Non-Contrast and Contrast-Enhanced Cardiac Computed Tomography Imaging in the Diagnostic and Prognostic Evaluation of Coronary Artery Disease
Abstract
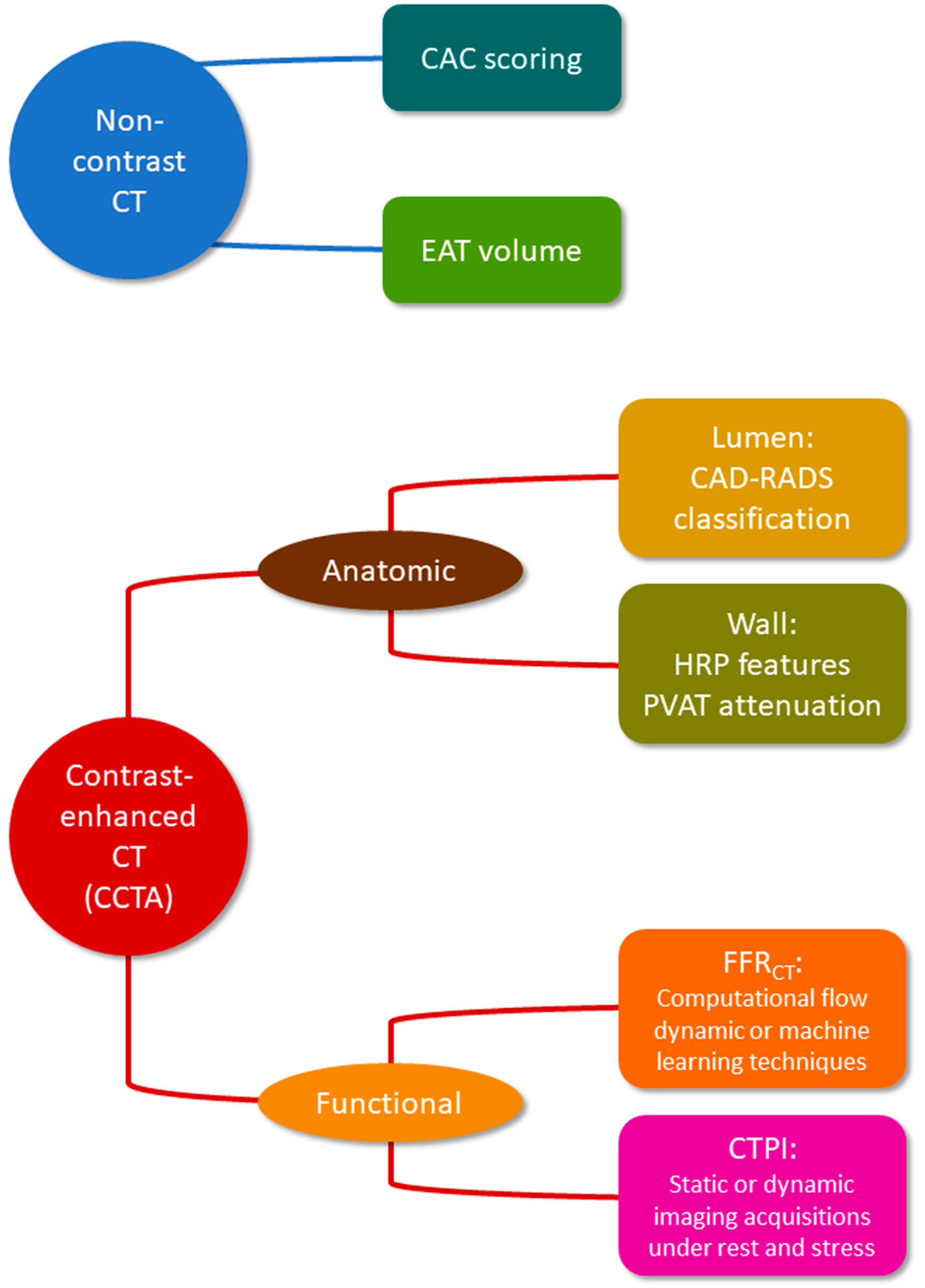
1. Introduction
2. Non-Contrast CT
2.1. Coronary Artery Calcium
2.2. Epicardial Adipose Tissue
3. Contrast-Enhanced CT
3.1. Lumen Stenosis
3.2. Myocardial Ischemia
3.3. Plaque Burden, Composition and Instability Features
3.4. Perivascular Adipose Tissue
4. Cardiac CT Positioning in Current Guidelines
4.1. CAC Score
4.2. CCTA
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report from the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Gao, Y.; Isakadze, N.; Duffy, E.; Sheng, Q.; Ding, J.; MacFarlane, Z.T.; Sang, Y.; McClure, S.T.; Selvin, E.; Matsushita, K.; et al. Secular Trends in Risk Profiles Among Adults with Cardiovascular Disease in the United States. J. Am. Coll. Cardiol. 2022, 80, 126–137. [Google Scholar] [CrossRef]
- Sofogianni, A.; Stalikas, N.; Antza, C.; Tziomalos, K. Cardiovascular Risk Prediction Models and Scores in the Era of Personalized Medicine. J. Pers. Med. 2022, 12, 1180. [Google Scholar] [CrossRef]
- Lloyd-Jones, D.M. Cardiovascular risk prediction: Basic concepts, current status, and future directions. Circulation 2010, 121, 1768–1777. [Google Scholar] [CrossRef]
- Devon, H.A.; Rosenfeld, A.; Steffen, A.D.; Daya, M. Sensitivity, specificity, and sex differences in symptoms reported on the 13-item acute coronary syndrome checklist. J. Am. Heart Assoc. 2014, 3, e000586. [Google Scholar] [CrossRef]
- Canto, J.G.; Shlipak, M.G.; Rogers, W.J.; Malmgren, J.A.; Frederick, P.D.; Lambrew, C.T.; Ornato, J.P.; Barron, H.V.; Kiefe, C.I. Prevalence, clinical characteristics, and mortality among patients with myocardial infarction presenting without chest pain. JAMA 2000, 283, 3223–3229. [Google Scholar] [CrossRef]
- Scirica, B.M. Prevalence, incidence, and implications of silent myocardial infarctions in patients with diabetes mellitus. Circulation 2013, 127, 965–967. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A. Stress testing and non-invasive coronary angiography in patients with suspected coronary artery disease: Time for a new paradigm. Heart Int. 2012, 7, e2. [Google Scholar] [CrossRef]
- Mintz, G.S.; Guagliumi, G. Intravascular imaging in coronary artery disease. Lancet 2017, 390, 793–809. [Google Scholar] [CrossRef]
- Desai, N.R.; Bradley, S.M.; Parzynski, C.S.; Nallamothu, B.K.; Chan, P.S.; Spertus, J.A.; Patel, M.R.; Ader, J.; Soufer, A.; Krumholz, H.M.; et al. Appropriate Use Criteria for Coronary Revascularization and Trends in Utilization, Patient Selection, and Appropriateness of Percutaneous Coronary Intervention. JAMA 2015, 314, 2045–2053. [Google Scholar] [CrossRef]
- Patel, M.R.; Peterson, E.D.; Dai, D.; Brennan, J.M.; Redberg, R.F.; Anderson, H.V.; Brindis, R.G.; Douglas, P.S. Low diagnostic yield of elective coronary angiography. N. Engl. J. Med. 2010, 362, 886–895. [Google Scholar] [CrossRef]
- Stocker, T.J.; Deseive, S.; Leipsic, J.; Hadamitzky, M.; Chen, M.Y.; Rubinshtein, R.; Heckner, M.; Bax, J.J.; Fang, X.M.; Grove, E.L.; et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: Results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur. Heart J. 2018, 39, 3715–3723. [Google Scholar] [CrossRef]
- Wong, N.D.; Budoff, M.J.; Ferdinand, K.; Graham, I.M.; Michos, E.D.; Reddy, T.; Shapiro, M.D.; Toth, P.P. Atherosclerotic cardiovascular disease risk assessment: An American Society for Preventive Cardiology clinical practice statement. Am. J. Prev. Cardiol. 2022, 10, 100335. [Google Scholar] [CrossRef]
- Demer, L.L.; Tintut, Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef]
- Mori, H.; Torii, S.; Kutyna, M.; Sakamoto, A.; Finn, A.V.; Virmani, R. Coronary Artery Calcification and its Progression: What Does it Really Mean? JACC Cardiovasc. Imaging 2018, 11, 127–142. [Google Scholar] [CrossRef]
- Aikawa, E.; Nahrendorf, M.; Figueiredo, J.L.; Swirski, F.K.; Shtatland, T.; Kohler, R.H.; Jaffer, F.A.; Aikawa, M.; Weissleder, R. Osteogenesis associates with inflammation in early-stage atherosclerosis evaluated by molecular imaging in vivo. Circulation 2007, 116, 2841–2850. [Google Scholar] [CrossRef]
- Shanahan, C.M. Inflammation ushers in calcification: A cycle of damage and protection? Circulation 2007, 116, 2782–2785. [Google Scholar] [CrossRef]
- Pugliese, G.; Iacobini, C.; Blasetti Fantauzzi, C.; Menini, S. The dark and bright side of atherosclerotic calcification. Atherosclerosis 2015, 238, 220–230. [Google Scholar] [CrossRef]
- Otsuka, F.; Sakakura, K.; Yahagi, K.; Joner, M.; Virmani, R. Has our understanding of calcification in human coronary atherosclerosis progressed? Arterioscler. Thromb. Vasc. Biol. 2014, 34, 724–736. [Google Scholar] [CrossRef]
- Agatston, A.S.; Janowitz, W.R.; Hildner, F.J.; Zusmer, N.R.; Viamonte, M., Jr.; Detrano, R. Quantification of coronary artery calcium using ultrafast computed tomography. J. Am. Coll. Cardiol. 1990, 15, 827–832. [Google Scholar] [CrossRef]
- Greenland, P.; Blaha, M.J.; Budoff, M.J.; Erbel, R.; Watson, K.E. Coronary Calcium Score and Cardiovascular Risk. J. Am. Coll. Cardiol. 2018, 72, 434–447. [Google Scholar] [CrossRef]
- Bild, D.E.; Detrano, R.; Peterson, D.; Guerci, A.; Liu, K.; Shahar, E.; Ouyang, P.; Jackson, S.; Saad, M.F. Ethnic differences in coronary calcification: The Multi-Ethnic Study of Atherosclerosis (MESA). Circulation 2005, 111, 1313–1320. [Google Scholar] [CrossRef]
- Detrano, R.; Guerci, A.D.; Carr, J.J.; Bild, D.E.; Burke, G.; Folsom, A.R.; Liu, K.; Shea, S.; Szklo, M.; Bluemke, D.A.; et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N. Engl. J. Med. 2008, 358, 1336–1345. [Google Scholar] [CrossRef]
- Loria, C.M.; Liu, K.; Lewis, C.E.; Hulley, S.B.; Sidney, S.; Schreiner, P.J.; Williams, O.D.; Bild, D.E.; Detrano, R. Early adult risk factor levels and subsequent coronary artery calcification: The CARDIA Study. J. Am. Coll. Cardiol. 2007, 49, 2013–2020. [Google Scholar] [CrossRef]
- Carr, J.J.; Jacobs, D.R., Jr.; Terry, J.G.; Shay, C.M.; Sidney, S.; Liu, K.; Schreiner, P.J.; Lewis, C.E.; Shikany, J.M.; Reis, J.P.; et al. Association of Coronary Artery Calcium in Adults Aged 32 to 46 Years with Incident Coronary Heart Disease and Death. JAMA Cardiol. 2017, 2, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Ceponiene, I.; Nakanishi, R.; Osawa, K.; Kanisawa, M.; Nezarat, N.; Rahmani, S.; Kissel, K.; Kim, M.; Jayawardena, E.; Broersen, A.; et al. Coronary Artery Calcium Progression Is Associated with Coronary Plaque Volume Progression: Results From a Quantitative Semiautomated Coronary Artery Plaque Analysis. JACC Cardiovasc. Imaging 2018, 11, 1785–1794. [Google Scholar] [CrossRef]
- Tramontano, L.; Punzo, B.; Clemente, A.; Seitun, S.; Saba, L.; Bossone, E.; Maffei, E.; Cavaliere, C.; Cademartiri, F. Prognostic Value of Coronary Calcium Score in Asymptomatic Individuals: A Systematic Review. J. Clin. Med. 2022, 11, 5842. [Google Scholar] [CrossRef]
- Peters, S.A.; Bakker, M.; den Ruijter, H.M.; Bots, M.L. Added value of CAC in risk stratification for cardiovascular events: A systematic review. Eur. J. Clin. Investig. 2012, 42, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; LaBree, L.; Azen, S.P.; Doherty, T.M.; Detrano, R.C. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. JAMA 2004, 291, 210–215. [Google Scholar] [CrossRef]
- Erbel, R.; Möhlenkamp, S.; Moebus, S.; Schmermund, A.; Lehmann, N.; Stang, A.; Dragano, N.; Grönemeyer, D.; Seibel, R.; Kälsch, H.; et al. Heinz Nixdorf Recall Study Investigative Group. Coronary risk stratification, discrimination, and reclassification improvement based on quantification of subclinical coronary atherosclerosis: The Heinz Nixdorf Recall study. J. Am. Coll. Cardiol. 2010, 56, 1397–1406. [Google Scholar] [CrossRef] [PubMed]
- McClelland, R.L.; Jorgensen, N.W.; Budoff, M.; Blaha, M.J.; Post, W.S.; Kronmal, R.A.; Bild, D.E.; Shea, S.; Liu, K.; Watson, K.E.; et al. 10-Year Coronary Heart Disease Risk Prediction Using Coronary Artery Calcium and Traditional Risk Factors: Derivation in the MESA (Multi-Ethnic Study of Atherosclerosis) With Validation in the HNR (Heinz Nixdorf Recall) Study and the DHS (Dallas Heart Study). J. Am. Coll. Cardiol. 2015, 66, 1643–1653. [Google Scholar] [PubMed]
- Shaw, L.J.; Min, J.K.; Nasir, K.; Xie, J.X.; Berman, D.S.; Miedema, M.D.; Whelton, S.P.; Dardari, Z.A.; Rozanski, A.; Rumberger, J.; et al. Sex differences in calcified plaque and long-term cardiovascular mortality: Observations from the CAC Consortium. Eur. Heart J. 2018, 39, 3727–3735. [Google Scholar] [CrossRef]
