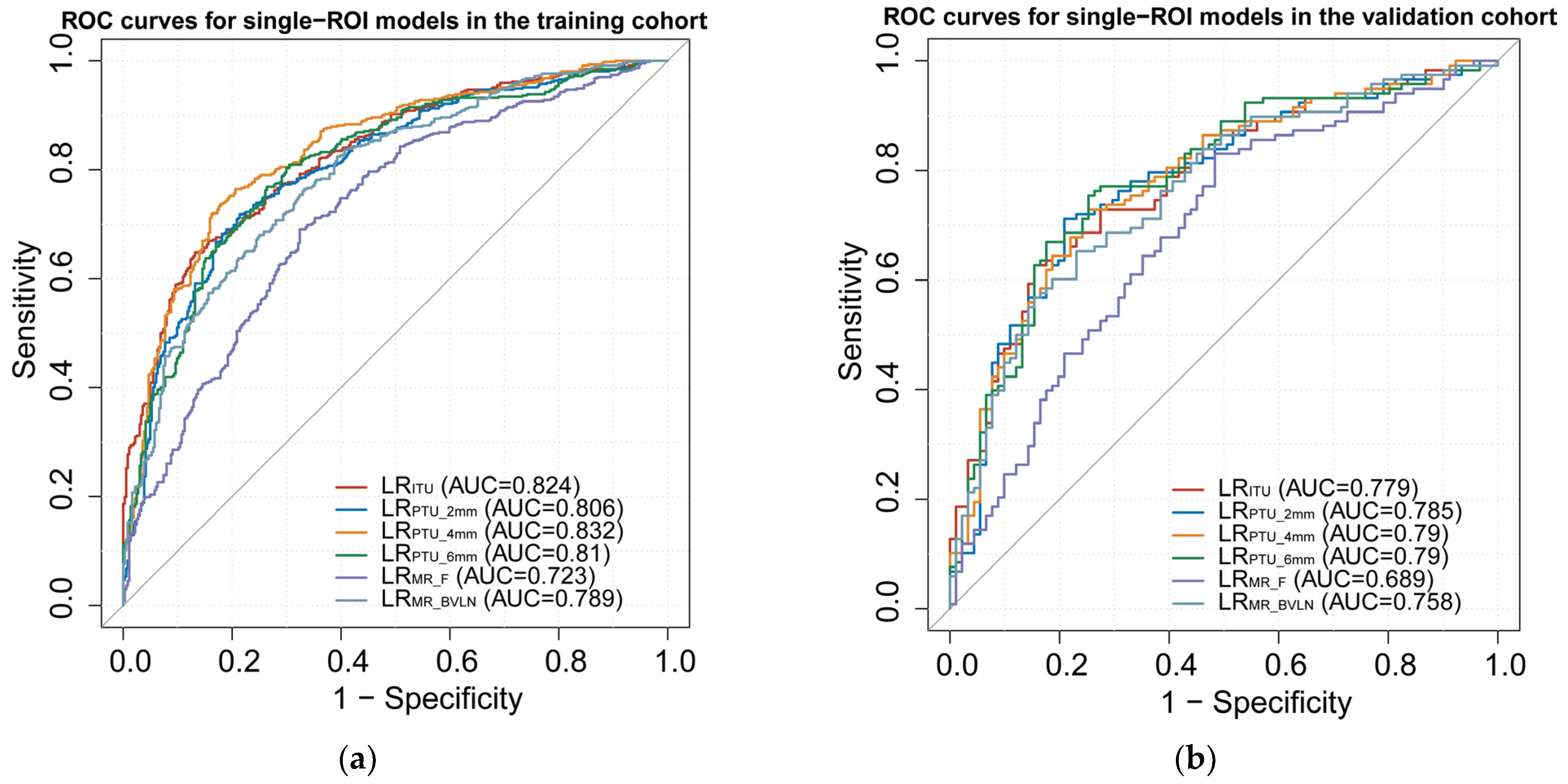
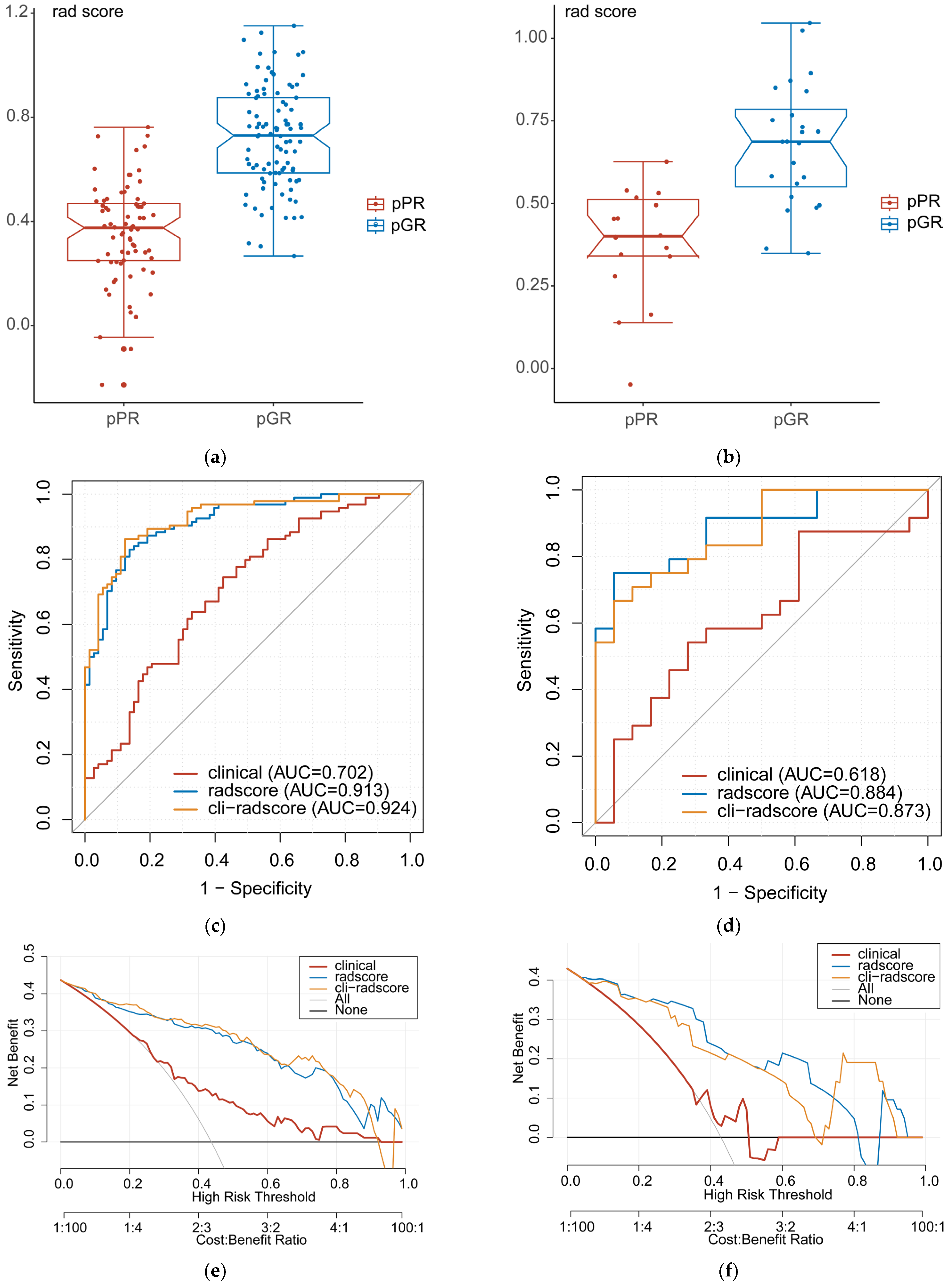
3.3. Model Construction and Assessment
3.4. Clinical, Radscore, and Cli–Radscore Models
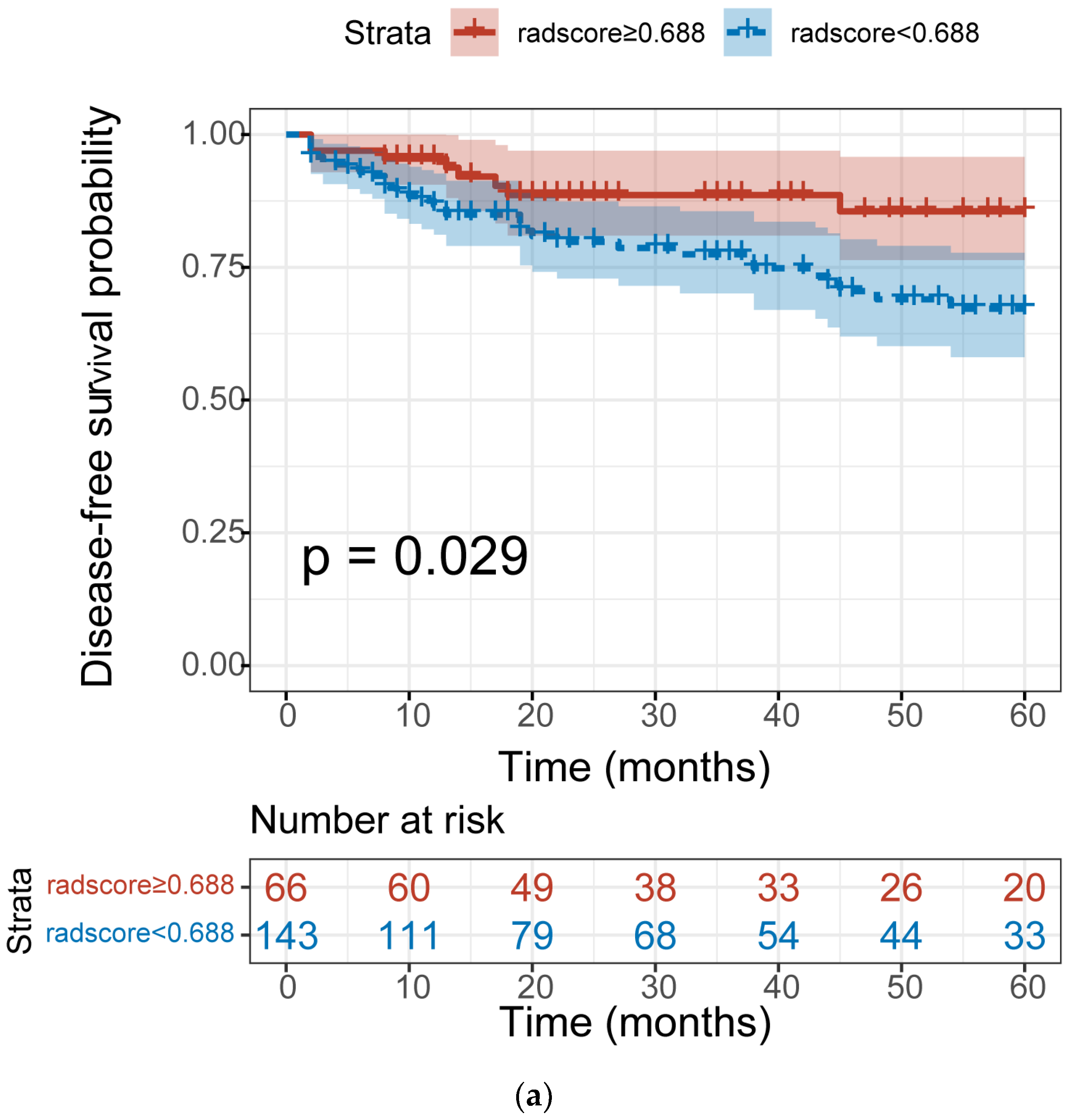
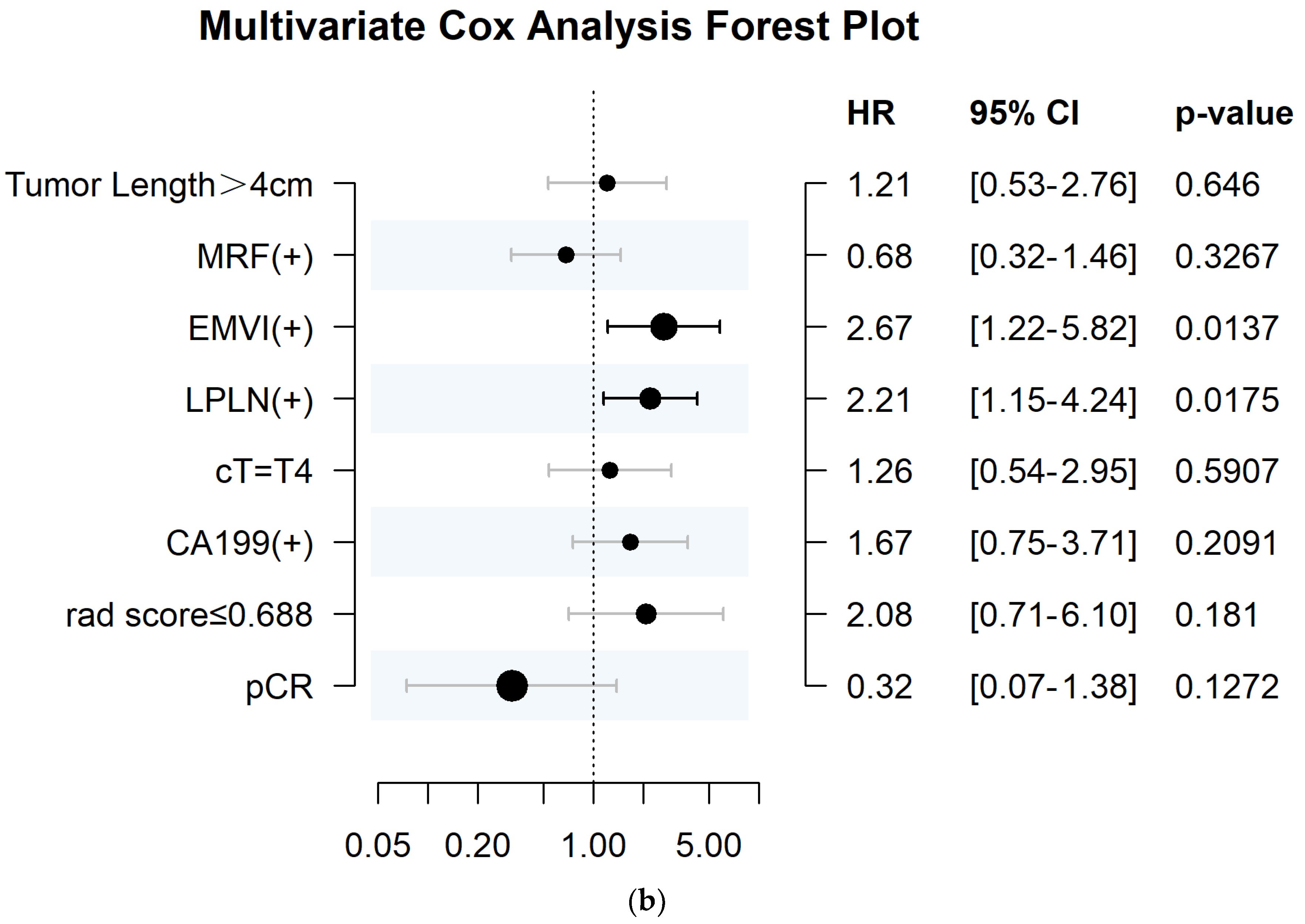
3.5. The Association between Radscore and Disease–Free Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Willett, C.G. Management of Locoregional Rectal Cancer. J. Natl. Compr. Canc. Netw. 2018, 16, 617–619. [Google Scholar] [CrossRef] [Green Version]
- Du, D.; Su, Z.; Wang, D.; Liu, W.; Wei, Z. Optimal Interval to Surgery After Neoadjuvant Chemoradiotherapy in Rectal Cancer: A Systematic Review and Meta–analysis. Clin. Colorectal. Cancer 2018, 17, 13–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, H.Y.; Feng, L.L.; Li, M.; Ju, H.Q.; Ding, Y.; Lan, M.; Song, S.M.; Han, W.D.; Yu, L.; Wei, M.B.; et al. College of American Pathologists Tumor Regression Grading System for Long–Term Outcome in Patients with Locally Advanced Rectal Cancer. Oncol. 2021, 26, e780–e793. [Google Scholar] [CrossRef]
- Fischer, J.; Eglinton, T.W.; Richards, S.J.; Frizelle, F.A. Predicting pathological response to chemoradiotherapy for rectal cancer: A systematic review. Expert. Rev. Anticancer Ther. 2021, 21, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Kennelly, R.P.; Heeney, A.; White, A.; Fennelly, D.; Sheahan, K.; Hyland, J.M.; O’Connell, P.R.; Winter, D.C. A prospective analysis of patient outcome following treatment of T3 rectal cancer with neo–adjuvant chemoradiotherapy and transanal excision. Int. J. Colorectal. Dis. 2012, 27, 759–764. [Google Scholar] [CrossRef] [PubMed]
- Denost, Q.; Kontovounisios, C.; Rasheed, S.; Chevalier, R.; Brasio, R.; Capdepont, M.; Rullier, E.; Tekkis, P.P. Individualizing surgical treatment based on tumour response following neoadjuvant therapy in T4 primary rectal cancer. Eur. J. Surg. Oncol. 2017, 43, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.G.; Wiig, J.N.; Emblemsvaag, H.L.; Grøholt, K.K.; Hole, K.H.; Bentsen, A.; Dueland, S.; Vetrhus, T.; Giercksky, K.E. Extended total mesorectal excision in locally advanced rectal cancer (T4a) and the clinical role of MRI–evaluated neo–adjuvant downstaging. Colorectal. Dis. 2009, 11, 759–767. [Google Scholar] [CrossRef]
