Detection of Biomarker Using Aptasensors to Determine the Type of Diabetes
Abstract
:1. Introduction
2. Methods
3. Result
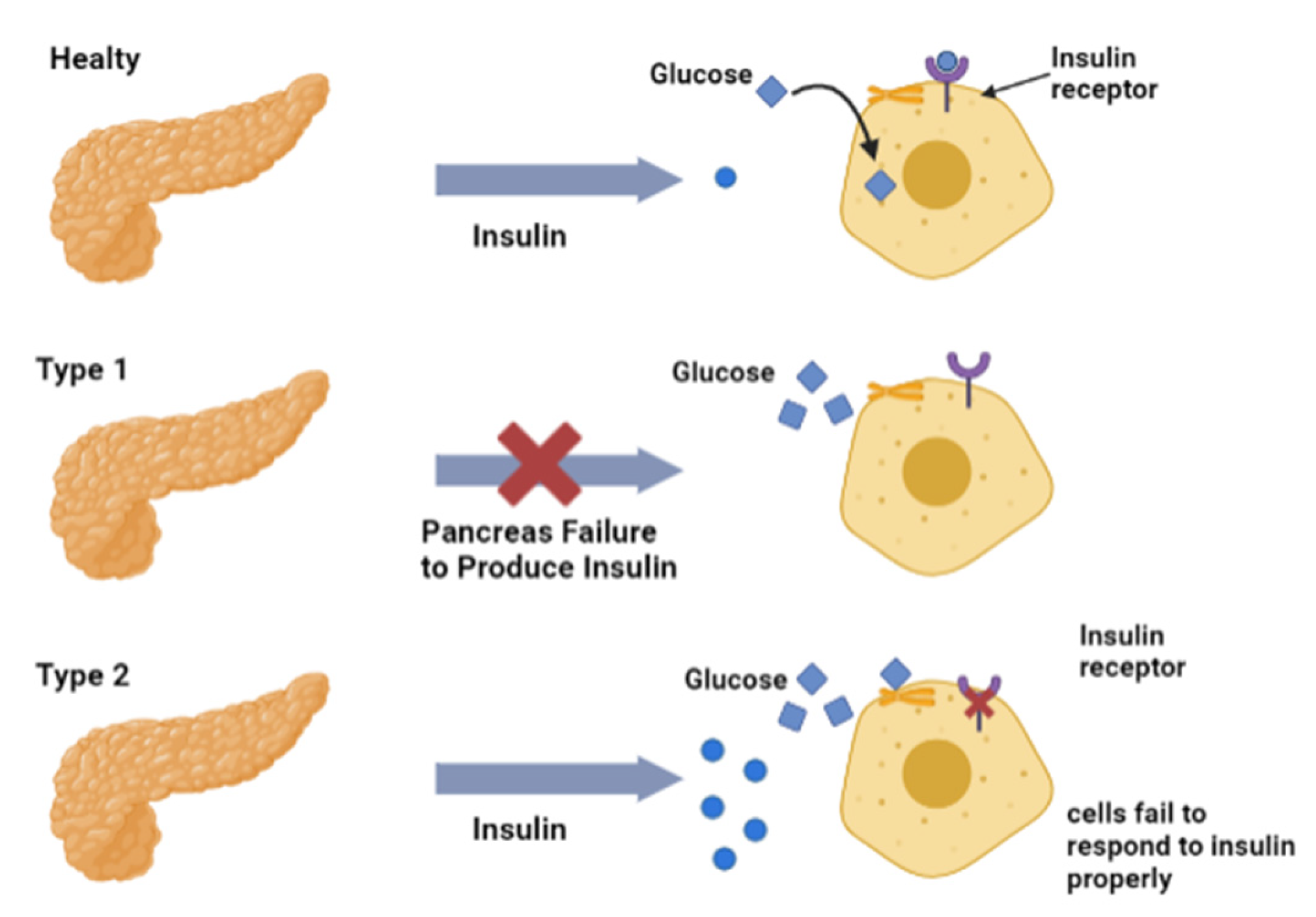
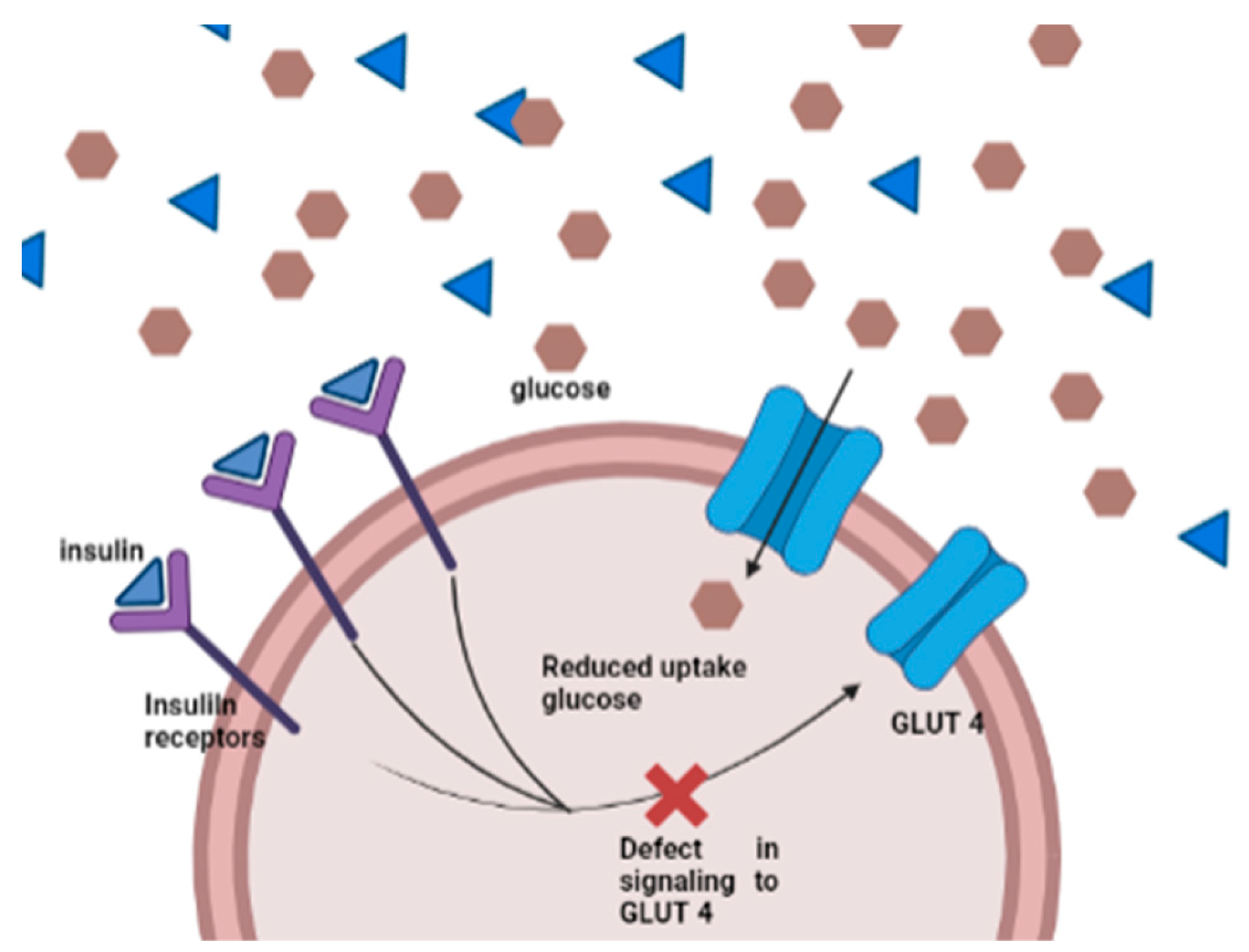
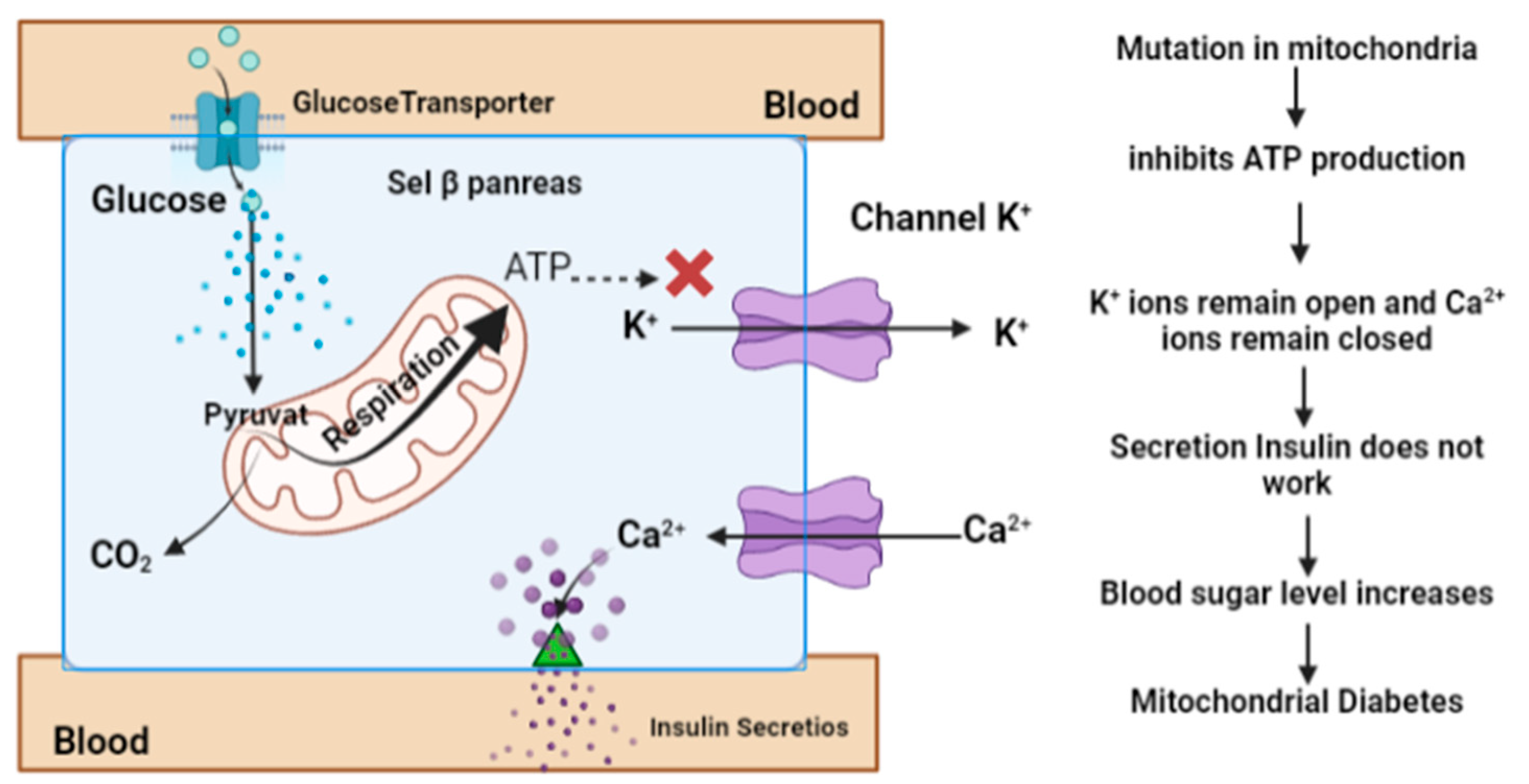
3.1. Diabetes Mellitus (DM)
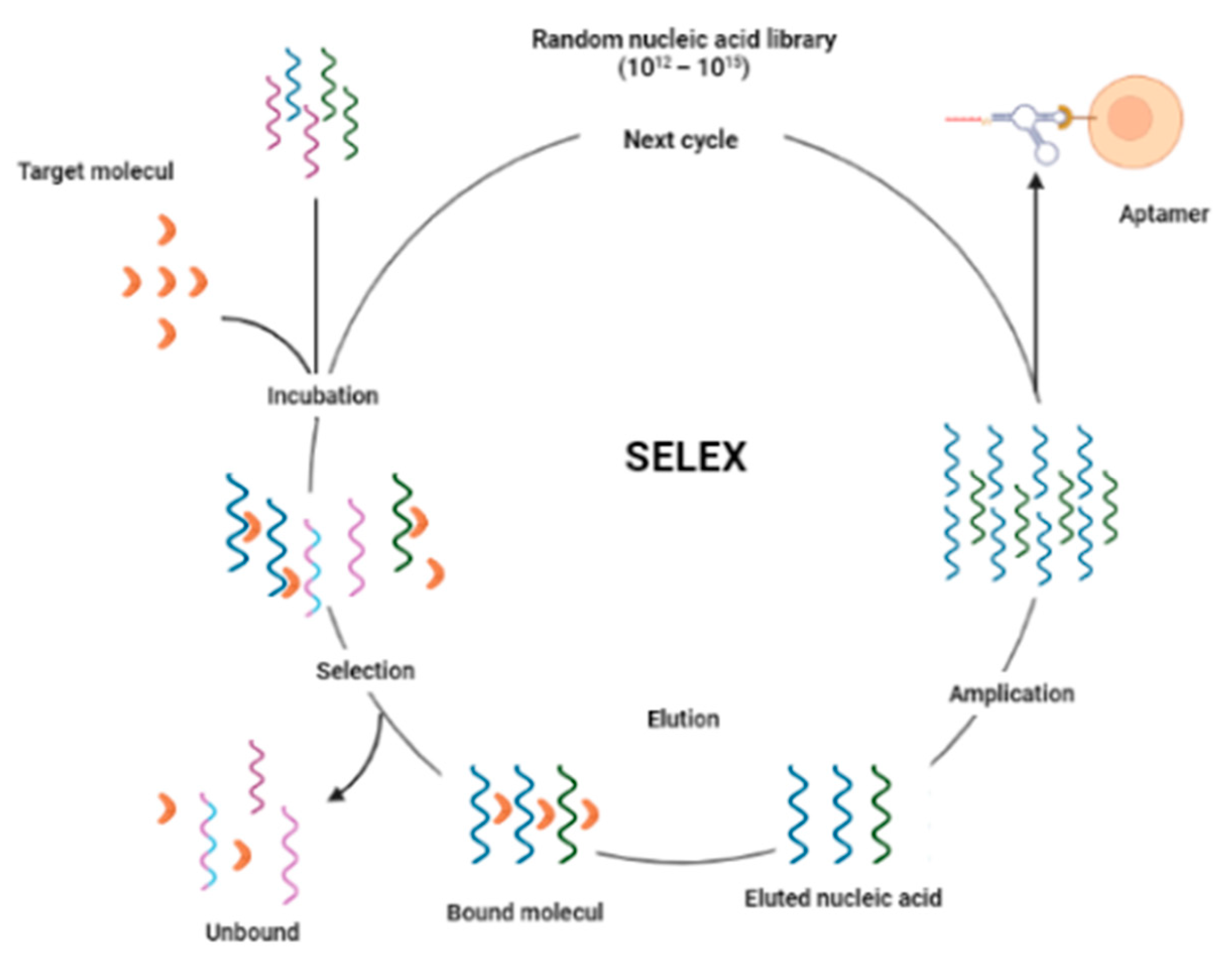
3.2. Aptasensor
3.3. Aptamer
3.4. Comparison of Aptamer with Antibodies
4. Aptasensor for Diagnosis of Diabetes Mellitus and Mitochondrial Diabetes
4.1. Aptasensor for Glucose Detection
4.2. Aptasensor for HbA1c Detection
4.3. Aptasensor for GHSA Detection
4.4. Aptasensor for Insulin Detection
4.5. Aptasensor for ATP Detection
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Smeltzer, S.C.; Bare, B.G. Buku Ajar Keperawatan Medikal Bedah Brunner & Suddarth, 8th ed.; Penerbit Buku Kedokteran: Jakarta, Indonesia, 2013; Volume 2. [Google Scholar]
- International Diabetes Federation. IDF Diabetes Atlas; International Diabetes Federation: Brussels, Belgium, 2021; Volume 102, ISBN 9782930229980. [Google Scholar]
- Powers, A.C.; Niswender, K.D.; Evans-Molina, C. Diabetes Mellitus: Diagnosis, Classification, and Pathophysiology. In Harrison’s Principles of Internal Medicine, 20th ed.; Jameson, J.L., Fauci, A.S., Kasper, D.L., Hauser, S.L., Longo, D.L., Loscalzo, J., Eds.; McGraw-Hill Education: New York, NY, USA, 2018. [Google Scholar]
- Persatuan Endokrinologi Indonesia. Konsensus Pengelolaan Dan Pencegahan Diabetes Melitus Tipe 2 Di Indonesia 2006; Persatuan Endokrinologi Indonesia: Jakarta, Indonesia, 2006. [Google Scholar]
- Juarez-Facio, A.T.; de Lagarde, V.M.; Monteil, C.; Vaugeois, J.-M.; Corbiere, C.; Rogez-Florent, T. Validation of a Fast and Simple HPLC-UV Method for the Quantification of Adenosine Phosphates in Human Bronchial Epithelial Cells. Molecules 2021, 26, 6324. [Google Scholar] [CrossRef] [PubMed]
- Vancraenenbroeck, R.; Webb, M.R. A Fluorescent, Reagentless Biosensor for ATP, Based on Malonyl-Coenzyme A Synthetase. ACS Chem. Biol. 2015, 10, 2650–2657. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Wei, K.; Cheng, L.; Hu, J.; Xiang, Q. Fluorometric aptamer based determination of adenosine triphosphate based on deoxyribonuclease I-aided target recycling and signal amplification using graphene oxide as a quencher. Microchim. Acta 2017, 184, 1847–1854. [Google Scholar] [CrossRef]
- Qu, F.; Sun, C.; Lv, X.; You, J. A terbium-based metal-organic framework@gold nanoparticle system as a fluorometric probe for aptamer based determination of adenosine triphosphate. Microchim. Acta 2018, 185, 359. [Google Scholar] [CrossRef]