- Wong, N.D.; Cordola Hsu, A.R.; Rozanski, A.; Shaw, L.J.; Whelton, S.P.; Budoff, M.J.; Nasir, K.; Miedema, M.D.; Rumberger, J.; Blaha, M.J.; et al. Sex Differences in Coronary Artery Calcium and Mortality from Coronary Heart Disease, Cardiovascular Disease, and All Causes in Adults With Diabetes: The Coronary Calcium Consortium. Diabetes Care 2020, 43, 2597–2606. [Google Scholar] [CrossRef]
- Kavousi, M.; Desai, C.S.; Ayers, C.; Blumenthal, R.S.; Budoff, M.J.; Mahabadi, A.A.; Ikram, M.A.; van der Lugt, A.; Hofman, A.; Erbel, R.; et al. Prevalence and Prognostic Implications of Coronary Artery Calcification in Low-Risk Women: A Meta-analysis. JAMA 2016, 316, 2126–2134. [Google Scholar] [CrossRef]
- Valenti, V.; Ó Hartaigh, B.; Heo, R.; Cho, I.; Schulman-Marcus, J.; Gransar, H.; Truong, Q.A.; Shaw, L.J.; Knapper, J.; Kelkar, A.A.; et al. A 15-Year Warranty Period for Asymptomatic Individuals Without Coronary Artery Calcium: A Prospective Follow-Up of 9715 Individuals. JACC Cardiovasc. Imaging 2015, 8, 900–909. [Google Scholar] [CrossRef]
- Yeboah, J.; McClelland, R.L.; Polonsky, T.S.; Burke, G.L.; Sibley, C.T.; O’Leary, D.; Carr, J.J.; Goff, D.C.; Greenland, P.; Herrington, D.M. Comparison of novel risk markers for improvement in cardiovascular risk assessment in intermediate-risk individuals. JAMA 2012, 308, 788–795. [Google Scholar] [CrossRef]
- Budoff, M.J.; Mayrhofer, T.; Ferencik, M.; Bittner, D.; Lee, K.L.; Lu, M.T.; Coles, A.; Jang, J.; Krishnam, M.; Douglas, P.S.; et al. PROMISE Investigators. Prognostic Value of Coronary Artery Calcium in the PROMISE Study (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 136, 1993–2005. [Google Scholar] [CrossRef]
- Lo-Kioeng-Shioe, M.S.; Rijlaarsdam-Hermsen, D.; van Domburg, R.T.; Hadamitzky, M.; Lima, J.A.C.; Hoeks, S.E.; Deckers, J.W. Prognostic value of coronary artery calcium score in symptomatic individuals: A meta-analysis of 34,000 subjects. Int. J. Cardiol. 2020, 299, 56–62. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Dzaye, O.; Steffensen, F.H.; Bøtker, H.E.; Jensen, J.M.; Rønnow Sand, N.P.; Kragholm, K.H.; Sørensen, H.T.; Leipsic, J.; Mæng, M.; et al. Impact of Plaque Burden Versus Stenosis on Ischemic Events in Patients with Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2020, 76, 2803–2813. [Google Scholar] [CrossRef]
- Agha, A.M.; Pacor, J.; Grandhi, G.R.; Mszar, R.; Khan, S.U.; Parikh, R.; Agrawal, T.; Burt, J.; Blankstein, R.; Blaha, M.J.; et al. The Prognostic Value of CAC Zero Among Individuals Presenting with Chest Pain: A Meta-Analysis. JACC Cardiovasc. Imaging 2022, 15, 1745–1757. [Google Scholar]
- Villines, T.C.; Hulten, E.A.; Shaw, L.J.; Goyal, M.; Dunning, A.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; Cademartiri, F.; et al. CONFIRM Registry Investigators. Prevalence and severity of coronary artery disease and adverse events among symptomatic patients with coronary artery calcification scores of zero undergoing coronary computed tomography angiography: Results from the CONFIRM (Coronary CT Angiography Evaluation for Clinical Outcomes: An International Multicenter) registry. J. Am. Coll. Cardiol. 2011, 58, 2533–2540. [Google Scholar] [PubMed]
- Mortensen, M.B.; Gaur, S.; Frimmer, A.; Bøtker, H.E.; Sørensen, H.T.; Kragholm, K.H.; Niels Peter, S.R.; Steffensen, F.H.; Jensen, R.V.; Mæng, M.; et al. Association of Age with the Diagnostic Value of Coronary Artery Calcium Score for Ruling Out Coronary Stenosis in Symptomatic Patients. JAMA Cardiol. 2022, 7, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.W.; Knez, A.; White, C.W.; Becker, A.; von Ziegler, F.; Muehling, O.; Becker, C.; Reiser, M.; Steinbeck, G.; Boekstegers, P. Composition of coronary atherosclerotic plaques in patients with acute myocardial infarction and stable angina pectoris determined by contrast-enhanced multislice computed tomography. Am. J. Cardiol. 2003, 91, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Shemesh, J.; Apter, S.; Itzchak, Y.; Motro, M. Coronary calcification compared in patients with acute versus in those with chronic coronary events by using dual-sector spiral CT. Radiology 2003, 226, 483–488. [Google Scholar] [CrossRef]
- Pugliese, L.; Spiritigliozzi, L.; Di Tosto, F.; Ricci, F.; Cavallo, A.U.; Di Donna, C.; De Stasio, V.; Presicce, M.; Benelli, L.; D’Errico, F.; et al. Association of plaque calcification pattern and attenuation with instability features and coronary stenosis and calcification grade. Atherosclerosis 2020, 311, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Houslay, E.S.; Cowell, S.J.; Prescott, R.J.; Reid, J.; Burton, J.; Northridge, D.B.; Boon, N.A.; Newby, D.E. Scottish Aortic Stenosis and Lipid Lowering Therapy, Impact on Regression trial Investigators. Progressive coronary calcification despite intensive lipid-lowering treatment: A randomised controlled trial. Heart 2006, 92, 1207–1212. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.; Granåsen, G.; Wiklund, U.; Schmermund, A.; Guerci, A.; Erbel, R.; Raggi, P. High dose and long-term statin therapy accelerate coronary artery calcification. Int. J. Cardiol. 2015, 184, 581–586. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Owen, A. Statins moderate coronary stenoses but not coronary calcification: Results from meta-analyses. Int. J. Cardiol. 2011, 153, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Osborne, M.T.; Tung, B.; Li, M.; Li, Y. Imaging Cardiovascular Calcification. J. Am. Heart Assoc. 2018, 7, 008564. [Google Scholar] [CrossRef]
- van Rosendael, A.R.; Narula, J.; Lin, F.Y.; van den Hoogen, I.J.; Gianni, U.; Al Hussein Alawamlh, O.; Dunham, P.C.; Peña, J.M.; Lee, S.E.; Andreini, D.; et al. Association of High-Density Calcified 1K Plaque with Risk of Acute Coronary Syndrome. JAMA Cardiol. 2020, 5, 282–290. [Google Scholar] [CrossRef]
- Criqui, M.H.; Denenberg, J.O.; Ix, J.H.; McClelland, R.L.; Wassel, C.L.; Rifkin, D.E.; Carr, J.J.; Budoff, M.J.; Allison, M.A. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 2014, 311, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G. Epicardial adipose tissue in contemporary cardiology. Nat. Rev. Cardiol. 2022, 19, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Iacobellis, G.; Bianco, A.C. Epicardial adipose tissue: Emerging physiological, pathophysiological and clinical features. Trends Endocrinol. Metab. 2011, 22, 450–457. [Google Scholar] [CrossRef]
- Antonopoulos, A.S.; Antoniades, C. The role of epicardial adipose tissue in cardiac biology: Classic concepts and emerging roles. J. Physiol. 2017, 595, 3907–3917. [Google Scholar] [CrossRef]
- Packer, M. Epicardial Adipose Tissue May Mediate Deleterious Effects of Obesity and Inflammation on the Myocardium. J. Am. Coll. Cardiol. 2018, 71, 2360–2372. [Google Scholar] [CrossRef] [PubMed]
- Bos, D.; Leening, M.J.G. Leveraging the coronary calcium scan beyond the coronary calcium score. Eur. Radiol. 2018, 28, 3082–3087. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Marwan, M.; Doris, M.K.; Cadet, S.; Commandeur, F.; Chen, X.; Slomka, P.J.; Gransar, H.; Cao, J.J.; et al. Epicardial adipose tissue density and volume are related to subclinical atherosclerosis, inflammation and major adverse cardiac events in asymptomatic subjects. J. Cardiovasc. Comput. Tomogr. 2018, 12, 67–73. [Google Scholar] [CrossRef]
- Bos, D.; Shahzad, R.; van Walsum, T.; van Vliet, L.J.; Franco, O.H.; Hofman, A.; Niessen, W.J.; Vernooij, M.W.; van der Lugt, A. Epicardial fat volume is related to atherosclerotic calcification in multiple vessel beds. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1264–1269. [Google Scholar] [CrossRef]
- Mancio, J.; Pinheiro, M.; Ferreira, W.; Carvalho, M.; Barros, A.; Ferreira, N.; Vouga, L.; Ribeiro, V.G.; Leite-Moreira, A.; Falcao-Pires, I.; et al. Gender differences in the association of epicardial adipose tissue and coronary artery calcification: EPICHEART study: EAT and coronary calcification by gender. Int. J. Cardiol. 2017, 249, 419–425. [Google Scholar] [CrossRef]
- Yerramasu, A.; Dey, D.; Venuraju, S.; Anand, D.V.; Atwal, S.; Corder, R.; Berman, D.S.; Lahiri, A. Increased volume of epicardial fat is an independent risk factor for accelerated progression of sub-clinical coronary atherosclerosis. Atherosclerosis 2012, 220, 223–230. [Google Scholar] [CrossRef]