- Deidda, S.; Elmore, U.; Rosati, R.; De Nardi, P.; Vignali, A.; Puccetti, F.; Spolverato, G.; Capelli, G.; Zuin, M.; Muratore, A.; et al. Association of Delayed Surgery With Oncologic Long–term Outcomes in Patients With Locally Advanced Rectal Cancer Not Responding to Preoperative Chemoradiation. JAMA Surg. 2021, 156, 1141–1149. [Google Scholar] [CrossRef]
- Tang, X.; Jiang, W.; Li, H.; Xie, F.; Dong, A.; Liu, L.; Li, L. Predicting poor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer: Model constructed using pre–treatment MRI features of structured report template. Radiother. Oncol. 2020, 148, 97–106. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L.; Wu, Y.; Xu, M.; Liu, X.; Guan, G. Worse treatment response to neoadjuvant chemoradiotherapy in young patients with locally advanced rectal cancer. BMC Cancer 2020, 20, 854. [Google Scholar] [CrossRef]
- Shin, J.K.; Huh, J.W.; Lee, W.Y.; Yun, S.H.; Kim, H.C.; Cho, Y.B.; Park, Y.A. Clinical prediction model of pathological response following neoadjuvant chemoradiotherapy for rectal cancer. Sci. Rep. 2022, 12, 7145. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Li, Y.; Zhu, H.; Pei, Q.; Tan, F.; Song, X.; Zhou, Z.; Zhou, Y.; Wang, D.; Pei, H. The Relationship between Primary Gross Tumor Volume and Tumor Response of Locally Advanced Rectal Cancer: pGTV as a More Accurate Tumor Size Indicator. J. Investig. Surg. 2021, 34, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.V.; Roxburgh, C.S.; Vakiani, E.; Shia, J.; Smith, J.J.; Temple, L.K.; Paty, P.; Garcia-Aguilar, J.; Nash, G.; Guillem, J.; et al. Distance to the anal verge is associated with pathologic complete response to neoadjuvant therapy in locally advanced rectal cancer. J. Surg. Oncol. 2016, 114, 637–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Xie, X.; Wu, Z.; Hu, H.; Cai, Y.; Li, J.; Ling, J.; Ding, M.; Li, W.; Deng, Y. Mucinous Adenocarcinoma Predicts Poor Response and Prognosis in Patients With Locally Advanced Rectal Cancer: A Pooled Analysis of Individual Participant Data From 3 Prospective Studies. Clin. Colorectal. Cancer 2021, 20, e240–e248. [Google Scholar] [CrossRef]