- Anand, A.; Chen, C.-Y.; Chen, T.-H.; Liu, Y.-C.; Sheu, S.-Y.; Chen, Y.-T. Detecting glycated hemoglobin in human blood samples using a transistor-based nanoelectronic aptasensor. Nano Today 2021, 41, 101294. [Google Scholar] [CrossRef]
- Srivastava, P.; Razi, S.S.; Ali, R.; Srivastav, S.; Patnaik, S.; Srikrishna, S.; Misra, A. Highly sensitive cell imaging “Off–On” fluorescent probe for mitochondria and ATP. Biosens. Bioelectron. 2015, 69, 179–185. [Google Scholar] [CrossRef]
- Rukmini, M.S.; Ashritha; Nishmitha, P.; Yalla, D.; Christy, A.; Manjrekar, P. Analytical Calibre of High Performance Liquid Chromatography and Ion Exchange Chromatography Resin Methods in Estimation of Glycated Hemoglobin: A Comparitive Study. Biomed. Res. 2017, 28, 1765–1769. [Google Scholar]
- Lakshmy, R.; Gupta, R. Measurement of Glycated Hemoglobin A1c from Dried Blood by Turbidimetric Immunoassay. J. Diabetes Sci. Technol. 2009, 3, 1203–1206. [Google Scholar] [CrossRef] [Green Version]
- Gilani, M.; Aamir, M.; Akram, A.; Haroon, Z.H.; Ijaz, A.; Khadim, M.T. Comparison of Turbidimetric Inhibition Immunoassay, High-Performance Liquid Chromatography, and Capillary Electrophoresis Methods for Glycated Hemoglobin Determination. Lab. Med. 2020, 51, 579–584. [Google Scholar] [CrossRef]
- Zhou, G.; Wang, Y.; Cui, L. Biomedical Sensor, Device and Measurement Systems; Serra, P.A., Ed.; IntechOpen: Rijeka, Croatia, 2015; p. 7. [Google Scholar]
- Velasco-Garcia, M.N.; Missailidis, S. New Trends in Aptamer-Based Electrochemical Biosensors. Gene Ther. Mol. Biol. 2009, 13, 1–10. [Google Scholar]
- Tandra, H. Strategi Mengalahkan Komplikasi Diabetes; Gramedia Pustaka Utama: Jakarta, Indonesia, 2014. [Google Scholar]
- American Diabetes Association. Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2014, 37 (Suppl. S1), S81–S90. [Google Scholar] [CrossRef] [Green Version]
- Forouhi, N.G.; Wareham, N.J. Epidemiology of diabetes. Medicine 2014, 42, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Bhatt, H.; Saklani, S.; Upadhayay, K. Anti-Oxidant and Anti-Diabetic Activities of Ethanolic Extract of Primula Denticulata Flowers. Indones. J. Pharm. 2016, 27, 74. [Google Scholar] [CrossRef] [Green Version]
- Forbes, J.M.; Cooper, M.E. Mechanisms of Diabetic Complications. Physiol. Rev. 2013, 93, 137–188. [Google Scholar] [CrossRef]