- Nakanishi, R.; Rajani, R.; Cheng, V.Y.; Gransar, H.; Nakazato, R.; Shmilovich, H.; Otaki, Y.; Hayes, S.W.; Thomson, L.E.; Friedman, J.D.; et al. Increase in epicardial fat volume is associated with greater coronary artery calcification progression in subjects at intermediate risk by coronary calcium score: A serial study using non-contrast cardiac CT. Atherosclerosis 2011, 218, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Mahabadi, A.A.; Lehmann, N.; Kälsch, H.; Robens, T.; Bauer, M.; Dykun, I.; Budde, T.; Moebus, S.; Jöckel, K.H.; Erbel, R.; et al. Association of epicardial adipose tissue with progression of coronary artery calcification is more pronounced in the early phase of atherosclerosis: Results from the Heinz Nixdorf recall study. JACC Cardiovasc. Imaging 2014, 7, 909–916. [Google Scholar] [CrossRef]
- Alexopoulos, N.; McLean, D.S.; Janik, M.; Arepalli, C.D.; Stillman, A.E.; Raggi, P. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis 2010, 210, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Yamamoto, H.; Ohashi, N.; Kitagawa, T.; Kunita, E.; Utsunomiya, H.; Yamazato, R.; Urabe, Y.; Horiguchi, J.; Awai, K.; et al. Association between epicardial adipose tissue volume and characteristics of non-calcified plaques assessed by coronary computed tomographic angiography. Int. J. Cardiol. 2012, 161, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Bachar, G.N.; Dicker, D.; Kornowski, R.; Atar, E. Epicardial adipose tissue as a predictor of coronary artery disease in asymptomatic subjects. Am. J. Cardiol. 2012, 110, 534–538. [Google Scholar] [CrossRef]
- Jin, X.; Gao, B.; Zheng, J.; Wu, X.; Zhang, N.; Zhu, L.; Zhu, X.; Xie, J.; Wang, Z.; Tong, G.; et al. Impact of epicardial adipose tissue volume on hemodynamically significant coronary artery disease in Chinese patients with known or suspected coronary artery disease. Front. Cardiovasc. Med. 2023, 10, 1088961. [Google Scholar] [CrossRef]
- Tesche, C.; Bauer, M.J.; Straube, F.; Rogowski, S.; Baumann, S.; Renker, M.; Fink, N.; Schoepf, U.J.; Hoffmann, E.; Ebersberger, U. Association of epicardial adipose tissue with coronary CT angiography plaque parameters on cardiovascular outcome in patients with and without diabetes mellitus. Atherosclerosis 2022, 363, 78–84. [Google Scholar] [CrossRef]
- Ito, T.; Suzuki, Y.; Ehara, M.; Matsuo, H.; Teramoto, T.; Terashima, M.; Nasu, K.; Kinoshita, Y.; Tsuchikane, E.; Suzuki, T.; et al. Impact of epicardial fat volume on coronary artery disease in symptomatic patients with a zero calcium score. Int. J. Cardiol. 2013, 167, 2852–2858. [Google Scholar] [CrossRef]
- Mancio, J.; Azevedo, D.; Saraiva, F.; Azevedo, A.I.; Pires-Morais, G.; Leite-Moreira, A.; Falcao-Pires, I.; Lunet, N.; Bettencourt, N. Epicardial adipose tissue volume assessed by computed tomography and coronary artery disease: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 490–497. [Google Scholar] [CrossRef]
- Glaser, R.; Selzer, F.; Faxon, D.P.; Laskey, W.K.; Cohen, H.A.; Slater, J.; Detre, K.M.; Wilensky, R.L. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation 2005, 111, 143–149. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. PROSPECT Investigators. A prospective natural-history study of coronary atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Uren, N.G.; Melin, J.A.; De Bruyne, B.; Wijns, W.; Baudhuin, T.; Camici, P.G. Relation between myocardial blood flow and the severity of coronary-artery stenosis. N. Engl. J. Med. 1994, 330, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Kochar, M.; Arsanjani, R.; Raman, S.V.; Shaw, L.J.; Berman, D.S.; Min, J.K. Identifying and redefining stenosis by CT angiography. Cardiol. Clin. 2012, 30, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Cury, R.C.; Abbara, S.; Achenbach, S.; Agatston, A.; Berman, D.S.; Budoff, M.J.; Dill, K.E.; Jacobs, J.E.; Maroules, C.D.; Rubin, G.D.; et al. CAD-RADS™: Coronary Artery Disease—Reporting and Data System: An Expert Consensus Document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Radiology (ACR) and the North American Society for Cardiovascular Imaging (NASCI). Endorsed by the American College of Cardiology. J. Am. Coll. Radiol. 2016, 13, 1458–1466.e9. [Google Scholar]
- Hamon, M.; Biondi-Zoccai, G.G.; Malagutti, P.; Agostoni, P.; Morello, R.; Valgimigli, M.; Hamon, M. Diagnostic performance of multislice spiral computed tomography of coronary arteries as compared with conventional invasive coronary angiography: A meta-analysis. J. Am. Coll. Cardiol. 2006, 48, 1896–1910. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.M.; Rochitte, C.E.; Dewey, M.; Arbab-Zadeh, A.; Niinuma, H.; Gottlieb, I.; Paul, N.; Clouse, M.E.; Shapiro, E.P.; Hoe, J.; et al. Diagnostic performance of coronary angiography by 64-row CT. N. Engl. J. Med. 2008, 359, 2324–2336. [Google Scholar] [CrossRef]
- Chao, S.P.; Law, W.Y.; Kuo, C.J.; Hung, H.F.; Cheng, J.J.; Lo, H.M.; Shyu, K.G. The diagnostic accuracy of 256-row computed tomographic angiography compared with invasive coronary angiography in patients with suspected coronary artery disease. Eur. Heart J. 2010, 31, 1916–1923. [Google Scholar] [CrossRef]
- Budoff, M.J.; Dowe, D.; Jollis, J.G.; Gitter, M.; Sutherland, J.; Halamert, E.; Scherer, M.; Bellinger, R.; Martin, A.; Benton, R.; et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: Results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J. Am. Coll. Cardiol. 2008, 52, 1724–1732. [Google Scholar]
- Meijboom, W.B.; Meijs, M.F.; Schuijf, J.D.; Cramer, M.J.; Mollet, N.R.; van Mieghem, C.A.; Nieman, K.; van Werkhoven, J.M.; Pundziute, G.; Weustink, A.C.; et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: A prospective, multicenter, multivendor study. J. Am. Coll. Cardiol. 2008, 52, 2135–2144. [Google Scholar] [CrossRef]
- Dewey, M.; Rief, M.; Martus, P.; Kendziora, B.; Feger, S.; Dreger, H.; Priem, S.; Knebel, F.; Böhm, M.; Schlattmann, P.; et al. Evaluation of computed tomography in patients with atypical angina or chest pain clinically referred for invasive coronary angiography: Randomised controlled trial. BMJ 2016, 355, i5441. [Google Scholar] [CrossRef]
- Chang, H.J.; Lin, F.Y.; Gebow, D.; An, H.Y.; Andreini, D.; Bathina, R.; Baggiano, A.; Beltrama, V.; Cerci, R.; Choi, E.Y.; et al. Selective Referral Using CCTA Versus Direct Referral for Individuals Referred to Invasive Coronary Angiography for Suspected CAD: A Randomized, Controlled, Open-Label Trial. JACC Cardiovasc. Imaging 2019, 12, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- DISCHARGE Trial Group; Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Štěchovský, C.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. N. Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [PubMed]
- Nørgaard, B.L.; Leipsic, J.; Achenbach, S. Coronary CT Angiography to Guide Treatment Decision Making: Lessons From the SYNTAX II Trial. J. Am. Coll. Cardiol. 2018, 71, 2770–2772. [Google Scholar] [CrossRef] [PubMed]
- Shalev, A.; Nakazato, R.; Arsanjani, R.; Nakanishi, R.; Park, H.B.; Otaki, Y.; Cheng, V.Y.; Gransar, H.; LaBounty, T.M.; Hayes, S.W.; et al. SYNTAX Score Derived from Coronary CT Angiography for Prediction of Complex Percutaneous Coronary Interventions. Acad. Radiol. 2016, 23, 1384–1392. [Google Scholar] [CrossRef] [PubMed]
- Suh, Y.J.; Han, K.; Chang, S.; Kim, J.Y.; Im, D.J.; Hong, Y.J.; Lee, H.J.; Hur, J.; Kim, Y.J.; Choi, B.W. SYNTAX score based on coronary computed tomography angiography may have a prognostic value in patients with complex coronary artery disease: An observational study from a retrospective cohort. Medicine 2017, 96, e7999. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Onuma, Y.; Andreini, D.; Sonck, J.; Pompilio, G.; Mushtaq, S.; La Meir, M.; Miyazaki, Y.; de Mey, J.; Gaemperli, O.; et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur. Heart J. 2018, 39, 3689–3698. [Google Scholar] [CrossRef]
- Farooq, V.; van Klaveren, D.; Steyerberg, E.W.; Meliga, E.; Vergouwe, Y.; Chieffo, A.; Kappetein, A.P.; Colombo, A.; Holmes, D.R., Jr.; Mack, M.; et al. Anatomical and clinical characteristics to guide decision making between coronary artery bypass surgery and percutaneous coronary intervention for individual patients: Development and validation of SYNTAX score II. Lancet 2013, 381, 639–650. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Ortner, N.; Nørgaard, B.L.; Achenbach, S.; Leipsic, J.; Abdulla, J. The diagnostic accuracy and outcomes after coronary computed tomography angiography vs. conventional functional testing in patients with stable angina pectoris: A systematic review and meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 961–971. [Google Scholar] [CrossRef]