- Ryan, J.E.; Warrier, S.K.; Lynch, A.C.; Ramsay, R.G.; Phillips, W.A.; Heriot, A.G. Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A systematic review. Colorectal. Dis. 2016, 18, 234–246. [Google Scholar] [CrossRef]
- Lambin, P.; Rios–Velazquez, E.; Leijenaar, R.; Carvalho, S.; Van Stiphout, R.G.; Granton, P.; Zegers, C.M.; Gillies, R.; Boellard, R.; Dekker, A.; et al. Radiomics: Extracting more information from medical images using advanced feature analysis. Eur. J. Cancer 2012, 48, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Feng, L.; Liu, Z.; Li, C.; Li, Z.; Lou, X.; Shao, L.; Wang, Y.; Huang, Y.; Chen, H.; Pang, X.; et al. Development and validation of a radiopathomics model to predict pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: A multicentre observational study. Lancet Digit. Health 2022, 4, e8–e17. [Google Scholar] [CrossRef]
- Shin, J.; Seo, N.; Baek, S.E.; Son, N.H.; Lim, J.S.; Kim, N.K.; Koom, W.S.; Kim, S. MRI Radiomics Model Predicts Pathologic Complete Response of Rectal Cancer Following Chemoradiotherapy. Radiology 2022, 303, 351–358. [Google Scholar] [CrossRef]
- Song, M.; Li, S.; Wang, H.; Hu, K.; Wang, F.; Teng, H.; Wang, Z.; Liu, J.; Jia, A.Y.; Cai, Y.; et al. MRI radiomics independent of clinical baseline characteristics and neoadjuvant treatment modalities predicts response to neoadjuvant therapy in rectal cancer. Br. J. Cancer 2022, 127, 249–257. [Google Scholar] [CrossRef]
- Bourbonne, V.; Schick, U.; Pradier, O.; Visvikis, D.; Metges, J.P.; Badic, B. Radiomics Approaches for the Prediction of Pathological Complete Response after Neoadjuvant Treatment in Locally Advanced Rectal Cancer: Ready for Prime Time? Cancers 2023, 15, 432. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.; Peng, Y.; Xu, X.; Tu, S.; Wei, Y.; Ma, Y. A Tumoral and Peritumoral CT–Based Radiomics and Machine Learning Approach to Predict the Microsatellite Instability of Rectal Carcinoma. Cancer Manag. Res. 2022, 14, 2409–2418. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, X.L.; Liu, H.; Lu, T.; Li, Z.L. MRI–based multiregional radiomics for predicting lymph nodes status and prognosis in patients with resectable rectal cancer. Front. Oncol. 2022, 12, 1087882. [Google Scholar] [CrossRef]
- Li, M.; Jin, Y.M.; Zhang, Y.C.; Zhao, Y.L.; Huang, C.C.; Liu, S.M.; Song, B. Radiomics for predicting perineural invasion status in rectal cancer. World J. Gastroenterol. 2021, 27, 5610–5621. [Google Scholar] [CrossRef] [PubMed]