- Akil, A.A.-S.; Yassin, E.; Al-Maraghi, A.; Aliyev, E.; Al-Malki, K.; Fakhro, K.A. Diagnosis and treatment of type 1 diabetes at the dawn of the personalized medicine era. J. Transl. Med. 2021, 19, 137. [Google Scholar] [CrossRef]
- Kahanovitz, L.M.; Sluss, P.M.; Russell, S.J. Type 1 Diabetes—A Clinical Perspective. Point Care J. Near-Patient Test. Technol. 2017, 16, 37–40. [Google Scholar] [CrossRef] [Green Version]
- Paschou, S.A.; Papadopoulou-Marketou, N.; Chrousos, G.P.; Kanaka-Gantenbein, C. On type 1 diabetes mellitus pathogenesis. Endocr. Connect. 2018, 7, R38–R46. [Google Scholar] [CrossRef] [Green Version]
- Yahaya, T.; Salisu, T. Genes predisposing to type 1 diabetes mellitus and pathophysiology: A narrative review. Med. J. Indones. 2020, 29, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Giwa, A.M.; Ahmed, R.; Omidian, Z.; Majety, N.; Karakus, K.E.; Omer, S.M.; Donner, T.; Hamad, A.R.A. Current understandings of the pathogenesis of type 1 diabetes: Genetics to environment. World J. Diabetes 2020, 11, 13–25. [Google Scholar] [CrossRef]
- Soelistijo, S.A.; Lindarto, D.; Decroli, E.; Permana, H.; Sucipto, K.W.; Kusnadi, Y. Pengelolaan Dan Pencegahan Diabetes Melitus Tipe 2 Dewasa Di Indonesia; Perkeni: Jakarta, Indonesia, 2019; p. 133. [Google Scholar]
- Ndisang, J.F.; Rastogi, S.; Vannacci, A. Insulin Resistance, Type 1 and Type 2 Diabetes, and Related Complications 2015. J. Diabetes Res. 2015, 2015, 234135. [Google Scholar] [CrossRef]
- Maxine, A.; Papadakis, M.D.; Stephen, J.; McPhee, M.D.; Michael, W.; Rabow, M. Current Medical Diagnosis & Treatment. In LANGE Medical Book; McGrow Hill: New York, NY, USA, 2022. [Google Scholar]
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of Type 2 Diabetes Mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Goyal, R.; Jialal, I. Diabetes Mellitus Type 2; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Hatting, M.; Tavares, C.D.; Sharabi, K.; Rines, A.K.; Puigserver, P. Insulin regulation of gluconeogenesis. Ann. N. Y. Acad. Sci. 2017, 1411, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Maassen, J.A.; Hart, L.M.; van Essen, E.; Heine, R.J.; Nijpels, G.; Tafrechi, R.S.J.; Raap, A.K.; Janssen, G.M.; Lemkes, H.H. Mitochondrial Diabetes. Diabetes 2004, 53, S103–S109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kindred Healthcare Pathophysiology of Diabetes Mellitus. Available online: https://www.kindredhospitals.com/resources/blog-kindred-continuum/2013/11/07/pathophysiology-of-diabetes-mellitus (accessed on 7 May 2023).