- Arbab-Zadeh, A.; Di Carli, M.F.; Cerci, R.; George, R.T.; Chen, M.Y.; Dewey, M.; Niinuma, H.; Vavere, A.L.; Betoko, A.; Plotkin, M.; et al. Accuracy of Computed Tomographic Angiography and Single-Photon Emission Computed Tomography-Acquired Myocardial Perfusion Imaging for the Diagnosis of Coronary Artery Disease. Circ. Cardiovasc. Imaging 2015, 8, e003533. [Google Scholar] [CrossRef]
- Danad, I.; Szymonifka, J.; Twisk, J.W.R.; Norgaard, B.L.; Zarins, C.K.; Knaapen, P.; Min, J.K. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: A meta-analysis. Eur. Heart J. 2017, 38, 991–998. [Google Scholar] [CrossRef]
- Hollander, J.E.; Gatsonis, C.; Greco, E.M.; Snyder, B.S.; Chang, A.M.; Miller, C.D.; Singh, H.; Litt, H.I. Coronary Computed Tomography Angiography Versus Traditional Care: Comparison of One-Year Outcomes and Resource Use. Ann. Emerg. Med. 2016, 67, 460–468.1. [Google Scholar] [CrossRef] [PubMed]
- Levsky, J.M.; Spevack, D.M.; Travin, M.I.; Menegus, M.A.; Huang, P.W.; Clark, E.T.; Kim, C.W.; Hirschhorn, E.; Freeman, K.D.; Tobin, J.N.; et al. Coronary Computed Tomography Angiography Versus Radionuclide Myocardial Perfusion Imaging in Patients with Chest Pain Admitted to Telemetry: A Randomized Trial. Ann. Intern. Med. 2015, 163, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Uretsky, S.; Argulian, E.; Supariwala, A.; Agarwal, S.K.; El-Hayek, G.; Chavez, P.; Awan, H.; Jagarlamudi, A.; Puppala, S.P.; Cohen, R.; et al. Comparative effectiveness of coronary CT angiography vs. stress cardiac imaging in patients following hospital admission for chest pain work-up: The Prospective First Evaluation in Chest Pain (PERFECT) Trial. J. Nucl. Cardiol. 2017, 24, 1267–1278. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Truong, Q.A.; Schoenfeld, D.A.; Chou, E.T.; Woodard, P.K.; Nagurney, J.T.; Pope, J.H.; Hauser, T.H.; White, C.S.; Weiner, S.G.; et al. ROMICAT-II Investigators. Coronary CT angiography versus standard evaluation in acute chest pain. N. Engl. J. Med. 2012, 367, 299–308. [Google Scholar] [CrossRef]
- Linde, J.J.; Hove, J.D.; Sørgaard, M.; Kelbæk, H.; Jensen, G.B.; Kühl, J.T.; Hindsø, L.; Køber, L.; Nielsen, W.B.; Kofoed, K.F. Long-Term Clinical Impact of Coronary CT Angiography in Patients with Recent Acute-Onset Chest Pain: The Randomized Controlled CATCH Trial. JACC Cardiovasc. Imaging 2015, 8, 1404–1413. [Google Scholar] [CrossRef]
- Hamilton-Craig, C.; Fifoot, A.; Hansen, M.; Pincus, M.; Chan, J.; Walters, D.L.; Branch, K.R. Diagnostic performance and cost of CT angiography versus stress ECG--a randomized prospective study of suspected acute coronary syndrome chest pain in the emergency department (CT-COMPARE). Int. J. Cardiol. 2014, 177, 867–873. [Google Scholar] [CrossRef]
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Dolor, R.J.; Fordyce, C.B.; Huang, M.; et al. PROMISE Investigators. Outcomes of anatomical versus functional testing for coronary artery disease. N. Engl. J. Med. 2015, 372, 1291–1300. [Google Scholar] [CrossRef]
- Min, J.K.; Koduru, S.; Dunning, A.M.; Cole, J.H.; Hines, J.L.; Greenwell, D.; Biga, C.; Fanning, G.; LaBounty, T.M.; Gomez, M.; et al. Coronary CT angiography versus myocardial perfusion imaging for near-term quality of life, cost and radiation exposure: A prospective multicenter randomized pilot trial. J. Cardiovasc. Comput. Tomogr. 2012, 6, 274–283. [Google Scholar] [CrossRef]
- SCOT-HEART Investigators; Newby, D.E.; Adamson, P.D.; Berry, C.; Boon, N.A.; Dweck, M.R.; Flather, M.; Forbes, J.; Hunter, A.; Lewis, S.; et al. Coronary CT Angiography and 5-Year Risk of Myocardial Infarction. N. Engl. J. Med. 2018, 379, 924–933. [Google Scholar]
- Lubbers, M.; Dedic, A.; Coenen, A.; Galema, T.; Akkerhuis, J.; Bruning, T.; Krenning, B.; Musters, P.; Ouhlous, M.; Liem, A.; et al. Calcium imaging and selective computed tomography angiography in comparison to functional testing for suspected coronary artery disease: The multicentre, randomized CRESCENT trial. Eur. Heart J. 2016, 37, 1232–1243. [Google Scholar] [CrossRef]
- McKavanagh, P.; Lusk, L.; Ball, P.A.; Verghis, R.M.; Agus, A.M.; Trinick, T.R.; Duly, E.; Walls, G.M.; Stevenson, M.; James, B.; et al. A comparison of cardiac computerized tomography and exercise stress electrocardiogram test for the investigation of stable chest pain: The clinical results of the CAPP randomized prospective trial. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 441–448. [Google Scholar] [CrossRef]
- Hwang, I.C.; Choi, S.J.; Choi, J.E.; Ko, E.B.; Suh, J.K.; Choi, I.; Kang, H.J.; Kim, Y.J.; Kim, J.Y. Comparison of mid- to long-term clinical outcomes between anatomical testing and usual care in patients with suspected coronary artery disease: A meta-analysis of randomized trials. Clin. Cardiol. 2017, 40, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Burch, R.A.; Siddiqui, T.A.; Tou, L.C.; Turner, K.B.; Umair, M. The Cost Effectiveness of Coronary CT Angiography and the Effective Utilization of CT-Fractional Flow Reserve in the Diagnosis of Coronary Artery Disease. J. Cardiovasc. Dev. Dis. 2023, 10, 25. [Google Scholar] [CrossRef] [PubMed]
- Andreini, D.; Pontone, G.; Mushtaq, S.; Bartorelli, A.L.; Bertella, E.; Antonioli, L.; Formenti, A.; Cortinovis, S.; Veglia, F.; Annoni, A.; et al. A long-term prognostic value of coronary CT angiography in suspected coronary artery disease. JACC Cardiovasc. Imaging 2012, 5, 690–701. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Shaw, L.J.; Devereux, R.B.; Okin, P.M.; Weinsaft, J.W.; Russo, D.J.; Lippolis, N.J.; Berman, D.S.; Callister, T.Q. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J. Am. Coll. Cardiol. 2007, 50, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Hadamitzky, M.; Achenbach, S.; Al-Mallah, M.; Berman, D.; Budoff, M.; Cademartiri, F.; Callister, T.; Chang, H.J.; Cheng, V.; Chinnaiyan, K.; et al. CONFIRM Investigators. Optimized prognostic score for coronary computed tomographic angiography: Results from the CONFIRM registry (COronary CT Angiography EvaluatioN for Clinical Outcomes: An InteRnational Multicenter Registry). J. Am. Coll. Cardiol. 2013, 62, 468–476. [Google Scholar] [CrossRef]
- Clerc, O.F.; Kaufmann, B.P.; Possner, M.; Liga, R.; Vontobel, J.; Mikulicic, F.; Gräni, C.; Benz, D.C.; Fuchs, T.A.; Stehli, J.; et al. Long-term prognostic performance of low-dose coronary computed tomography angiography with prospective electrocardiogram triggering. Eur. Radiol. 2017, 27, 4650–4660. [Google Scholar] [CrossRef]
- Nielsen, L.H.; Bøtker, H.E.; Sørensen, H.T.; Schmidt, M.; Pedersen, L.; Sand, N.P.; Jensen, J.M.; Steffensen, F.H.; Tilsted, H.H.; Bøttcher, M.; et al. Prognostic assessment of stable coronary artery disease as determined by coronary computed tomography angiography: A Danish multicentre cohort study. Eur. Heart J. 2017, 38, 413–421. [Google Scholar] [CrossRef]
- Ostrom, M.P.; Gopal, A.; Ahmadi, N.; Nasir, K.; Yang, E.; Kakadiaris, I.; Flores, F.; Mao, S.S.; Budoff, M.J. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J. Am. Coll. Cardiol. 2008, 52, 1335–1343. [Google Scholar] [CrossRef]
- Bittner, D.O.; Mayrhofer, T.; Budoff, M.; Szilveszter, B.; Foldyna, B.; Hallett, T.R.; Ivanov, A.; Janjua, S.; Meyersohn, N.M.; Staziaki, P.V.; et al. PROMISE Investigators. Prognostic Value of Coronary CTA in Stable Chest Pain: CAD-RADS, CAC, and Cardiovascular Events in PROMISE. JACC Cardiovasc. Imaging 2020, 13, 1534–1545. [Google Scholar] [CrossRef]
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F.; et al. PROMISE Investigators. Prognostic Value of Noninvasive Cardiovascular Testing in Patients with Stable Chest Pain: Insights From the PROMISE Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 135, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, V.; Green, R.; Acampa, W.; Petretta, M.; Bonaduce, D.; Salvatore, M.; Cuocolo, A. Long-term prognostic value of stress myocardial perfusion imaging and coronary computed tomography angiography: A meta-analysis. J. Nucl. Cardiol. 2016, 23, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, U.; Bamberg, F.; Chae, C.U.; Nichols, J.H.; Rogers, I.S.; Seneviratne, S.K.; Truong, Q.A.; Cury, R.C.; Abbara, S.; Shapiro, M.D.; et al. Coronary computed tomography angiography for early triage of patients with acute chest pain: The ROMICAT (Rule Out Myocardial Infarction using Computer Assisted Tomography) trial. J. Am. Coll. Cardiol. 2009, 53, 1642–1650. [Google Scholar] [CrossRef]
