A Rare Complication of Ascariasis: A Case of Acute Interstitial Nephritis
Abstract
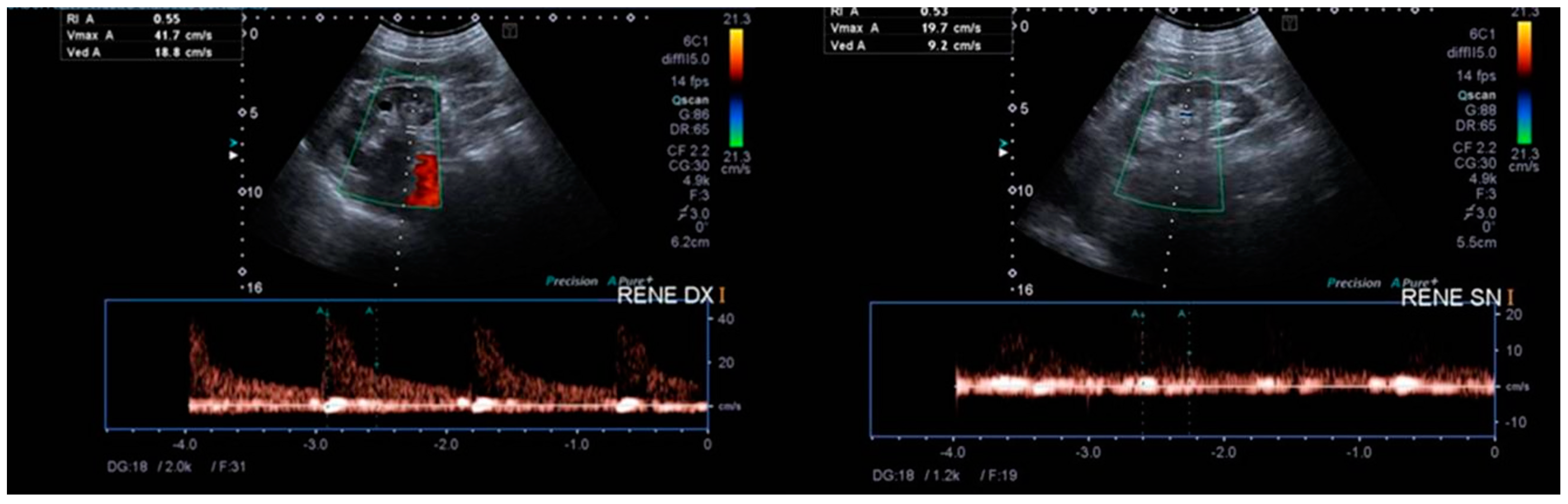
:


Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lynn, M.K.; Morrissey, J.A.; Conserve, D.F. Soil-Transmitted Helminths in the USA: A Review of Five Common Parasites and Future Directions for Avenues of Enhanced Epidemiologic Inquiry. Curr. Trop. Med. Rep. 2021, 8, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Kolleri, J.J.; Thabet, A.M.J.; Mohammedain, S.; Sajid, S.; Ahmed, Z.; Momin, U. A Case Report on Biliary Ascariasis. Cureus. 2023, 15, e33323. [Google Scholar] [CrossRef] [PubMed]
- Raghavan, R.; Eknoyan, G. Acute interstitial nephritis—A reappraisal and update. Clin. Nephrol. 2014, 82, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Ditting, T.; Gröne, H.J.; Geiger, H.; Hauser, I.A. Acute interstitial nephritis in a case of Ascaris lumbricoides infection. Nephrol. Dial. Transplant. 2004, 19, 1625–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Duffey, M.; Alex, S.E.; Suarez-Reyes, C.; Clark, E.H.; Weatherhead, J.E. The role of helminths in the development of non-communicable diseases. Front. Immunol. 2022, 13, 941977. [Google Scholar] [CrossRef] [PubMed]
- Turgut, F.; Awad, A.S.; Abdel-Rahman, E.M. Acute Kidney Injury: Medical Causes and Pathogenesis. J. Clin. Med. 2023, 12, 375. [Google Scholar] [CrossRef] [PubMed]
- Goicoechea, M.; Rivera, F.; López-Gómez, J.M. Spanish Registry of Glomerulonephritis. Increased prevalence of acute tubulointerstitial nephritis. Nephrol. Dial. Transplant. 2013, 28, 112–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moledina, D.G.; Parikh, C.R. Differentiating Acute Interstitial Nephritis from Acute Tubular Injury: A Challenge for Clinicians. Nephron. 2019, 143, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Prasad, N.; Patel, M.R. Infection-Induced Kidney Diseases. Front. Med. 2018, 5, 327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carullo, N.; Divenuto, F.; Marascio, N.; Adams, N.J.; Giancotti, A.; Comi, N.; Faga, T.; Bolignano, D.; Coppolino, G.; Serapide, F.; et al. A Rare Complication of Ascariasis: A Case of Acute Interstitial Nephritis. Diagnostics 2023, 13, 2054. https://doi.org/10.3390/diagnostics13122054
Carullo N, Divenuto F, Marascio N, Adams NJ, Giancotti A, Comi N, Faga T, Bolignano D, Coppolino G, Serapide F, et al. A Rare Complication of Ascariasis: A Case of Acute Interstitial Nephritis. Diagnostics. 2023; 13(12):2054. https://doi.org/10.3390/diagnostics13122054
Chicago/Turabian StyleCarullo, Nazareno, Francesca Divenuto, Nadia Marascio, Neill James Adams, Aida Giancotti, Nicolino Comi, Teresa Faga, Davide Bolignano, Giuseppe Coppolino, Francesca Serapide, and et al. 2023. "A Rare Complication of Ascariasis: A Case of Acute Interstitial Nephritis" Diagnostics 13, no. 12: 2054. https://doi.org/10.3390/diagnostics13122054