- Chae, J.H.; Hwang, H.; Lim, B.C.; Cheong, H.I.; Hwang, Y.S.; Kim, K.J. Clinical features of A3243G mitochondrial tRNA mutation. Brain Dev. 2004, 26, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Cox, M. Lehninger Principles of Biochemistry; Freeman and Company: New York, NY, USA, 2013. [Google Scholar]
- Martinkova, P.; Pohanka, M. Biosensors for Blood Glucose and Diabetes Diagnosis: Evolution, Construction, and Current Status. Anal. Lett. 2015, 48, 2509–2532. [Google Scholar] [CrossRef]
- Iswantini, D.; Tri, W.; Purwaningsih, H.; Nurhidayat, N. Biosensor: Prinsip Dan Aplikasinya; IPB Press: Bogor, Indonesia, 2020. [Google Scholar]
- Ferentinos, K.; Yialouris, C.; Blouchos, P.; Moschopoulou, G.; Tsourou, V.; Kintzios, S. The Use of Artificial Neural Networks as a Component of a Cell-based Biosensor Device for the Detection of Pesticides. Proc. Eng. 2012, 47, 989–992. [Google Scholar] [CrossRef] [Green Version]
- Migliozzi, D.; Guibentif, T. Assessing the Potential Deployment of Biosensors for Point-of-Care Diagnostics in Developing Countries: Technological, Economic and Regulatory Aspects. Biosensors 2018, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Drummond, T.G.; Hill, M.G.; Barton, J.K. Electrochemical DNA sensors. Nat. Biotechnol. 2003, 21, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Santosh, B.; Yadava, P.K. Nucleic Acid Aptamers: Research Tools in Disease Diagnostics and Therapeutics. BioMed Res. Int. 2014, 2014, 540451. [Google Scholar] [CrossRef] [Green Version]
- Oleszek, M.; Kowalska, I.; Oleszek, W. Phytochemicals in Bioenergy Crops; Springer: Berlin/Heidelberg, Germany, 2019; Volume 18, ISBN 0123456789. [Google Scholar]
- Toh, S.Y.; Citartan, M.; Gopinath, S.C.; Tang, T.-H. Aptamers as a replacement for antibodies in enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2015, 64, 392–403. [Google Scholar] [CrossRef]
- Witt, M.; Walter, J.-G.; Stahl, F. Aptamer Microarrays—Current Status and Future Prospects. Biotech 2015, 4, 115–132. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.; Xi, Z.; He, N. Applications of aptamers for chemistry analysis, medicine and food security. Sci. China Chem. 2015, 58, 1122–1130. [Google Scholar] [CrossRef]
- Suaebah, E.; Naramura, T.; Myodo, M.; Hasegawa, M.; Shoji, S.; Buendia, J.J.; Kawarada, H. Aptamer-Based Carboxyl-Terminated Nanocrystalline Diamond Sensing Arrays for Adenosine Triphosphate Detection. Sensors 2017, 17, 1686. [Google Scholar] [CrossRef] [Green Version]
- Radi, A.-E. Electrochemical Aptamer-Based Biosensors: Recent Advances and Perspectives. Int. J. Electrochem. 2011, 2011, 863196. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.R.; Jimenez, R.M.; Chaput, J.C. Analysis of aptamer discovery and technology. Nat. Rev. Chem. 2017, 1, 76. [Google Scholar] [CrossRef]
- Cai, S.; Yan, J.; Xiong, H.; Liu, Y.; Peng, D.; Liu, Z. Investigations on the interface of nucleic acid aptamers and binding targets. Analyst 2018, 143, 5317–5338. [Google Scholar] [CrossRef]
- Villalonga, A.; Pérez-Calabuig, A.M.; Villalonga, R. Electrochemical biosensors based on nucleic acid aptamers. Anal. Bioanal. Chem. 2020, 412, 55–72. [Google Scholar] [CrossRef]
- Byun, J. Recent Progress and Opportunities for Nucleic Acid Aptamers. Life 2021, 11, 193. [Google Scholar] [CrossRef]
- Pividori, M. Electrochemical genosensor design: Immobilisation of oligonucleotides onto transducer surfaces and detection methods. Biosens. Bioelectron. 2000, 15, 291–303. [Google Scholar] [CrossRef]
- Nogues, C.; Leh, H.; Lautru, J.; Delelis, O.; Buckle, M. Efficient Antifouling Surface for Quantitative Surface Plasmon Resonance Based Biosensor Analysis. PLoS ONE 2012, 7, e44287. [Google Scholar] [CrossRef]
- Milne, N.; Di Rosa, F. The Diabetes Review: A Guide to the Basics. J. Diabetes Nurs. 2020, 24, JDN161. [Google Scholar]
- Liu, S.; Shen, Z.; Deng, L.; Liu, G. Smartphone assisted portable biochip for non-invasive simultaneous monitoring of glucose and insulin towards precise diagnosis of prediabetes/diabetes. Biosens. Bioelectron. 2022, 209, 114251. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-Y.; Chang, B.-Y.; Nam, H.; Park, S.-M. Selective Electrochemical Sensing of Glycated Hemoglobin (HbA1c) on Thiophene-3-Boronic Acid Self-Assembled Monolayer Covered Gold Electrodes. Anal. Chem. 2008, 80, 8035–8044. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, N.; Kimura, T.; Umehara, F.; Katayama, Y.; Nagai, G.; Suzuki, K.; Aisaka, K.; Maruyama, Y.; Itoh, T.; Hashimoto, W.; et al. Creation of haemoglobin A1c direct oxidase from fructosyl peptide oxidase by combined structure-based site specific mutagenesis and random mutagenesis. Sci. Rep. 2019, 9, 942. [Google Scholar] [CrossRef] [Green Version]
- Sherwani, S.I.; Khan, H.A.; Ekhzaimy, A.; Masood, A.; Sakharkar, M.K. Significance of HbA1c Test in Diagnosis and Prognosis of Diabetic Patients. Biomark. Insights 2016, 11, BMI-S38440. [Google Scholar] [CrossRef]
- Hörber, S.; Achenbach, P.; Schleicher, E.; Peter, A. Harmonization of immunoassays for biomarkers in diabetes mellitus. Biotechnol. Adv. 2019, 39, 107359. [Google Scholar] [CrossRef]
- Pohanka, M. Glycated Hemoglobin and Methods for Its Point of Care Testing. Biosensors 2021, 11, 70. [Google Scholar] [CrossRef]
- Duanghathaipornsuk, S.; Reaver, N.G.F.; Cameron, B.D.; Kim, D.-S. Adsorption Kinetics of Glycated Hemoglobin on Aptamer Microarrays with Antifouling Surface Modification. Langmuir 2021, 37, 4647–4657. [Google Scholar] [CrossRef]
- Suryathi, N.M.A. Hemoglobin Glikosilat Yang Tinggi Meningkatkan Prevalensi Retinopati Diabetik Proliferatif; Universitas Udayana: Denpasar, Indonesia, 2015; pp. 1–92. [Google Scholar]
- Thiruppathi, M.; Lin, P.-Y.; Chou, Y.-T.; Ho, H.-Y.; Wu, L.-C.; Ho, J.-A.A. Simple aminophenol-based electrochemical probes for non-enzymatic, dual amperometric detection of NADH and hydrogen peroxide. Talanta 2019, 200, 450–457. [Google Scholar] [CrossRef]