- Smulders, M.W.; Jaarsma, C.; Nelemans, P.J.; Bekkers, S.C.A.M.; Bucerius, J.; Leiner, T.; Crijns, H.J.G.M.; Wildberger, J.E.; Schalla, S. Comparison of the prognostic value of negative non-invasive cardiac investigations in patients with suspected or known coronary artery disease-a meta-analysis. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 980–987. [Google Scholar] [CrossRef]
- Hadamitzky, M.; Täubert, S.; Deseive, S.; Byrne, R.A.; Martinoff, S.; Schömig, A.; Hausleiter, J. Prognostic value of coronary computed tomography angiography during 5 years of follow-up in patients with suspected coronary artery disease. Eur. Heart J. 2013, 34, 3277–3285. [Google Scholar] [CrossRef] [PubMed]
- Finck, T.; Hardenberg, J.; Will, A.; Hendrich, E.; Haller, B.; Martinoff, S.; Hausleiter, J.; Hadamitzky, M. 10-Year Follow-Up After Coronary Computed Tomography Angiography in Patients with Suspected Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 1330–1338. [Google Scholar] [CrossRef]
- Jeremias, A.; Kirtane, A.J.; Stone, G.W. A Test in Context: Fractional Flow Reserve: Accuracy, Prognostic Implications, and Limitations. J. Am. Coll. Cardiol. 2017, 69, 2748–2758. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Shaw, L.J.; Min, J.K.; Page, C.B.; Berman, D.S.; Chaitman, B.R.; Picard, M.H.; Kwong, R.Y.; O’Brien, S.M.; Huang, Z.; et al. Outcomes in the ISCHEMIA Trial Based on Coronary Artery Disease and Ischemia Severity. Circulation 2021, 144, 1024–1038. [Google Scholar] [CrossRef]
- Bech, G.J.; De Bruyne, B.; Pijls, N.H.; de Muinck, E.D.; Hoorntje, J.C.; Escaned, J.; Stella, P.R.; Boersma, E.; Bartunek, J.; Koolen, J.J.; et al. Fractional flow reserve to determine the appropriateness of angioplasty in moderate coronary stenosis: A randomized trial. Circulation 2001, 103, 2928–2934. [Google Scholar] [CrossRef]
- Tonino, P.A.; De Bruyne, B.; Pijls, N.H.; Siebert, U.; Ikeno, F.; van’t Veer, M.; Klauss, V.; Manoharan, G.; Engstrøm, T.; Oldroyd, K.G.; et al. FAME Study Investigators. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention. N. Engl. J. Med. 2009, 360, 213–224. [Google Scholar] [CrossRef]
- Seitun, S.; Clemente, A.; De Lorenzi, C.; Benenati, S.; Chiappino, D.; Mantini, C.; Sakellarios, A.I.; Cademartiri, F.; Bezante, G.P.; Porto, I. Cardiac CT perfusion and FFRCTA: Pathophysiological features in ischemic heart disease. Cardiovasc. Diagn. Ther. 2020, 10, 1954–1978. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, X.; Zhang, Y.; Hou, L.; Li, W.; Fan, B.; Zhang, T.; Xu, Y. Enhanced diagnostic utility achieved by myocardial blood analysis: A meta-analysis of noninvasive cardiac imaging in the detection of functional coronary artery disease. Int. J. Cardiol. 2016, 221, 665–673. [Google Scholar] [CrossRef]
- Gonzalez, J.A.; Lipinski, M.J.; Flors, L.; Shaw, P.W.; Kramer, C.M.; Salerno, M. Meta-Analysis of Diagnostic Performance of Coronary Computed Tomography Angiography, Computed Tomography Perfusion, and Computed Tomography-Fractional Flow Reserve in Functional Myocardial Ischemia Assessment Versus Invasive Fractional Flow Reserve. Am. J. Cardiol. 2015, 116, 1469–1478. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, B.; Wang, S.; Zhao, S.; Lu, M. Computed tomography angiography-derived fractional flow reserve (CT-FFR) for the detection of myocardial ischemia with invasive fractional flow reserve as reference: Systematic review and meta-analysis. Eur. Radiol. 2020, 30, 712–725. [Google Scholar] [CrossRef]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ. Cardiovasc. Imaging 2015, 8, 002666. [Google Scholar] [CrossRef]
- Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Nørgaard, B.L.; Berman, D.S.; Raff, G.; Hurwitz-Koweek, L.M.; Pontone, G.; Kawasaki, T.; Sand, N.P.; et al. Real-world clinical utility and impact on clinical decision-making of coronary computed tomography angiography-derived fractional flow reserve: Lessons from the ADVANCE Registry. Eur. Heart J. 2018, 39, 3701–3711. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. NXT Trial Study Group. Diagnostic performance of noninvasive fractional flow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: The NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar]
- Driessen, R.S.; Danad, I.; Stuijfzand, W.J.; Raijmakers, P.G.; Schumacher, S.P.; van Diemen, P.A.; Leipsic, J.A.; Knuuti, J.; Underwood, S.R.; van de Ven, P.M.; et al. Comparison of Coronary Computed Tomography Angiography, Fractional Flow Reserve, and Perfusion Imaging for Ischemia Diagnosis. J. Am. Coll. Cardiol. 2019, 73, 161–173. [Google Scholar] [CrossRef]
- Patel, M.R.; Nørgaard, B.L.; Fairbairn, T.A.; Nieman, K.; Akasaka, T.; Berman, D.S.; Raff, G.L.; Hurwitz Koweek, L.M.; Pontone, G.; Kawasaki, T.; et al. 1-Year Impact on Medical Practice and Clinical Outcomes of FFRCT: The ADVANCE Registry. JACC Cardiovasc. Imaging 2020, 13, 97–105. [Google Scholar] [CrossRef]
- Ihdayhid, A.R.; Norgaard, B.L.; Gaur, S.; Leipsic, J.; Nerlekar, N.; Osawa, K.; Miyoshi, T.; Jensen, J.M.; Kimura, T.; Shiomi, H.; et al. Prognostic Value and Risk Continuum of Noninvasive Fractional Flow Reserve Derived from Coronary CT Angiography. Radiology 2019, 292, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. Clinical Validation of a Virtual Planner for Coronary Interventions Based on Coronary CT Angiography. JACC Cardiovasc. Imaging 2022, 15, 1242–1255. [Google Scholar] [CrossRef]
- Li, S.J.; Ge, Z.; Kan, J.; Zhang, J.J.; Ye, F.; Kwan, T.W.; Santoso, T.; Yang, S.; Sheiban, I.; Qian, X.S.; et al. Cutoff Value and Long-Term Prediction of Clinical Events by FFR Measured Immediately After Implantation of a Drug-Eluting Stent in Patients with Coronary Artery Disease: 1- to 3-Year Results From the DKCRUSH VII Registry Study. JACC Cardiovasc. Interv. 2017, 10, 986–995. [Google Scholar] [CrossRef]
- Piroth, Z.; Toth, G.G.; Tonino, P.A.L.; Barbato, E.; Aghlmandi, S.; Curzen, N.; Rioufol, G.; Pijls, N.H.J.; Fearon, W.F.; Jüni, P.; et al. Prognostic Value of Fractional Flow Reserve Measured Immediately After Drug-Eluting Stent Implantation. Circ. Cardiovasc. Interv. 2017, 10, e005233. [Google Scholar] [CrossRef] [PubMed]
- Mileva, N.; Ohashi, H.; Paolisso, P.; Leipsic, J.; Mizukami, T.; Sonck, J.; Norgaard, B.L.; Otake, H.; Ko, B.; Maeng, M.; et al. Relationship between coronary volume, myocardial mass, and post-PCI fractional flow reserve. Catheter. Cardiovasc. Interv. 2023, 101, 1182–1192. [Google Scholar] [CrossRef]
- Ide, S.; Sumitsuji, S.; Yamaguchi, O.; Sakata, Y. Cardiac computed tomography-derived myocardial mass at risk using the Voronoi-based segmentation algorithm: A histological validation study. J. Cardiovasc. Comput. Tomogr. 2017, 11, 179–182. [Google Scholar] [CrossRef]
- Zhou, T.; Wang, X.; Wu, T.; Yang, Z.; Li, S.; Li, Y.; He, F.; Zhang, M.; Yang, C.; Jia, S.; et al. Clinical application of computed tomography angiography and fractional flow reserve computed tomography in patients with coronary artery disease: A meta-analysis based on pre- and post-test probability. Eur. J. Radiol. 2021, 139, 109712. [Google Scholar] [CrossRef]
- Ko, B.S.; Cameron, J.D.; Meredith, I.T.; Leung, M.; Antonis, P.R.; Nasis, A.; Crossett, M.; Hope, S.A.; Lehman, S.J.; Troupis, J.; et al. Computed tomography stress myocardial perfusion imaging in patients considered for revascularization: A comparison with fractional flow reserve. Eur. Heart J. 2012, 33, 67–77. [Google Scholar] [CrossRef]
- Bettencourt, N.; Chiribiri, A.; Schuster, A.; Ferreira, N.; Sampaio, F.; Pires-Morais, G.; Santos, L.; Melica, B.; Rodrigues, A.; Braga, P.; et al. Direct comparison of cardiac magnetic resonance and multidetector computed tomography stress-rest perfusion imaging for detection of coronary artery disease. J. Am. Coll. Cardiol. 2013, 61, 1099–1107. [Google Scholar] [CrossRef]
- Rief, M.; Zimmermann, E.; Stenzel, F.; Martus, P.; Stangl, K.; Greupner, J.; Knebel, F.; Kranz, A.; Schlattmann, P.; Laule, M.; et al. Computed tomography angiography and myocardial computed tomography perfusion in patients with coronary stents: Prospective intraindividual comparison with conventional coronary angiography. J. Am. Coll. Cardiol. 2013, 62, 1476–1485. [Google Scholar] [CrossRef] [PubMed]
- Rochitte, C.E.; George, R.T.; Chen, M.Y.; Arbab-Zadeh, A.; Dewey, M.; Miller, J.M.; Niinuma, H.; Yoshioka, K.; Kitagawa, K.; Nakamori, S.; et al. Computed tomography angiography and perfusion to assess coronary artery stenosis causing perfusion defects by single photon emission computed tomography: The CORE320 study. Eur. Heart J. 2014, 35, 1120–1130. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, C.N.; Harris, B.S.; Schoepf, U.J.; Silverman, J.R.; McWhite, C.B.; Krazinski, A.W.; Bayer, R.R.; Meinel, F.G. Incremental value of pharmacological stress cardiac dual-energy CT over coronary CT angiography alone for the assessment of coronary artery disease in a high-risk population. AJR Am. J. Roentgenol. 2014, 203, W70–W77. [Google Scholar] [CrossRef]