- Shaish, H.; Aukerman, A.; Vanguri, R.; Spinelli, A.; Armenta, P.; Jambawalikar, S.; Makkar, J.; Bentley–Hibbert, S.; Del Portillo, A.; Kiran, R.; et al. Radiomics of MRI for pretreatment prediction of pathologic complete response, tumor regression grade, and neoadjuvant rectal score in patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation: An international multicenter study. Eur. Radiol. 2020, 30, 6263–6273. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.A.; Tan, T.T.; Rabson, A.B.; Anderson, D.; Degenhardt, K.; White, E. Hypoxia and defective apoptosis drive genomic instability and tumorigenesis. Genes Dev. 2004, 18, 2095–2107. [Google Scholar] [CrossRef] [Green Version]
- Jayaprakasam, V.S.; Paroder, V.; Gibbs, P.; Bajwa, R.; Gangai, N.; Sosa, R.E.; Petkovska, I.; Golia Pernicka, J.S.; Fuqua, J.L.; Bates, D.D.; et al. MRI radiomics features of mesorectal fat can predict response to neoadjuvant chemoradiation therapy and tumor recurrence in patients with locally advanced rectal cancer. Eur. Radiol. 2022, 32, 971–980. [Google Scholar] [CrossRef]
- Kluza, E.; Kleijnen, J.P.J.; Martens, M.H.; Rennspiess, D.; Maas, M.; Jeukens, C.R.; Riedl, R.G.; zur Hausen, A.; Beets, G.L.; Beets–Tan, R.G. Non–invasive MR assessment of macroscopic and microscopic vascular abnormalities in the rectal tumour–surrounding mesorectum. Eur. Radiol. 2016, 26, 1311–1319. [Google Scholar] [CrossRef]
- Smith, N.J.; Barbachano, Y.; Norman, A.R.; Swift, R.I.; Abulafi, A.M.; Brown, G. Prognostic significance of magnetic resonance imaging–detected extramural vascular invasion in rectal cancer. Br. J. Surg. 2008, 95, 229–236. [Google Scholar] [CrossRef]
- Ogawa, S.; Hida, J.I.; Ike, H.; Kinugasa, T.; Ota, M.; Shinto, E.; Itabashi, M.; Kameoka, S.; Sugihara, K. Selection of Lymph Node–Positive Cases Based on Perirectal and Lateral Pelvic Lymph Nodes Using Magnetic Resonance Imaging: Study of the Japanese Society for Cancer of the Colon and Rectum. Ann. Surg. Oncol. 2016, 23, 1187–1194. [Google Scholar] [CrossRef]