- Destiani, S.; Maksum, I.P.; Hartati, Y.W. Biosensor Elektrokimia untuk Memonitor Level Hemoglobin Terglikasi (HbA1c) pada Penyakit Diabetes Melitus. Alchemy J. Penelit. Kim. 2023, 19, 94–107. [Google Scholar] [CrossRef]
- Eissa, S.; Zourob, M. Aptamer- Based Label-Free Electrochemical Biosensor Array for the Detection of Total and Glycated Hemoglobin in Human Whole Blood. Sci. Rep. 2017, 7, 1016. [Google Scholar] [CrossRef] [Green Version]
- Eissa, S.; Almusharraf, A.Y.; Zourob, M. A comparison of the performance of voltammetric aptasensors for glycated haemoglobin on different carbon nanomaterials-modified screen printed electrodes. Mater. Sci. Eng. C 2019, 101, 423–430. [Google Scholar] [CrossRef]
- Bai, X.; Wang, Z.; Huang, C.; Wang, Z.; Chi, L. Investigation of Non-Enzymatic Glycosylation of Human Serum Albumin Using Ion Trap-Time of Flight Mass Spectrometry. Molecules 2012, 17, 8782–8794. [Google Scholar] [CrossRef] [Green Version]
- Koga, M.; Kasayama, S. Clinical impact of glycated albumin as another glycemic control marker. Endocr. J. 2010, 57, 751–762. [Google Scholar] [CrossRef] [Green Version]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Pu, L.J.; Lu, L.; Shen, W.F.; Zhang, Q.; Zhang, R.Y.; Zhang, J.S.; Hu, J.; Yang, Z.K.; Ding, F.H.; Chen, Q.J.; et al. Increased Serum Glycated Albumin Level is Associated with the Presence and Severity of Coronary Artery Disease in Type 2 Diabetic Patients. Circ. J. 2007, 71, 1067–1073. [Google Scholar] [CrossRef] [Green Version]
- Hatada, M.; Loew, N.; Okuda-Shimazaki, J.; Khanwalker, M.; Tsugawa, W.; Mulchandani, A.; Sode, K. Development of an Interdigitated Electrode-Based Disposable Enzyme Sensor Strip for Glycated Albumin Measurement. Molecules 2021, 26, 734. [Google Scholar] [CrossRef]
- Ghosh, S.; Datta, D.; Cheema, M.; Dutta, M.; Stroscio, M.A. Aptasensor based optical detection of glycated albumin for diabetes mellitus diagnosis. Nanotechnology 2017, 28, 435505. [Google Scholar] [CrossRef]
- Japrung, D.; Apiwat, C.; Treerattrakoon, K.; Dharakul, T.; Luksirikul, P. Aptasensor for diabetes mellitus detection and monitoring. In Proceedings of the 2015 IEEE 15th International Conference on Nanotechnology (IEEE-NANO), Rome, Italy, 27–30 July 2015; pp. 1521–1524. [Google Scholar] [CrossRef]
- Apiwat, C.; Luksirikul, P.; Kankla, P.; Pongprayoon, P.; Treerattrakoon, K.; Paiboonsukwong, K.; Fucharoen, S.; Dharakul, T.; Japrung, D. Graphene based aptasensor for glycated albumin in diabetes mellitus diagnosis and monitoring. Biosens. Bioelectron. 2016, 82, 140–145. [Google Scholar] [CrossRef]
- Bunyarataphan, S.; Dharakul, T.; Fucharoen, S.; Paiboonsukwong, K.; Japrung, D. Glycated Albumin Measurement Using an Electrochemical Aptasensor for Screening and Monitoring of Diabetes Mellitus. Electroanalysis 2019, 31, 2254–2261. [Google Scholar] [CrossRef]
- Aye, N.N.; Maraming, P.; Tavichakorntrakool, R.; Chaibunruang, A.; Boonsiri, P.; Daduang, S.; Teawtrakul, N.; Prasongdee, P.; Amornkitbamrung, V.; Daduang, J. A Simple Graphene Functionalized Electrochemical Aptasensor for the Sensitive and Se-lective Detection of Glycated Albumin. Appl. Sci. 2021, 11, 10315. [Google Scholar] [CrossRef]
- Zhou, L.; Figueroa-Miranda, G.; Chen, S.; Neis, M.; Hu, Z.; Zhu, R.; Li, Y.; Prömpers, M.; Offenhäusser, A.; Mayer, D. Flexible multielectrode arrays based electrochemical aptasensor for glycated human serum albumin detection. Sens. Actuators B Chem. 2023, 386, 133730. [Google Scholar] [CrossRef]
- Fargion, S.; Dongiovanni, P.; Guzzo, A.; Colombo, S.; Valenti, L.; Fracanzani, A.L. Iron and insulin resistance. Aliment. Pharmacol. Ther. 2005, 22, 61–63. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, W.; Mochizuki, E.; Takase, M.; Hasegawa, H.; Morita, Y.; Yamazaki, H.; Sode, K.; Ikebukuro, K. Selection of DNA aptamers against insulin and construction of an aptameric enzyme subunit for insulin sensing. Biosens. Bioelectron. 2009, 24, 1116–1120. [Google Scholar] [CrossRef]
- Kubo, I.; Eguchi, T. Study on Electrochemical Insulin Sensing Utilizing a DNA Aptamer-Immobilized Gold Electrode. Materials 2015, 8, 4710–4719. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Xu, Y.; Zhang, M.; Xiang, J.; Deng, C.; Wu, H. An electrochemical dual-signaling aptasensor for the ultrasensitive detection of insulin. Anal. Biochem. 2019, 573, 30–36. [Google Scholar] [CrossRef]
- Asadpour, F.; Mazloum-Ardakani, M.; Hoseynidokht, F.; Moshtaghioun, S.M. In situ monitoring of gating approach on mesoporous silica nanoparticles thin-film generated by the EASA method for electrochemical detection of insulin. Biosens. Bioelectron. 2021, 180, 113124. [Google Scholar] [CrossRef]
- Ishizaka, A.; Tono-Oka, T.; Matsumoto, S. Evaluation of the proliferative response of lymphocytes by measurement of intracellular ATP. J. Immunol. Methods 1984, 72, 127–132. [Google Scholar] [CrossRef]
- Nakamura, N.; Wada, Y. Properties of DNA fragmentation activity generated by ATP depletion. Cell Death Differ. 2000, 7, 477–484. [Google Scholar] [CrossRef] [Green Version]
- Garland, J.M.; Halestrap, A. Energy Metabolism during Apoptosis. J. Biol. Chem. 1997, 272, 4680–4688. [Google Scholar] [CrossRef] [Green Version]
- Crouch, S.P.M.; Kozlowski, R.; Slater, K.J.; Fletcher, J. The use of ATP bioluminescence as a measure of cell proliferation and cytotoxicity. J. Immunol. Methods 1993, 160, 81–88. [Google Scholar] [CrossRef]
- Manson, J.; Thiemermann, C.; Brohi, K. Trauma alarmins as activators of damage-induced inflammation. Br. J. Surg. 2011, 99, 12–20. [Google Scholar] [CrossRef]
- Ellsworth, M.L.; Ellis, C.G.; Goldman, D.; Stephenson, A.H.; Dietrich, H.H.; Sprague, R.S. Erythrocytes: Oxygen Sensors and Modulators of Vascular Tone. Physiology 2009, 24, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Chida, J.; Ono, R.; Yamane, K.; Hiyoshi, M.; Nishimura, M.; Onodera, M.; Nakataki, E.; Shichijo, K.; Matushita, M.; Kido, H. Blood Lactate/ATP Ratio, as an Alarm Index and Real-Time Biomarker in Critical Illness. PLoS ONE 2013, 8, e60561. [Google Scholar] [CrossRef] [Green Version]