- Wichmann, J.L.; Meinel, F.G.; Schoepf, U.J.; Lo, G.G.; Choe, Y.H.; Wang, Y.; Vliegenthart, R.; Varga-Szemes, A.; Muscogiuri, G.; Cannaò, P.M.; et al. Absolute Versus Relative Myocardial Blood Flow by Dynamic CT Myocardial Perfusion Imaging in Patients with Anatomic Coronary Artery Disease. AJR Am. J. Roentgenol. 2015, 205, W67–W72. [Google Scholar] [CrossRef]
- Pontone, G.; Andreini, D.; Guaricci, A.I.; Baggiano, A.; Fazzari, F.; Guglielmo, M.; Muscogiuri, G.; Berzovini, C.M.; Pasquini, A.; Mushtaq, S.; et al. Incremental Diagnostic Value of Stress Computed Tomography Myocardial Perfusion with Whole-Heart Coverage CT Scanner in Intermediate- to High-Risk Symptomatic Patients Suspected of Coronary Artery Disease. JACC Cardiovasc. Imaging 2019, 12, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Danad, I.; Szymonifka, J.; Schulman-Marcus, J.; Min, J.K. Static and dynamic assessment of myocardial perfusion by computed tomography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 836–844. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, S.; Sirajuddin, A.; Arai, A.E.; Zhao, S. Dynamic stress computed tomography myocardial perfusion for detecting myocardial ischemia: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 258, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Lubbers, M.; Coenen, A.; Kofflard, M.; Bruning, T.; Kietselaer, B.; Galema, T.; Kock, M.; Niezen, A.; Das, M.; van Gent, M.; et al. Comprehensive Cardiac CT With Myocardial Perfusion Imaging Versus Functional Testing in Suspected Coronary Artery Disease: The Multicenter, Randomized CRESCENT-II Trial. JACC Cardiovasc. Imaging 2018, 11, 1625–1636. [Google Scholar] [CrossRef]
- Nakamura, S.; Kitagawa, K.; Goto, Y.; Omori, T.; Kurita, T.; Yamada, A.; Takafuji, M.; Uno, M.; Dohi, K.; Sakuma, H. Incremental Prognostic Value of Myocardial Blood Flow Quantified with Stress Dynamic Computed Tomography Perfusion Imaging. JACC Cardiovasc. Imaging 2019, 12, 1379–1387. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Dou, G.; He, B.; Jin, Q.; Chen, Z.; Jing, J.; Di Carli, M.F.; Chen, Y.; Blankstein, R. Stress Myocardial Blood Flow Ratio by Dynamic CT Perfusion Identifies Hemodynamically Significant CAD. JACC Cardiovasc. Imaging 2020, 13, 966–976. [Google Scholar] [CrossRef]
- Yang, D.H.; Kim, Y.H.; Roh, J.H.; Kang, J.W.; Ahn, J.M.; Kweon, J.; Lee, J.B.; Choi, S.H.; Shin, E.S.; Park, D.W.; et al. Diagnostic performance of on-site CT-derived fractional flow reserve versus CT perfusion. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 432–440. [Google Scholar] [CrossRef]
- Coenen, A.; Rossi, A.; Lubbers, M.M.; Kurata, A.; Kono, A.K.; Chelu, R.G.; Segreto, S.; Dijkshoorn, M.L.; Wragg, A.; van Geuns, R.M.; et al. Integrating CT Myocardial Perfusion and CT-FFR in the Work-Up of Coronary Artery Disease. JACC Cardiovasc. Imaging 2017, 10, 760–770. [Google Scholar] [CrossRef]
- Pontone, G.; Baggiano, A.; Andreini, D.; Guaricci, A.I.; Guglielmo, M.; Muscogiuri, G.; Fusini, L.; Fazzari, F.; Mushtaq, S.; Conte, E.; et al. Stress Computed Tomography Perfusion Versus Fractional Flow Reserve CT Derived in Suspected Coronary Artery Disease: The PERFECTION Study. JACC Cardiovasc. Imaging 2019, 12, 1487–1497. [Google Scholar] [CrossRef] [PubMed]
- Glagov, S.; Bassiouny, H.S.; Sakaguchi, Y.; Goudet, C.A.; Vito, R.P. Mechanical determinants of plaque modeling, remodeling and disruption. Atherosclerosis 1997, 131, S13–S14. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, A.; Argulian, E.; Leipsic, J.; Newby, D.E.; Narula, J. From Subclinical Atherosclerosis to Plaque Progression and Acute Coronary Events: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1608–1617. [Google Scholar] [CrossRef] [PubMed]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Shah, P.K. Pathophysiology of coronary thrombosis: Role of plaque rupture and plaque erosion. Prog. Cardiovasc. Dis. 2002, 44, 357–368. [Google Scholar] [CrossRef]
- Burke, A.P.; Kolodgie, F.D.; Farb, A.; Weber, D.; Virmani, R. Morphological predictors of arterial remodeling in coronary atherosclerosis. Circulation 2002, 105, 297–303. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- Ehara, S.; Kobayashi, Y.; Yoshiyama, M.; Shimada, K.; Shimada, Y.; Fukuda, D.; Nakamura, Y.; Yamashita, H.; Yamagishi, H.; Takeuchi, K.; et al. Spotty calcification typifies the culprit plaque in patients with acute myocardial infarction: An intravascular ultrasound study. Circulation 2004, 110, 3424–3429. [Google Scholar] [CrossRef]
- Baumann, S.; Renker, M.; Meinel, F.G.; Wichmann, J.L.; Fuller, S.R.; Bayer, R.R.; Schoepf, U.J.; Steinberg, D.H. Computed tomography imaging of coronary artery plaque: Characterization and prognosis. Radiol. Clin. North Am. 2015, 53, 307–315. [Google Scholar] [CrossRef]
- Schuhbaeck, A.; Dey, D.; Otaki, Y.; Slomka, P.; Kral, B.G.; Achenbach, S.; Berman, D.S.; Fishman, E.K.; Lai, S.; Lai, H. Interscan reproducibility of quantitative coronary plaque volume and composition from CT coronary angiography using an automated method. Eur. Radiol. 2014, 24, 2300–2308. [Google Scholar] [CrossRef]
- Nakanishi, R.; Ceponiene, I.; Osawa, K.; Luo, Y.; Kanisawa, M.; Megowan, N.; Nezarat, N.; Rahmani, S.; Broersen, A.; Kitslaar, P.H.; et al. Plaque progression assessed by a novel semi-automated quantitative plaque software on coronary computed tomography angiography between diabetes and non-diabetes patients: A propensity-score matching study. Atherosclerosis 2016, 255, 73–79. [Google Scholar] [CrossRef] [PubMed]
- van Rosendael, A.R.; Lin, F.Y.; Ma, X.; van den Hoogen, I.J.; Gianni, U.; Al Hussein, O.; Al’Aref, S.J.; Peña, J.M.; Andreini, D.; Al-Mallah, M.H.; et al. Percent atheroma volume: Optimal variable to report whole-heart atherosclerotic plaque burden with coronary CTA, the PARADIGM study. J. Cardiovasc. Comput. Tomogr. 2020, 14, 400–406. [Google Scholar] [CrossRef]
- Saremi, F.; Achenbach, S. Coronary plaque characterization using CT. AJR Am. J. Roentgenol. 2015, 204, W249–W260. [Google Scholar] [CrossRef] [PubMed]
- Schlett, C.L.; Maurovich-Horvat, P.; Ferencik, M.; Alkadhi, H.; Stolzmann, P.; Scheffel, H.; Seifarth, H.; Nakano, M.; Do, S.; Vorpahl, M.; et al. Histogram analysis of lipid-core plaques in coronary computed tomographic angiography: Ex vivo validation against histology. Investig. Radiol. 2013, 48, 646–653. [Google Scholar] [CrossRef]
- Motoyama, S.; Kondo, T.; Sarai, M.; Sugiura, A.; Harigaya, H.; Sato, T.; Inoue, K.; Okumura, M.; Ishii, J.; Anno, H.; et al. Multislice computed tomographic characteristics of coronary lesions in acute coronary syndromes. J. Am. Coll. Cardiol. 2007, 50, 319–326. [Google Scholar] [CrossRef] [PubMed]
- Maurovich-Horvat, P.; Hoffmann, U.; Vorpahl, M.; Nakano, M.; Virmani, R.; Alkadhi, H. The napkin-ring sign: CT signature of high-risk coronary plaques? JACC Cardiovasc. Imaging 2010, 3, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Narula, J.; Shaw, L.J.; Bax, J.J.; Min, J.K.; Chang, H.J. Quantitative assessment of coronary plaque volume change related to triglyceride glucose index: The Progression of AtheRosclerotic PlAque DetermIned by Computed TomoGraphic Angiography IMaging (PARADIGM) registry. Cardiovasc. Diabetol. 2020, 19, 113. [Google Scholar]
- Petranovic, M.; Soni, A.; Bezzera, H.; Loureiro, R.; Sarwar, A.; Raffel, C.; Pomerantsev, E.; Jang, I.K.; Brady, T.J.; Achenbach, S.; et al. Assessment of nonstenotic coronary lesions by 64-slice multidetector computed tomography in comparison to intravascular ultrasound: Evaluation of nonculprit coronary lesions. J. Cardiovasc. Comput. Tomogr. 2009, 3, 24–31. [Google Scholar] [CrossRef]
- Enrico, B.; Suranyi, P.; Thilo, C.; Bonomo, L.; Costello, P.; Schoepf, U.J. Coronary artery plaque formation at coronary CT angiography: Morphological analysis and relationship to hemodynamics. Eur. Radiol. 2009, 19, 837–844. [Google Scholar] [CrossRef]
- Henzler, T.; Porubsky, S.; Kayed, H.; Harder, N.; Krissak, U.R.; Meyer, M.; Sueselbeck, T.; Marx, A.; Michaely, H.; Schoepf, U.J.; et al. Attenuation-based characterization of coronary atherosclerotic plaque: Comparison of dual source and dual energy CT with single-source CT and histopathology. Eur. J. Radiol. 2011, 80, 54–59. [Google Scholar] [CrossRef]