- Van Griethuysen, J.J.; Fedorov, A.; Parmar, C.; Hosny, A.; Aucoin, N.; Narayan, V.; Beets–Tan, R.G.; Fillion–Robin, J.C.; Pieper, S.; Aerts, H.J. Computational Radiomics System to Decode the Radiographic Phenotype. Cancer Res. 2017, 77, e104–e107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stollfuss, J.C.; Becker, K.; Sendler, A.; Seidl, S.; Settles, M.; Auer, F.; Beer, A.; Rummeny, E.J.; Woertler, K. Rectal carcinoma: High–spatial–resolution MR imaging and T2 quantification in rectal cancer specimens. Radiology 2006, 241, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kalisz, K.R.; Enzerra, M.D.; Paspulati, R.M. MRI Evaluation of the Response of Rectal Cancer to Neoadjuvant Chemoradiation Therapy. Radiographics 2019, 39, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Miranda, J.; Tan, G.X.V.; Fernandes, M.C.; Yildirim, O.; Sims, J.A.; Araujo–Filho, J.D.A.B.; Machado, F.A.D.M.; Assuncao–Jr, A.N.; Nomura, C.H.; Horvat, N. Rectal MRI radiomics for predicting pathological complete response: Where we are. Clin. Imaging 2022, 82, 141–149. [Google Scholar] [CrossRef]
- Defeudis, A.; Mazzetti, S.; Panic, J.; Micilotta, M.; Vassallo, L.; Giannetto, G.; Gatti, M.; Faletti, R.; Cirillo, S.; Regge, D.; et al. MRI–based radiomics to predict response in locally advanced rectal cancer: Comparison of manual and automatic segmentation on external validation in a multicentre study. Eur. Radiol. Exp. 2022, 6, 19. [Google Scholar] [CrossRef]
- Wang, T.; She, Y.; Yang, Y.; Liu, X.; Chen, S.; Zhong, Y.; Deng, J.; Zhao, M.; Sun, X.; Xie, D.; et al. Radiomics for Survival Risk Stratification of Clinical and Pathologic Stage IA Pure–Solid Non–Small Cell Lung Cancer. Radiology 2022, 302, 425–434. [Google Scholar] [CrossRef]
- Li, N.; Wan, X.; Zhang, H.; Zhang, Z.; Guo, Y.; Hong, D. Tumor and peritumor radiomics analysis based on contrast–enhanced CT for predicting early and late recurrence of hepatocellular carcinoma after liver resection. BMC Cancer 2022, 22, 664. [Google Scholar] [CrossRef]
- Ma, Y.; Guan, Z.; Liang, H.; Cao, H. Predicting the WHO/ISUP Grade of Clear Cell Renal Cell Carcinoma Through CT–Based Tumoral and Peritumoral Radiomics. Front. Oncol. 2022, 12, 831112. [Google Scholar] [CrossRef]
