Muscle Coactivation Index during Walking in People with Multiple Sclerosis with Mild Disability, a Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Procedure
2.4. Outcome Measures
2.5. Data Analysis
2.6. Sample Size Determination
2.7. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Eken, M.M.; Richards, R.; Beckerman, H.; van der Krogt, M.; Gerrits, K.; Rietberg, M.; de Groot, V.; Heine, M. Quantifying muscle fatigue during walking in people with multiple sclerosis. Clin. Biomech. 2020, 72, 94–101. [Google Scholar] [CrossRef]
- Giovannoni, G.; Butzkueven, H.; Dhib-Jalbut, S.; Hobart, J.; Kobelt, G.; Pepper, G.; Sormani, M.P.; Thalheim, C.; Traboulsee, A.; Vollmer, T. Brain Health: Time Matters in Multiple Sclerosis. Mult. Scler. Relat. Disord. 2016, 9, S5–S48. [Google Scholar] [CrossRef]
- Chalah, M.A.; Riachi, N.; Ahdab, R.; Créange, A.; Lefaucheur, J.P.; Ayache, S.S. Fatigue in Multiple Sclerosis: Neural Correlates and the Role of Non-Invasive Brain Stimulation. Front. Cell. Neurosci. 2015, 9, 460. [Google Scholar] [CrossRef] [PubMed]
- Coca-Tapia, M.; Cuesta-Gómez, A.; Molina-Rueda, F.; Carratalá-Tejada, M. Gait Pattern in People with Multiple Sclerosis: A Systematic Review. Diagnostics 2021, 11, 584. [Google Scholar] [CrossRef]
- Molina-Rueda, F.; Fernández-Vázquez, D.; Navarro-López, V.; Miangolarra-Page, J.C.; Carratalá-Tejada, M. The Timing of Kinematic and Kinetic Parameters during Gait Cycle as a Marker of Early Gait Deterioration in Multiple Sclerosis Subjects with Mild Disability. J. Clin. Med. 2022, 11, 1892. [Google Scholar] [CrossRef] [PubMed]
- Benedetti, M.G.; Piperno, R.; Simoncini, L.; Bonato, P.; Tonini, A.; Giannini, S. Gait abnormalities in minimally impaired multiple sclerosis patients. Mult. Scler. J. 1999, 5, 363–368. [Google Scholar] [CrossRef]
- Pau, M.; Coghe, G.; Corona, F.; Marrosu, M.G.; Cocco, E. Effect of spasticity on kinematics of gait and muscular activation in people with Multiple Sclerosis. J. Neurol. Sci. 2015, 358, 339–344. [Google Scholar] [CrossRef] [PubMed]
- Severini, G.; Manca, M.; Ferraresi, G.; Caniatti, L.M.; Cosma, M.; Baldasso, F.; Straudi, S.; Morelli, M.; Basaglia, N. Evaluation of Clinical Gait Analysis parameters in patients affected by Multiple Sclerosis: Analysis of kinematics. Clin. Biomech. 2017, 45, 1–8. [Google Scholar] [CrossRef]
- Huisinga, J.M.; Schmid, K.K.; Filipi, M.L.; Stergiou, N. Gait mechanics are different between healthy controls and patients with multiple sclerosis. J. Appl. Biomech. 2013, 29, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Sosnoff, J.J.; Sandroff, B.; Motl, R.W. Quantifying gait abnormalities in persons with multiple sclerosis with minimal disability. Gait Posture 2012, 36, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Kempen, J.C.; Doorenbosch, C.A.; Knol, D.L.; de Groot, V.; Beckerman, H. Newly Identified Gait Patterns in Patients With Multiple Sclerosis May Be Related to Push-off Quality. Phys. Ther. 2016, 96, 1744–1752. [Google Scholar] [CrossRef]
- Lencioni, T.; Jonsdottir, J.; Cattaneo, D.; Crippa, A.; Gervasoni, E.; Rovaris, M.; Bizzi, E.; Ferrarin, M. Are Modular Activations Altered in Lower Limb Muscles of Persons with Multiple Sclerosis during Walking? Evidence from Muscle Synergies and Biomechanical Analysis. Front. Hum. Neurosci. 2016, 10, 620. [Google Scholar] [CrossRef]
- Smith, A.M. The coactivation of antagonist muscles. Can. J. Physiol. Pharmacol. 1981, 59, 733–747. [Google Scholar] [CrossRef]
- Hortobágyi, T.; Devita, P. Mechanisms responsible for the age-associated increase in coactivation of antagonist muscles. Exerc. Sport Sci. Rev. 2006, 34, 29–35. [Google Scholar] [CrossRef]