- Nakazato, R.; Shalev, A.; Doh, J.H.; Koo, B.K.; Dey, D.; Berman, D.S.; Min, J.K. Quantification and characterisation of coronary artery plaque volume and adverse plaque features by coronary computed tomographic angiography: A direct comparison to intravascular ultrasound. Eur. Radiol. 2013, 23, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Doh, J.H.; Koo, B.K.; Nam, C.W.; Kim, J.H.; Min, J.K.; Nakazato, R.; Silalahi, T.; Prawira, H.; Choi, H.; Lee, S.Y.; et al. Diagnostic value of coronary CT angiography in comparison with invasive coronary angiography and intravascular ultrasound in patients with intermediate coronary artery stenosis: Results from the prospective multicentre FIGURE-OUT (Functional Imaging criteria for GUiding REview of invasive coronary angiOgraphy, intravascular Ultrasound, and coronary computed Tomographic angiography) study. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 870–877. [Google Scholar]
- Conte, E.; Mushtaq, S.; Pontone, G.; Li Piani, L.; Ravagnani, P.; Galli, S.; Collet, C.; Sonck, J.; Di Odoardo, L.; Guglielmo, M.; et al. Plaque quantification by coronary computed tomography angiography using intravascular ultrasound as a reference standard: A comparison between standard and last generation computed tomography scanners. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 191–201. [Google Scholar] [CrossRef]
- Gao, D.; Ning, N.; Guo, Y.; Ning, W.; Niu, X.; Yang, J. Computed tomography for detecting coronary artery plaques: A meta-analysis. Atherosclerosis 2011, 219, 603–609. [Google Scholar] [CrossRef]
- Fischer, C.; Hulten, E.; Belur, P.; Smith, R.; Voros, S.; Villines, T.C. Coronary CT angiography versus intravascular ultrasound for estimation of coronary stenosis and atherosclerotic plaque burden: A meta-analysis. J. Cardiovasc. Comput. Tomogr. 2013, 7, 256–266. [Google Scholar] [CrossRef]
- Voros, S.; Rinehart, S.; Qian, Z.; Vazquez, G.; Anderson, H.; Murrieta, L.; Wilmer, C.; Carlson, H.; Taylor, K.; Ballard, W.; et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: Results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc. Interv. 2011, 4, 198–208. [Google Scholar]
- Obaid, D.R.; Calvert, P.A.; Gopalan, D.; Parker, R.A.; Hoole, S.P.; West, N.E.; Goddard, M.; Rudd, J.H.; Bennett, M.R. Atherosclerotic plaque composition and classification identified by coronary computed tomography: Assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ. Cardiovasc. Imaging 2013, 6, 655–664. [Google Scholar] [CrossRef]
- de Graaf, M.A.; Broersen, A.; Kitslaar, P.H.; Roos, C.J.; Dijkstra, J.; Lelieveldt, B.P.; Jukema, J.W.; Schalij, M.J.; Delgado, V.; Bax, J.J.; et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: Cross-correlation with intravascular ultrasound virtual histology. Int. J. Cardiovasc. Imaging 2013, 29, 1177–1190. [Google Scholar] [CrossRef]
- Bamberg, F.; Sommer, W.H.; Hoffmann, V.; Achenbach, S.; Nikolaou, K.; Conen, D.; Reiser, M.F.; Hoffmann, U.; Becker, C.R. Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J. Am. Coll. Cardiol. 2011, 57, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Min, J.K.; Chang, H.J.; Andreini, D.; Pontone, G.; Guglielmo, M.; Bax, J.J.; Knaapen, P.; Raman, S.V.; Chazal, R.A.; Freeman, A.M.; et al. Coronary CTA plaque volume severity stages according to invasive coronary angiography and FFR. J. Cardiovasc. Comput. Tomogr. 2022, 16, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Vattay, B.; Borzsák, S.; Boussoussou, M.; Vecsey-Nagy, M.; Jermendy, Á.L.; Suhai, F.I.; Maurovich-Horvat, P.; Merkely, B.; Kolossvary, M.; Szilveszter, B. Association between coronary plaque volume and myocardial ischemia detected by dynamic perfusion CT imaging. Front. Cardiovasc. Med. 2022, 9, 974805. [Google Scholar] [CrossRef] [PubMed]
- Deseive, S.; Kupke, M.; Straub, R.; Stocker, T.J.; Broersen, A.; Kitslaar, P.; Martinoff, S.; Massberg, S.; Hadamitzky, M.; Hausleiter, J. Quantified coronary total plaque volume from computed tomography angiography provides superior 10-year risk stratification. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 314–321. [Google Scholar] [CrossRef] [PubMed]
- Halon, D.A.; Lavi, I.; Barnett-Griness, O.; Rubinshtein, R.; Zafrir, B.; Azencot, M.; Lewis, B.S. Plaque Morphology as Predictor of Late Plaque Events in Patients with Asymptomatic Type 2 Diabetes: A Long-Term Observational Study. JACC Cardiovasc. Imaging 2019, 12, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Puri, R.; Nissen, S.E.; Shao, M.; Ballantyne, C.M.; Barter, P.J.; Chapman, M.J.; Erbel, R.; Libby, P.; Raichlen, J.S.; Uno, K.; et al. Coronary atheroma volume and cardiovascular events during maximally intensive statin therapy. Eur. Heart J. 2013, 34, 3182–3190. [Google Scholar] [CrossRef]
- Chow, B.J.; Wells, G.A.; Chen, L.; Yam, Y.; Galiwango, P.; Abraham, A.; Sheth, T.; Dennie, C.; Beanlands, R.S.; Ruddy, T.D. Prognostic value of 64-slice cardiac computed tomography severity of coronary artery disease, coronary atherosclerosis, and left ventricular ejection fraction. J. Am. Coll. Cardiol. 2010, 55, 1017–1028. [Google Scholar] [CrossRef]
- Hou, Z.H.; Lu, B.; Gao, Y.; Jiang, S.L.; Wang, Y.; Li, W.; Budoff, M.J. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. JACC Cardiovasc. Imaging 2012, 5, 990–999. [Google Scholar] [CrossRef]
- Chang, H.J.; Lin, F.Y.; Lee, S.E.; Andreini, D.; Bax, J.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Coronary Atherosclerotic Precursors of Acute Coronary Syndromes. J. Am. Coll. Cardiol. 2018, 71, 2511–2522. [Google Scholar] [CrossRef]
- Hell, M.M.; Motwani, M.; Otaki, Y.; Cadet, S.; Gransar, H.; Miranda-Peats, R.; Valk, J.; Slomka, P.J.; Cheng, V.Y.; Rozanski, A.; et al. Quantitative global plaque characteristics from coronary computed tomography angiography for the prediction of future cardiac mortality during long-term follow-up. Eur. Heart J. Cardiovasc. Imaging 2017, 18, 1331–1339. [Google Scholar] [CrossRef]
- Ahmadi, A.; Leipsic, J.; Øvrehus, K.A.; Gaur, S.; Bagiella, E.; Ko, B.; Dey, D.; LaRocca, G.; Jensen, J.M.; Bøtker, H.E.; et al. Lesion-Specific and Vessel-Related Determinants of Fractional Flow Reserve Beyond Coronary Artery Stenosis. JACC Cardiovasc. Imaging 2018, 11, 521–530. [Google Scholar] [CrossRef]
- Bakhshi, H.; Meyghani, Z.; Kishi, S.; Magalhães, T.A.; Vavere, A.; Kitslaar, P.H.; George, R.T.; Niinuma, H.; Reiber, J.H.C.; Betoko, A.; et al. Comparative Effectiveness of CT-Derived Atherosclerotic Plaque Metrics for Predicting Myocardial Ischemia. JACC Cardiovasc. Imaging 2019, 12, 1367–1376. [Google Scholar] [CrossRef]
- Benedek, T.; Gyöngyösi, M.; Benedek, I. Multislice computed tomographic coronary angiography for quantitative assessment of culprit lesions in acute coronary syndromes. Can. J. Cardiol. 2013, 29, 364–371. [Google Scholar] [CrossRef]
- Motoyama, S.; Ito, H.; Sarai, M.; Kondo, T.; Kawai, H.; Nagahara, Y.; Harigaya, H.; Kan, S.; Anno, H.; Takahashi, H.; et al. Plaque Characterization by Coronary Computed Tomography Angiography and the Likelihood of Acute Coronary Events in Mid-Term Follow-Up. J. Am. Coll. Cardiol. 2015, 66, 337–346. [Google Scholar] [CrossRef]
- Ferencik, M.; Mayrhofer, T.; Bittner, D.O.; Emami, H.; Puchner, S.B.; Lu, M.T.; Meyersohn, N.M.; Ivanov, A.V.; Adami, E.C.; Patel, M.R.; et al. Use of High-Risk Coronary Atherosclerotic Plaque Detection for Risk Stratification of Patients with Stable Chest Pain: A Secondary Analysis of the PROMISE Randomized Clinical Trial. JAMA Cardiol. 2018, 3, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.V.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated with Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Fukuda, S.; Tanaka, A.; Nakanishi, K.; Taguchi, H.; Yoshikawa, J.; Shimada, K.; Yoshiyama, M. Napkin-ring sign on coronary CT angiography for the prediction of acute coronary syndrome. JACC Cardiovasc. Imaging 2013, 6, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Puchner, S.B.; Liu, T.; Mayrhofer, T.; Truong, Q.A.; Lee, H.; Fleg, J.L.; Nagurney, J.T.; Udelson, J.E.; Hoffmann, U.; Ferencik, M. High-risk plaque detected on coronary CT angiography predicts acute coronary syndromes independent of significant stenosis in acute chest pain: Results from the ROMICAT-II trial. J. Am. Coll. Cardiol. 2014, 64, 684–692. [Google Scholar] [CrossRef]
- Erlinge, D.; Maehara, A.; Ben-Yehuda, O.; Bøtker, H.E.; Maeng, M.; Kjøller-Hansen, L.; Engstrøm, T.; Matsumura, M.; Crowley, A.; Dressler, O.; et al. PROSPECT II Investigators. Identification of vulnerable plaques and patients by intracoronary near-infrared spectroscopy and ultrasound (PROSPECT II): A prospective natural history study. Lancet 2021, 397, 985–995. [Google Scholar] [CrossRef] [PubMed]