- Delli Pizzi, A.; Chiarelli, A.M.; Chiacchiaretta, P.; d’Annibale, M.; Croce, P.; Rosa, C.; Mastrodicasa, D.; Trebeschi, S.; Lambregts, D.M.J.; Caposiena, D.; et al. MRI–based clinical–radiomics model predicts tumor response before treatment in locally advanced rectal cancer. Sci. Rep. 2021, 11, 5379. [Google Scholar] [CrossRef]
- Braman, N.; Prasanna, P.; Bera, K.; Alilou, M.; Khorrami, M.; Leo, P.; Etesami, M.; Vulchi, M.; Turk, P.; Gupta, A.; et al. Novel Radiomic Measurements of Tumor–Associated Vasculature Morphology on Clinical Imaging as a Biomarker of Treatment Response in Multiple Cancers. Clin. Cancer Res. 2022, 28, 4410–4424. [Google Scholar] [CrossRef]
- Marques, F.G.; Poli, E.; Malaquias, J.; Carvalho, T.; Portêlo, A.; Ramires, A.; Aldeia, F.; Ribeiro, R.M.; Vitorino, E.; Diegues, I.; et al. Low doses of ionizing radiation activate endothelial cells and induce angiogenesis in peritumoral tissues. Radiother. Oncol. 2020, 151, 322–327. [Google Scholar] [CrossRef] [PubMed]

| Features | pPR (n = 91 a) | pGR (n = 118 a) | p |
|---|---|---|---|
| Gender | 0.373 | ||
| Male | 67 (74%) | 79 (67%) | |
| Female | 24 (26%) | 39 (33%) | |
| Age (years) | 59.66 ± 11.83 | 60.32 ± 10.64 | 0.671 |
| BMI (kg/m2) | 24.39 ± 3.11 | 24.21 ± 3.17 | 0.682 |
| cT | 0.508 | ||
| T3 | 79 (87%) | 107 (91%) | |
| T4 | 12 (13%) | 11 (9.3%) | |
| cN | 0.771 | ||
| N0 | 17 (19%) | 22 (19%) | |
| N1 | 68 (75%) | 85 (72%) | |
| N2 | 74 (7%) | 96 (9%) | |
| DTAV | <0.001 | ||
| ≤4 cm | 35 (38%) | 19 (16%) | |
| >4 cm | 56 (62%) | 99 (84%) | |
| Tumor length | 0.049 | ||
| ≤4 cm | 30 (33%) | 56 (47%) | |
| >4 cm | 61 (67%) | 62 (53%) | |
| MRF | 0.007 | ||
| Negative | 50 (55%) | 87 (74%) | |
| Positive | 41 (45%) | 31 (26%) | |
| EMVI | 0.029 | ||
| Negative | 44 (48%) | 76 (64%) | |
| Positive | 47 (52%) | 42 (36%) | |
| LPLN | 0.698 | ||
| Negative | 64 (70%) | 88 (74%) | |
| Positive | 27 (30%) | 31 (26%) | |
| CEA | 0.647 | ||
| ≤5 ng/mL | 51 (56%) | 71 (60%) | |
| >5 ng/mL | 40 (44%) | 47 (40%) | |
| CA19–9 | 0.182 | ||
| ≤39 ng/mL | 77 (85%) | 108 (92%) | |
| >39 ng/mL | 14 (15%) | 10 (8.5%) | |
| WBC (×109/L) | 6.69 ± 1.80 | 6.30 ± 1.63 | 0.100 |
| HGB (g/L) | 136.73 ± 16.97 | 133.99 ± 21.19 | 0.315 |
| PLT (×109/L) | 256.91 ± 70.17 | 233.87 ± 66.45 | 0.016 |
| Lymphocyte (×109/L) | 1.74 ± 0.55 | 1.83 ± 0.59 | 0.278 |
| Neutrophil (×109/L) | 4.34 ± 1.51 | 3.91 ± 1.38 | 0.036 |