- Naing, A.; Kenchaiah, M.; Krishnan, B.; Mir, F.; Charnley, A.; Egan, C.; Bano, G. Maternally inherited diabetes and deafness (MIDD): Diagnosis and management. J. Diabetes Its Complicat. 2014, 28, 542–546. [Google Scholar] [CrossRef]
- Maksum, I.P.; Natradisastra, S.G.; Nuswantara, Y.N. The Effect of A3243G Mutation of Mitochondrial DNA to the Clinical Features of Type-2 Diabetes Mellitus and Cataract. Pap. Knowl. Towar. Media Hist. Doc. 2013, 96, 591–599. [Google Scholar]
- Frazier, A.E.; Thorburn, D.R.; Compton, A.G. Mitochondrial energy generation disorders: Genes, mechanisms, and clues to pathology. J. Biol. Chem. 2019, 294, 5386–5395. [Google Scholar] [CrossRef] [Green Version]
- Maksum, I.P.; Maulana, A.F.; Yusuf, M.; Mulyani, R.; Destiarani, W.; Rustaman, R. Molecular Dynamics Simulation of a tRNA-Leucine Dimer with an A3243G Heteroplasmy Mutation in Human Mitochondria Using a Secondary Structure Prediction Approach. Indones. J. Chem. 2021, 22, 1043–1051. [Google Scholar] [CrossRef]
- Sari, R.P.; Maulana, A.F.; Yusuf, M.; Maksum, I.P. Simulation Modeling of A3243g Mutations on tRNALeu (UUR) against Type 2 Diabetes Mellitus using In Silico Method. Res. J. Chem. Environ. 2023, 27, 65–71. [Google Scholar] [CrossRef]
- Destiarani, W.; Mulyani, R.; Yusuf, M.; Maksum, I.P. Molecular Dynamics Simulation of T10609C and C10676G Mutations of Mitochondrial ND4L Gene Associated with Proton Translocation in Type 2 Diabetes Mellitus and Cataract Patients. Bioinform. Biol. Insights 2020, 14, 1–8. [Google Scholar] [CrossRef]
- Azizah, M.I.; Mulyani, R.; Maksum, I.P. Design and Optimization of PCR-RFLP Assay for Detection of G9053A and T15663C Mutation in Mitochondrial DNA. Res. J. Chem. Environ. 2023, 27, 1–5. [Google Scholar] [CrossRef]
- Maksum, I.P.; Farhani, A.; Rachman, S.D.; Ngili, Y. Making of the A3243g Mutant Template through Site Directed Mutagenesis as Positive Control in PASA-Mismatch Three Bases. Int. J. PharmTech Res. 2013, 5, 441–450. [Google Scholar]
- Maksum, I.P.; Saputra, S.R.; Indrayati, N.; Yusuf, M.; Subroto, T. Bioinformatics Study of m.9053G>A Mutation at the ATP6 Gene in Relation to Type 2 Diabetes Mellitus and Cataract Diseases. Bioinform. Biol. Insights 2017, 11, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.; Lim, H.S.; Ma, Q.; Gao, Z. Optical Aptasensors for Adenosine Triphosphate. Theranostics 2016, 6, 1683–1702. [Google Scholar] [CrossRef]
- Sazani, P.L.; Larralde, R.; Szostak, J.W. A Small Aptamer with Strong and Specific Recognition of the Triphosphate of ATP. J. Am. Chem. Soc. 2004, 126, 8370–8371. [Google Scholar] [CrossRef] [Green Version]
- Zhao, M.; Liao, L.; Wu, M.; Lin, Y.; Xiao, X.; Nie, C. Double-receptor sandwich supramolecule sensing method for the determination of ATP based on uranyl–salophen complex and aptamer. Biosens. Bioelectron. 2012, 34, 106–111. [Google Scholar] [CrossRef]
- Sessler, J.L.; Melfi, P.J.; Pantos, G.D. Uranium complexes of multidentate N-donor ligands. Co-Ord. Chem. Rev. 2006, 250, 816–843. [Google Scholar] [CrossRef]
- Rudkevich, D.M.; Verboom, W.; Brzozka, Z.; Palys, M.J.; Stauthamer, W.P.R.V.; van Hummel, G.J.; Franken, S.M.; Harkema, S.; Engbersen, J.F.J.; Reinhoudt, D.N. Functionalized UO2 Salenes: Neutral Receptors for Anions. J. Am. Chem. Soc. 1994, 116, 4341–4351. [Google Scholar] [CrossRef] [Green Version]
- Kashefi-Kheyrabadi, L.; Mehrgardi, M.A. Aptamer-based electrochemical biosensor for detection of adenosine triphosphate using a nanoporous gold platform. Bioelectrochemistry 2013, 94, 47–52. [Google Scholar] [CrossRef]
- Xie, Y.-C.; Eriksson, L.A.; Zhang, R.-B. Molecular dynamics study of the recognition of ATP by nucleic acid aptamers. Nucleic Acids Res. 2020, 48, 6471–6480. [Google Scholar] [CrossRef]
- Kanyong, P.; Rawlinson, S.; Davis, J. Gold nanoparticle modified screen-printed carbon arrays for the simultaneous electrochemical analysis of lead and copper in tap water. Microchim. Acta 2016, 183, 2361–2368. [Google Scholar] [CrossRef]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Al Bawab, A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef] [Green Version]
- Mulyani, R.; Yumna, N.; Maksum, I.P.; Subroto, T.; Hartati, Y.W. Optimization of Aptamer-Based Electrochemical Biosensor for ATP Detection Using Screen-Printed Carbon Electrode/Gold Nanoparticles (SPCE/AuNP). Indones. J. Chem. 2022, 22, 1256–1268. [Google Scholar] [CrossRef]
- Rustaman, R.; Rahmawan, R.R.; Maksum, I.P. In Silico Study of Aptamer Specificity for De-Tection of Adenosine Triphosphate (Atp) As Biosensor Development for Mitochondria. Turk. Comput. Theor. Chem. 2023, 7, 58–69. [Google Scholar]
- Zeng, X.; Wang, H.; Zeng, Y.; Yang, Y.; Zhang, Z.; Li, L. Label-free Aptasensor for the Ultrasensitive Detection of Insulin Via a Synergistic Fluorescent Turn-on Strategy Based on G-quadruplex and AIEgens. J. Fluoresc. 2022, 33, 955–963. [Google Scholar] [CrossRef]
- He, Y.; Cheng, Y.; Wen, X. A design of red emission CDs-based aptasensor for sensitive detection of insulin via fluorescence resonance energy transfer. Spectrochim. Acta Part A: Mol. Biomol. Spectrosc. 2022, 280, 121497. [Google Scholar] [CrossRef]
- Liu, J.; Zhu, B.; Dong, H.; Zhang, Y.; Xu, M.; Travas-Sejdic, J.; Chang, Z. A novel electrochemical insulin aptasensor: From glassy carbon electrodes to disposable, single-use laser-scribed graphene electrodes. Bioelectrochemistry 2021, 143, 107995. [Google Scholar] [CrossRef]
- Mandani, S.; Rezaei, B.; Ensafi, A.A. Developing a highly-sensitive aptasensor based on surface energy transfer between InP/ZnS quantum dots and Ag-nanoplates for the determination of insulin. J. Photochem. Photobiol. A Chem. 2021, 423, 113601. [Google Scholar] [CrossRef]