- Antonopoulos, A.S.; Sanna, F.; Sabharwal, N.; Thomas, S.; Oikonomou, E.K.; Herdman, L.; Margaritis, M.; Shirodaria, C.; Kampoli, A.M.; Akoumianakis, I.; et al. Detecting human coronary inflammation by imaging perivascular fat. Sci. Transl. Med. 2017, 9, eaal2658. [Google Scholar] [CrossRef]
- Akoumianakis, I.; Antoniades, C. The interplay between adipose tissue and the cardiovascular system: Is fat always bad? Cardiovasc. Res. 2017, 113, 999–1008. [Google Scholar] [CrossRef]
- Antoniades, C.; Kotanidis, C.P.; Berman, D.S. State-of-the-art review article. Atherosclerosis affecting fat: What can we learn by imaging perivascular adipose tissue? J. Cardiovasc. Comput. Tomogr. 2019, 13, 288–296. [Google Scholar] [CrossRef]
- Libby, P. The changing landscape of atherosclerosis. Nature 2021, 592, 524–533. [Google Scholar] [CrossRef]
- Mazurek, T.; Kobylecka, M.; Zielenkiewicz, M.; Kurek, A.; Kochman, J.; Filipiak, K.J.; Mazurek, K.; Huczek, Z.; Królicki, L.; Opolski, G. PET/CT evaluation of 18F-FDG uptake in pericoronary adipose tissue in patients with stable coronary artery disease: Independent predictor of atherosclerotic lesions’ formation? J. Nucl. Cardiol. 2017, 24, 1075–1084. [Google Scholar] [CrossRef] [PubMed]
- Kwiecinski, J.; Dey, D.; Cadet, S.; Lee, S.E.; Otaki, Y.; Huynh, P.T.; Doris, M.K.; Eisenberg, E.; Yun, M.; Jansen, M.A.; et al. Peri-Coronary Adipose Tissue Density Is Associated With 18F-Sodium Fluoride Coronary Uptake in Stable Patients with High-Risk Plaques. JACC Cardiovasc. Imaging 2019, 12, 2000–2010. [Google Scholar] [CrossRef] [PubMed]
- Goeller, M.; Rahman Ihdayhid, A.; Cadet, S.; Lin, A.; Adams, D.; Thakur, U.; Yap, G.; Marwan, M.; Achenbach, S.; Damini, D.; et al. Pericoronary adipose tissue and quantitative global non-calcified plaque characteristics from CT angiography do not differ in matched South Asian, East Asian and European-origin Caucasian patients with stable chest pain. Eur. J. Radiol. 2020, 125, 108874. [Google Scholar] [CrossRef]
- Goeller, M.; Achenbach, S.; Cadet, S.; Kwan, A.C.; Commandeur, F.; Slomka, P.J.; Gransar, H.; Albrecht, M.H.; Tamarappoo, B.K.; Berman, D.S.; et al. Pericoronary Adipose Tissue Computed Tomography Attenuation and High-Risk Plaque Characteristics in Acute Coronary Syndrome Compared with Stable Coronary Artery Disease. JAMA Cardiol. 2018, 3, 858–863. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Antonopoulos, A.S.; Schottlander, D.; Marwan, M.; Mathers, C.; Tomlins, P.; Siddique, M.; Klüner, L.V.; Shirodaria, C.; Mavrogiannis, M.C.; et al. Standardized measurement of coronary inflammation using cardiovascular computed tomography: Integration in clinical care as a prognostic medical device. Cardiovasc. Res. 2021, 117, 2677–2690. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Hutt Centeno, E.; Thomas, S.; Herdman, L.; Kotanidis, C.P.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Desai, M.Y.; Marwan, M.; Kotanidis, C.P.; Antonopoulos, A.S.; Schottlander, D.; Channon, K.M.; Neubauer, S.; Achenbach, S.; Antoniades, C. Perivascular Fat Attenuation Index Stratifies Cardiac Risk Associated with High-Risk Plaques in the CRISP-CT Study. J. Am. Coll. Cardiol. 2020, 76, 755–757. [Google Scholar] [CrossRef]
- Oikonomou, E.K.; Williams, M.C.; Kotanidis, C.P.; Desai, M.Y.; Marwan, M.; Antonopoulos, A.S.; Thomas, K.E.; Thomas, S.; Akoumianakis, I.; Fan, L.M.; et al. A novel machine learning-derived radiotranscriptomic signature of perivascular fat improves cardiac risk prediction using coronary CT angiography. Eur. Heart J. 2019, 40, 3529–3543. [Google Scholar] [CrossRef]
- Antoniades, C.; Antonopoulos, A.S.; Deanfield, J. Imaging residual inflammatory cardiovascular risk. Eur. Heart J. 2020, 41, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Greenland, P.; Alpert, J.S.; Beller, G.A.; Benjamin, E.J.; Budoff, M.J.; Fayad, Z.A.; Foster, E.; Hlatky, M.A.; Hodgson, J.M.; Kushner, F.G.; et al. American College of Cardiology Foundation; American Heart Association. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2010, 56, e50–e103. [Google Scholar] [PubMed]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2019, 74, 1376–1414. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.M.; Capodanno, D.; et al. ESC National Cardiac Societies; ESC Scientific Document Group. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Cosentino, F.; Grant, P.J.; Aboyans, V.; Bailey, C.J.; Ceriello, A.; Delgado, V.; Federici, M.; Filippatos, G.; Grobbee, D.E.; Hansen, T.B.; et al. ESC Scientific Document Group. 2019 ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur. Heart J. 2020, 41, 255–323. [Google Scholar] [CrossRef] [PubMed]
- Kramer, H.; Toto, R.; Peshock, R.; Cooper, R.; Victor, R. Association between chronic kidney disease and coronary artery calcification: The Dallas Heart Study. J. Am. Soc. Nephrol. 2005, 16, 507–513. [Google Scholar] [CrossRef]
- Writing Committee Members; Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 78, e187–e285. [Google Scholar]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Knuuti, J. ESC 2019 guidelines for the diagnosis and management of chronic coronary syndromes: Recommendations for cardiovascular imaging. Herz 2020, 45, 409–420. [Google Scholar] [CrossRef]
- Narula, J.; Chandrashekhar, Y.; Ahmadi, A.; Abbara, S.; Berman, D.S.; Blankstein, R.; Leipsic, J.; Newby, D.; Nicol, E.D.; Nieman, K.; et al. SCCT 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography: A Report of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2021, 15, 192–217. [Google Scholar] [CrossRef]
- Maroules, C.D.; Rybicki, F.J.; Ghoshhajra, B.B.; Batlle, J.C.; Branch, K.; Chinnaiyan, K.; Hamilton-Craig, C.; Hoffmann, U.; Litt, H.; Meyersohn, N.; et al. 2022 use of coronary computed tomographic angiography for patients presenting with acute chest pain to the emergency department: An expert consensus document of the Society of cardiovascular computed tomography (SCCT): Endorsed by the American College of Radiology (ACR) and North American Society for cardiovascular Imaging (NASCI). J. Cardiovasc. Comput. Tomogr. 2022, 17, 146–163. [Google Scholar]
- Andreini, D.; Collet, C.; Leipsic, J.; Nieman, K.; Bittencurt, M.; De Mey, J.; Buls, N.; Onuma, Y.; Mushtaq, S.; Conte, E.; et al. Pre-procedural planning of coronary revascularization by cardiac computed tomography: An expert consensus document of the Society of Cardiovascular Computed Tomography. J. Cardiovasc. Comput. Tomogr. 2022, 16, 558–572. [Google Scholar] [CrossRef] [PubMed]

| CAD-RADS Category | Degree of Maximal Coronary Stenosis (%) | Interpretation in Acute Chest Pain (ACS) | Interpretation in Stable Chest Pain (CAD) |
|---|---|---|---|
| 0 | 0 | Highly unlikely | Absence of CAD |
| 1 | 1–24 | Highly unlikely | Minimal non-obstructive CAD |
| 2 | 25–49 | Unlikely | Mild non-obstructive CAD |
| 3 | 50–69 | Possible | Moderate stenosis |
| 4A | One or two vessels: 70–99 | Likely | Severe stenosis |
| 4B | Left main artery: >50 or three vessels ≥70 | Likely | Severe stenosis |
| 5 | 100 | Very likely | Total occlusion |
| N | Non-diagnostic | Cannot be excluded | Cannot be excluded |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pugliese, L.; Ricci, F.; Sica, G.; Scaglione, M.; Masala, S. Non-Contrast and Contrast-Enhanced Cardiac Computed Tomography Imaging in the Diagnostic and Prognostic Evaluation of Coronary Artery Disease. Diagnostics 2023, 13, 2074. https://doi.org/10.3390/diagnostics13122074
Pugliese L, Ricci F, Sica G, Scaglione M, Masala S. Non-Contrast and Contrast-Enhanced Cardiac Computed Tomography Imaging in the Diagnostic and Prognostic Evaluation of Coronary Artery Disease. Diagnostics. 2023; 13(12):2074. https://doi.org/10.3390/diagnostics13122074
Chicago/Turabian StylePugliese, Luca, Francesca Ricci, Giacomo Sica, Mariano Scaglione, and Salvatore Masala. 2023. "Non-Contrast and Contrast-Enhanced Cardiac Computed Tomography Imaging in the Diagnostic and Prognostic Evaluation of Coronary Artery Disease" Diagnostics 13, no. 12: 2074. https://doi.org/10.3390/diagnostics13122074
APA StylePugliese, L., Ricci, F., Sica, G., Scaglione, M., & Masala, S. (2023). Non-Contrast and Contrast-Enhanced Cardiac Computed Tomography Imaging in the Diagnostic and Prognostic Evaluation of Coronary Artery Disease. Diagnostics, 13(12), 2074. https://doi.org/10.3390/diagnostics13122074