| Eosinophilic granulocyte (×109/L) | 0.16 ± 0.12 | 0.14 ± 0.10 | 0.100 |
| Monocyte (×109/L) | 0.39 ± 0.15 | 0.41 ± 0.16 | 0.41 |
| NLR | 2.73 ± 1.44 | 2.37 ± 1.16 | 0.047 |
| LMR | 4.97 ± 2.17 | 4.97 ± 2.01 | 0.997 |
| PLR | 162.98 ± 77.89 | 139.78 ± 58.86 | 0.015 |
| TRG | <0.001 | ||
| 0 | 0 (0%) | 44 (37%) | |
| 1 | 0 (0%) | 74 (63%) | |
| 2 | 71 (78%) | 0 (0%) | |
| 3 | 20 (22%) | 0 (0%) |
| Models | Cohort | Cut–Off | AUC (95%CI) | F1 Score | Sensitivity | Specificity | Accuracy |
|---|---|---|---|---|---|---|---|
| LRITU | Training | 0.48 | 0.824 (0.764–0.887) | 0.774 | 0.775 | 0.703 | 0.744 |
| Validation | 0.779 (0.634–0.924) | 0.741 | 0.728 | 0.705 | 0.718 | ||
| LRPTU_2mm | Training | 0.52 | 0.806 (0.74–0.873) | 0.761 | 0.722 | 0.772 | 0.744 |
| Validation | 0.785 (0.638–0.93) | 0.758 | 0.72 | 0.769 | 0.742 | ||
| LRPTU_4mm | Training | 0.48 | 0.832 (0.776–0.899) | 0.789 | 0.754 | 0.797 | 0.773 |
| Validation | 0.79 (0.649–0.93) | 0.749 | 0.712 | 0.757 | 0.732 | ||
| LRPTU_6mm | Training | 0.48 | 0.81 (0.745–0.877) | 0.779 | 0.775 | 0.72 | 0.751 |
| Validation | 0.79 (0.647–0.933) | 0.772 | 0.762 | 0.725 | 0.746 | ||
| LRMR_F | Training | 0.50 | 0.723 (0.645–0.801) | 0.745 | 0.822 | 0.5 | 0.682 |
| Validation | 0.689 (0.522–0.854) | 0.751 | 0.83 | 0.517 | 0.694 | ||
| LRMR_BVLN | Training | 0.54 | 0.789 (0.722–0.858) | 0.695 | 0.614 | 0.802 | 0.696 |
| Validation | 0.758 (0.61–0.906) | 0.685 | 0.602 | 0.815 | 0.694 | ||
| LRITU+PTU_2mm | Training | 0.49 | 0.831 (0.771–0.893) | 0.779 | 0.754 | 0.764 | 0.758 |
| Validation | 0.795 (0.654–0.935) | 0.764 | 0.745 | 0.737 | 0.741 | ||
| LR ITU+PTU_4mm | Training | 0.50 | 0.832 (0.771–0.895) | 0.778 | 0.714 | 0.843 | 0.77 |
| Validation | 0.805 (0.667–0.944) | 0.755 | 0.695 | 0.825 | 0.751 | ||
| LR ITU+PTU_6mm | Training | 0.51 | 0.874 (0.825–0.927) | 0.807 | 0.801 | 0.761 | 0.783 |
| Validation | 0.795 (0.659–0.931) | 0.761 | 0.754 | 0.704 | 0.732 | ||
| LR ITU+MR_F | Training | 0.52 | 0.842 (0.784–0.903) | 0.794 | 0.771 | 0.777 | 0.774 |
| Validation | 0.79 (0.643–0.935) | 0.745 | 0.712 | 0.748 | 0.727 | ||
| LR ITU+MR_BVLN | Training | 0.52 | 0.936 (0.904–0.972) | 0.878 | 0.85 | 0.89 | 0.867 |
| Validation | 0.859 (0.745–0.974) | 0.811 | 0.789 | 0.803 | 0.794 | ||