- Şahin, S.; Kaya, Ş.; Üstündağ, Z.; Caglayan, M.O. An electrochemical signal switch–based (on–off) aptasensor for sensitive detection of insulin on gold-deposited screen-printed electrodes. J. Solid State Electrochem. 2022, 26, 907–915. [Google Scholar] [CrossRef]
- Salandari-Jolge, N.; Ensafi, A.A.; Rezaei, B. An ultrasensitive electrochemical aptasensor based on a single-stranded aptamer-Au@Fe-MIL-88 complex using methylene blue as an electrochemical probe for insulin detection. Anal. Bioanal. Chem. 2021, 413, 7451–7462. [Google Scholar] [CrossRef]
- Meng, A.; Hong, X.; Zhang, Y.; Yin, J.; Sheng, L.; Li, Z. An antifouling electrochemical aptasensor based on poly (glutamic acid) and peptide for the sensitive detection of adenosine triphosphate. Microchem. J. 2021, 168, 106365. [Google Scholar] [CrossRef]
- Li, J.; Wang, X.; Liu, W.; Li, X.; Yang, L.; Ma, H.; Wu, R.; Wei, Q. Highly selective electrochemiluminescence aptasensor coupled with mesoporous Fe3O4@Cu@Cu2O as co-reaction accelerator for ATP assay based on target-triggered emitter release. Sens. Actuators B Chem. 2021, 346, 130581. [Google Scholar] [CrossRef]
- Wang, X.; Mao, Z.; Chen, R.; Li, S.; Ren, S.; Liang, J.; Gao, Z. Self-assembled DNA origami-based duplexed aptasensors combined with centrifugal filters for efficient and rechargeable ATP detection. Biosens. Bioelectron. 2022, 211, 114336. [Google Scholar] [CrossRef] [PubMed]


| Current Plasma Glucose (mg/dL) | Glucose Tolerance Impaired (mg/dL) | Fasting Blood Glucose (mg/dL) | HbA1c (%) | |
|---|---|---|---|---|
| Diabetes | ≥200 | ≥200 | ≥126 | ≥6.5 |
| Prediabetes | Not applicable | 140–199 | 100–125 | 5.7–6.4 |
| Diabetes during pragnency | ≥200 | ≥200 | ≥126 | ≥6.5 |
| Normal | Not applicable | <140 | <100 | <5.7 |
| Characteristics | Aptamer | Antibody |
|---|---|---|
| Toxic or poorly immunogenic immunogenogenic target | No | Yes |
| Isolation process | In vitro selection under various conditions | Limited to psychological conditions with animal immunization |
| Immunogenicity | Little to none | Significant |
| Batch activity | Uniform | Varies |
| Stability | Insensitive to redox reactions. Difficult to aggregate due to lack of hydrophobicity. Resistant to changes in pH and temperature. | Sensitive to redox reactions. Easy to form aggregates. Sensitive to changes in pH and temperature. |
| Screening time snd cost | Limited screening processes | Time-comsuming and expensive |
| Shelf life | Long | Limited |
| Controllable binding and release condition | Can be changed on demand | Difficult to modify |
| Chemical modifications | Easy to modify with low cost | Difficult and expensive to modify |
| Detection Principle | Biomarker | Aptamer Sequence | Result | Ref |
|---|---|---|---|---|
| Surface modification of SPCE with gold nanoparticles using methylene blue as a redox probe. The prepared SPCE is integrated with a micro-device containing a bluetooth transmission system for smartphone signal reading. | Glucose and Insulin | Glucose: 5′–HS–HS-C6-CTCTCGGGACGACCGTGTGTGTTGCTCTGTAACAGTGTCCATTGTCGTCCC-MB-3′ Insulin: 5′ –HS–HS-C6-AAAAGGTGGTGGGGGGGGTTGGTAGGGTGTCTTCT-MB3′ | Good stability and high sensitivity. | [55] |
| Apatamers modified with thiol groups were immobilized on AuNP electrodes and measured using square wave voltammetry (SWV). | HbA1c | 5′-GGGGACACAGCAAC ACACCCACCCACCAGCCCCAGCATCATGCCCATCCGTCGTGTGTG-3′ | High sensitivity and selectivity, with an LoD of 0.2 mL−1 and a linear range of 100 pg mL−1–10µg mL−1. | [65] |
| The aptamer was non-covalently immobilized on six electrodes of nanomaterials via π–π stacking interactions between DNA nucleobase and carbon material surface. Measurements were performed using SWV. | HbA1c | 5′-ACACACCCACCCA CAGCCCCAGCATCATGCCCATCCGTCGTGTGT-3′ | The SWCNT aptasensor showed the highest selectivity and sensitivity, with a limit of detection (LoD) of 0.03 pg mL−1. | [66] |
| Aptamer immobilization on silicon nanowire field effect transistors. Aptamer was also modified with polyethylene glycol. Docking and molecular dynamics simulations were performed. | HbA1c | 5′-HS-GGT GAG TTA AGG AAT CAG CGG CTC AGA CGA CCC GAC GCA C-3′ | Aptamer has a binding energy of 12.30 ± 0.05 kcal/mol, which is consistent with theoretical calculations. | [9] |
| Immobilization of thiolated aptamer on gold electrode surface and quantum dot. Measurement conducted using Ocean Optics USB4000 spectrophotometer. | GHSA | 5′-Thiol C6/TGCGGTTGTAGTACTCGTGCCCG/Thiol C6 SS 3’ | High selectivity to GHSA. Concentration range of 1008 nM–4500 nM and limit of detection of 1 nM. | [72] |
| Immobilization of fluorescently labeled aptamer on graphene oxide surface. When GHSA is present, the fluorescence-labeled aptamer will bind to GHSA and produce a signal. | GHSA | 5′-GGTGCGGTTCGTGCGGTTGTAGTACTCGTGGCCGATAGAGGTAGTTTCG-3′ | High sensitivity when detecting human serum with an LoD of 50 μg mL−1 | [73] |
| Immobilization of biotinylated aptamer on screen-printed carbon electrode modified with streptavidin and measurement using SWV. | GHSA | 5’ TGCGGTTGTAGTAC TCGTGCCCG-3 | High selectivity for GHSA compared to glucose, glycine, folic acid and ampicillin. | [75] |
| Immobilization of thiol aptamer on the surface with graphene oxide was measured using SWV. | GHSA | 5′ -NH2-TGC GGT TGT AGT ACT CGT GGC CG–3 | High selectivity, with an LoD of 0.031 μg mL−1. | [76] |
| Immobilization of aptamer on the surface of the pollimer chip modified with gold electrode. Measurement conducted by differential pulse voltammetry (DPV). | GHSA and HSA | GHSA: 5′-ferrosen-(CH2)6-GTC TCA GCT ACC TTA CCG TAT GTG GCC CAA AGC GTC TGG ATG GCT ATG AA-(CH2)6-SS-(CH2)6-OH-3′ HSA: 5′-ferrosen-(CH2)6-TGC GGT TCG TGC GGT TGT AGT ACT CGT GGC CGA T-(CH2)6-SS-(CH2)6-OH-3′. | Selective to the target in diluted blood samples with an LoD for HSA of 13 nM and 25 nM for GHSA. | [77] |