| LR ITU+MR_F +MR_BVLN | Training | 0.55 | 0.873 (0.825–0.926) | 0.782 | 0.705 | 0.874 | 0.779 |
| Validation | 0.85 (0.736–0.967) | 0.763 | 0.679 | 0.879 | 0.766 |
| Factor | Number (n) | Univariate Cox Analysis | Multivariate Cox Analysis | |||
|---|---|---|---|---|---|---|
| HR (95%CI) | p | C–Index | HR (95%CI) | p | ||
| Tumor length | 0.016 | 0.585 | 0.53 | |||
| ≤4 cm | 86 | 1 | ||||
| >4 cm | 123 | 2.328 (1.144–4.736) | 1.304 (0.57–2.985) | |||
| MRF | 0.018 | 0.591 | 0.356 | |||
| Negative | 137 | 1 | ||||
| Positive | 72 | 2.05 (1.119–3.755) | 0.704 (0.335–1.482) | |||
| EMVI | <0.001 | 0.678 | 0.009 | |||
| Negative | 120 | 1 | ||||
| Positive | 89 | 4.102 (2.098–8.018) | 2.799 (1.292–6.062) | |||
| LPLN | <0.001 | 0.636 | 0.015 | |||
| Negative | 151 | 1 | ||||
| Positive | 58 | 3.42 (1.863–6.281) | 2.251 (1.17–4.332) | |||
| cT | 0.036 | 0.555 | 0.654 | |||
| T3 | 186 | 1 | ||||
| T4 | 23 | 2.245 (1.036–4.862) | 1.211 (0.524–2.801) | |||
| CA19–9 | 0.014 | 0.234 | ||||
| Negative | 185 | 1 | ||||
| Positive | 24 | 2.461 (1.177–5.146) | 1.629 (0.73–3.638) | |||
| radscore | 0.034 | 0.576 | 0.78 | |||
| ≥0.688 | 52 | 1 | ||||
| <0.688 | 157 | 2.303 (1.065–4.977) | 1.126 (0.489–2.595) | |||
| pCR | 0.007 | 0.59 | 0.103 | |||
| No | 165 | 1 | ||||
| Yes | 44 | 0.176 (0.043–0.728) | 0.294 (0.068–1.279) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, S.; Lu, S.; Liu, K.; Zhou, Y.; Wang, Q.; Chen, Y.; Zhang, E.; Wang, H.; Lang, N. Radiomics from Mesorectal Blood Vessels and Lymph Nodes: A Novel Prognostic Predictor for Rectal Cancer with Neoadjuvant Therapy. Diagnostics 2023, 13, 1987. https://doi.org/10.3390/diagnostics13121987
Qin S, Lu S, Liu K, Zhou Y, Wang Q, Chen Y, Zhang E, Wang H, Lang N. Radiomics from Mesorectal Blood Vessels and Lymph Nodes: A Novel Prognostic Predictor for Rectal Cancer with Neoadjuvant Therapy. Diagnostics. 2023; 13(12):1987. https://doi.org/10.3390/diagnostics13121987
Chicago/Turabian StyleQin, Siyuan, Siyi Lu, Ke Liu, Yan Zhou, Qizheng Wang, Yongye Chen, Enlong Zhang, Hao Wang, and Ning Lang. 2023. "Radiomics from Mesorectal Blood Vessels and Lymph Nodes: A Novel Prognostic Predictor for Rectal Cancer with Neoadjuvant Therapy" Diagnostics 13, no. 12: 1987. https://doi.org/10.3390/diagnostics13121987