| Selection of aptamers by SELEX process; the process of measuring circular dichroism (CD) is used to select specific aptamers. | Insulin | 5′-GGTGGTGGGGGGGG TTGGTAGGGTGTCTTC-3′ | The aptamer folded well into a quartet structure. | [79] |
| The aptamer was immobilized on a gold electrode. Measurements were made using cyclic voltammetry and activity was confirmed using spectrophotometry. | Insulin | 5′-GGTGGTGGGGGGGGTTGGTAGGGTGTCTTC-3′ | The cathodic peak in IGA3–hemin complex decreased with insulin concentration. | [80] |
| Immobilization of insulin aptamer on graphene oxide with quaternary tetraphenylethine salt probe. | Insulin | 5′-GGTGGTGGGGGGG GTTGGTAGGGTGTCTTC-3′ | Good linear relationship, with an insulin concentration ranging from 1.0 pM to 1.0 μM with a low LOD of 0.42 pM. | [110] |
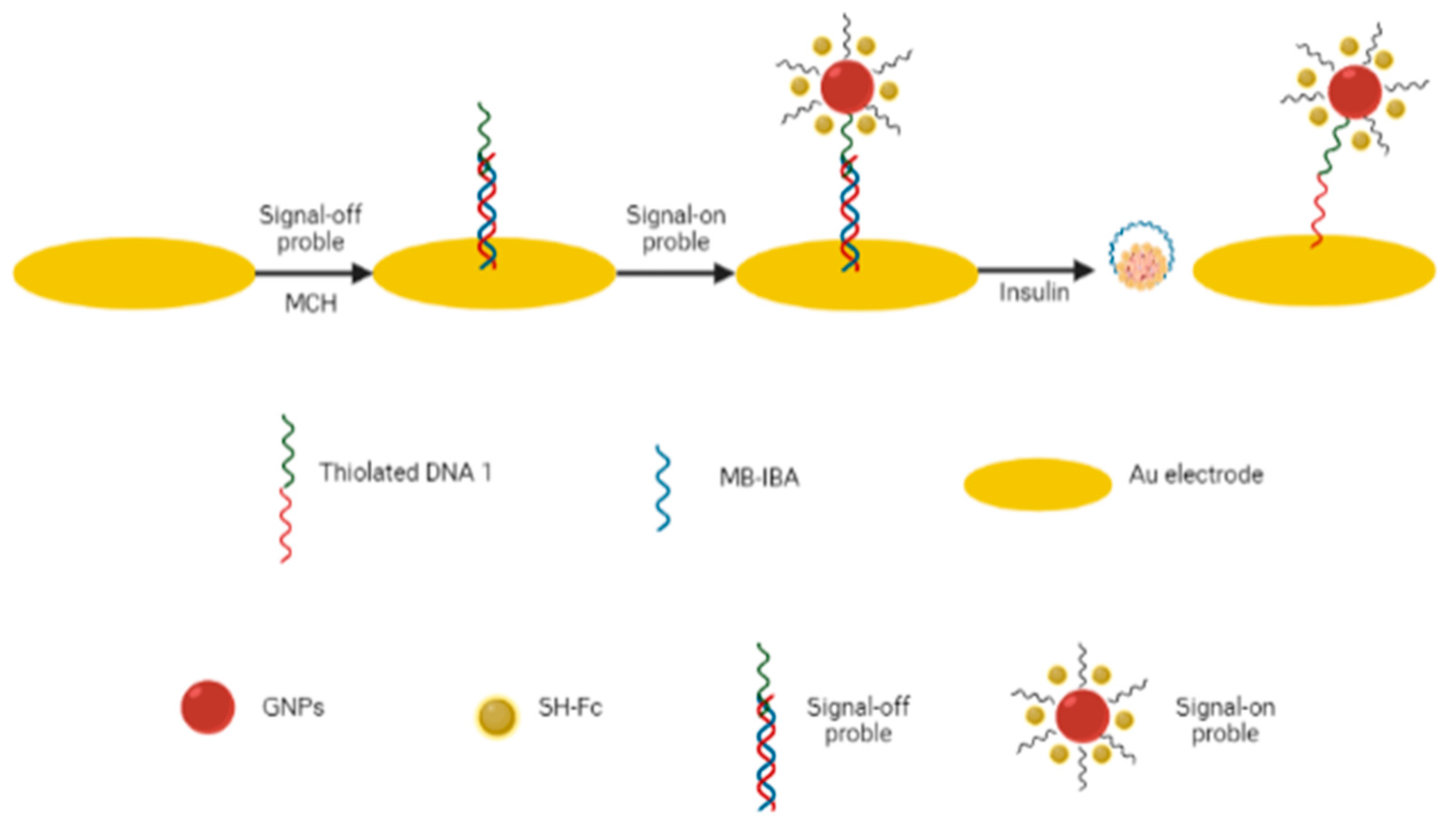
| Immobilized insulin binding aptamer (IBA) modified with methylene blue (MB) as “signal off” probe and aptamer/ferrocene modified with gold nanoparticles as “signal on” probe. Measurements were made using SWV. | Insulin | Aptamer IBA: 5′-SH-TTTTTTCAC CCT ACC ACC CCCTATGTAATA AGA GCT AAA-3′ | High sensitivity and selectivity; good reproducibility with an LoD of 0.1 pM and linear range of 10 pM–10 nM. | [81] |
| The aptamer hybridizes with cDNA on the mesoporous silica surface as a molecule that limits the diffusion of the probe towards the electrode by closing the mesochannels. When binding to insulin, the hybridization of cDNA with the aptamer will be destroyed and open the nano-channels, resulting in an increase in DPV signal. | Insulin | 5′-GGT GGT GGG GGG GGT TGG TAG GGT GTC TTC-3′ | High selectivity and sensitivity, with an LoD of 3 nM and linear range of 10.0–350.0 nM. | [82] |
| Immobilization of aptamer-labeled red emission carbon dots (R-CDs) on graphene oxidase. Effectively detected insulin (INS) generates fluorescence resonance energy transfer (FRET). | Insulin | 5′-(CH2)6-NH2GGT GGT GGG GGG GGT TGG TAG GGT GTC TTC-3′ | High sensitivity and anti-interference, with an LOD of 1.1 nM and a linear range of 1.3–150 nM. | [111] |
| Immobilization of aptamer on glass carbon electrode (GCE); when insulin binds to aptamer, it will undergo hydrolysis with the help of Exo 1 to cut the aptame that does not bind. Gold nanoparticle-probe aptamer is added so that a complex is formed to form a sandwich assay. Measurement with DPV. | Insulin | 5′-SH-(CH2)6-GGT GGT GGG GGG GGT TGG TAG GGT GTC TTC 3′ | High selectivity and sensitivity for the detection of insulin, with an LoD of 9.8 fM and linear range from 0.1 pM to 1.0 μM. | [112] |
| Immobilization of aptamer on quantum dot surface. Fluorescence signal measurement. | Insulin | NH2-5’-GGT GGT GGG GGG GGT TGG TAG GGT GTC TTC-3’ | High sensitivity and selectivity, with a linear range of 0.001–5000 nM and LoD of 0.5 pM. | [113] |
| Methylene blue (MB)-modifed insulin-specifc aptamer immobilized on gold-deposited screen-printed electrodes. | Insulin | SH-(CH2)6–GGT GGT GGG GGG GGT TGG TAG GGT GTC TTC—AttoMB2 | The stability of the sensor was good for 10 days, remaining at 92% with high sensitivity and selectivity. | [114] |
| Aptamer with MB probe immobilized on Au@Fe-MIL-88. Measurements by cyclic voltametry and DPV. | Insulin | 5′-NH2 GGTGGTGGGG GGGGTTGGTAGGGTGTTTTC-3′ | High-sensitivity dan reproducibility, with an LoD 1.3 × 10−16 mol L−1. | [115] |
| Immobilization of aptamer on modified SPCE on gold nanoparticles. Measurement with DPV. | ATP | F1: 5′-HS-(CH2)6-ACCTGGGGGAGTAT-3′ F2: 5′-TGCGGAGGAAGGT-CH2)2-NH2-3′ | High selectivity for ATP compared to UTP, CTP, and GTP. Good reproducibility and stability. | [104] |
| Immobilization of aptamer on modified SPCE on gold nanoparticles. Measurement with DPV. | ATP | F1: 5′-HS-(CH2)6-ACCTGGGGGAGTAT-3′ F2: 5′-TGCGGAGGAAGGT-CH2)2-NH2-3′ | High selectivity for ATP compared to UTP, CTP, and GTP, with LOD and LOQ values of 7.43 and 24.78 μM, respectively. | [108] |
| Molecular dynamics simulation between aptamer and ATP, forming a sandwich conformation assay to determine the interaction. | ATP | F1: 5′-HS-(CH2)6-ACCTGGGGGAGTAT-3′ F2: 5′-TGCGGAGGAAGGT-CH2)2-NH2-3′ | High specificity to ATP compared to ADP and AMP. | [109] |
| Molecular dynamics simulation between aptamer and ATP to determine the interaction and binding energy. | ATP | 5′-ACCTGGGGGAGTAT TGCGGAGGAAGGT-3′ | Aptamer has a high degree of rigidity due to the influence of ATP. Interacts on nucleobases at G6, A23, and forms hydrographic bonds at G22. | [105] |
| The aptamer was immobilized on a modified glassy carbon electrode. Measurement conducted by DPV. | ATP | 5′-NH2-ACC TGG GGG AGT ATT GCG GAG GAA GGT-3′ | High selectivity, stability and reproducibility with an LoD of 0.01 pM and linear range of 0.01 pM–1 μM. | [116] |
| Immobilization of aptamer on modified GCE with Fe3HAI4@ Cu@Cu2O nanocomposite. | ATP | 5′-NH2-TGGAAGGAGGCGTTATGAGGGGGTCCA-3′ | High sensitivity and excellent specificity, with an LoD of 0.17 nmol/L and a linear range of 0.5–2500 nmol/L. | [117] |
| Immobilization of aptamer labeled with FAM aptamer on origami-based duplex. | ATP | 5′-CACTGACCTGGGGGAGTATTGCGGAGGAAGGT-3′ | High sensitivity and selectivity with LoD 0.29 ng mL−1 and a linear range of 0.1 ng mL−1. | [118] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mulyani, D.E.; Maksum, I.P. Detection of Biomarker Using Aptasensors to Determine the Type of Diabetes. Diagnostics 2023, 13, 2035. https://doi.org/10.3390/diagnostics13122035
Mulyani DE, Maksum IP. Detection of Biomarker Using Aptasensors to Determine the Type of Diabetes. Diagnostics. 2023; 13(12):2035. https://doi.org/10.3390/diagnostics13122035
Chicago/Turabian StyleMulyani, Dinda Exelsa, and Iman Permana Maksum. 2023. "Detection of Biomarker Using Aptasensors to Determine the Type of Diabetes" Diagnostics 13, no. 12: 2035. https://doi.org/10.3390/diagnostics13122035