- Lang, K.C.; Hackney, M.E.; Ting, L.H.; McKay, J.L. Antagonist muscle activity during reactive balance responses is elevated in Parkinson’s disease and in balance impairment. PLoS ONE 2019, 14, e0211137. [Google Scholar] [CrossRef]
- Keloth, S.M.; Arjunan, S.P.; Raghav, S.; Kumar, D.K. Muscle activation strategies of people with early-stage Parkinson’s during walking. J. NeuroEng. Rehabil. 2021, 18, 133. [Google Scholar] [CrossRef] [PubMed]
- Latash, M.L. Muscle coactivation: Definitions, mechanisms, and functions. J. Neurophysiol. 2018, 120, 88–104. [Google Scholar] [CrossRef]
- Busse, M.E.; Wiles, C.M.; van Deursen, R.W. Co-activation: Its association with weakness and specific neurological pathology. J. Neuroeng. Rehabil. 2006, 3, 26. [Google Scholar] [CrossRef] [PubMed]
- Ervilha, U.F.; Graven-Nielsen, T.; Duarte, M. A simple test of muscle coactivation estimation using electromyography. Braz. J. Med. Biol. Res. 2012, 45, 977–981. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Takahashi, M.; Shinkoda, K. Differences of muscle co-contraction of the ankle joint between young and elderly adults during dynamic postural control at different speeds. J. Physiol. Anthropol. 2017, 36, 32. [Google Scholar] [CrossRef] [PubMed]
- Tramonti, C.; Imperatori, L.S.; Fanciullacci, C.; Lamola, G.; Lettieri, G.; Bernardi, G.; Cecchetti, L.; Ricciardi, E.; Chisari, C. Predictive Value of Electroencephalography Connectivity Measures for Motor Training Outcome in Multiple Sclerosis: An Observational Longitudinal Study. Eur. J. Phys. Rehabil. Med. 2019, 55, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Boudarham, J.; Hameau, S.; Zory, R.; Hardy, A.; Bensmail, D.; Roche, N. Coactivation of Lower Limb Muscles during Gait in Patients with Multiple Sclerosis. PLoS ONE 2016, 11, e0158267. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Cohen, J.A.; Fazecas, F.; Filippi, M.; Freedman, M.; Fujihara, K.; et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef] [PubMed]
- Gribble, P.L.; Mullin, L.I.; Cothros, N.; Mattar, A. Role of cocontraction in arm movement accuracy. J. Neurophysiol. 2003, 89, 2396–2405. [Google Scholar] [CrossRef]
- Perry, J. Gait Analysis: Normal and Pathological Function. J. Sports Sci. Med. 2010, 9, 353. [Google Scholar] [CrossRef]
- Numpy.org. Available online: https://numpy.org/doc/stable/reference/generated/numpy.trapz.html (accessed on 15 April 2023).
- Rose, W. Mathematics and Signal Processing for Biomechanics Electromyogram analysis. Available online: https://www1.udel.edu/biology/rosewc/kaap686/notes/EMG%20analysis.pdf/ (accessed on 15 April 2023).
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. J. Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef]
- Halki, M.; Ginn, K. Normalization of EMG Signals: To Normalize or Not to Normalize and What to Normalize to? In Computational Intelligence in Electromyography Analysis; Naik, R.G., Ed.; InTech Open: London, UK, 2012. [Google Scholar] [CrossRef]
- Institut Hospital del Mar d’Investigacions Mèdiques. “Granmo.”. Available online: https://www.imim.es/ofertadeserveis/software-public/granmo/ (accessed on 15 April 2023).
- Nogueira, L.A.C.; Dos Santos, L.T.; Sabino, P.G.; Alvarenga, R.M.P.; Santos Thuler, L.C. Factors for lower walking speed in persons with multiple sclerosis. Mult. Scler. Int. 2013, 2013, 875648. [Google Scholar] [CrossRef]
- Theunissen, K.; Plasqui, G.; Boonen, A.; Brauwers, B.; Timmermans, A.; Meyns, P.; Meijer, K.; Feys, P. The Relationship Between Walking Speed and the Energetic Cost of Walking in Persons With Multiple Sclerosis and Healthy Controls: A Systematic Review. Neurorehabilit. Neural Repair 2021, 35, 486–500. [Google Scholar] [CrossRef] [PubMed]
- Higginson, J.S.; Zajac, F.E.; Neptune, R.R.; Kautz, S.A.; Delp, S.L. Muscle contributions to support during gait in an individual with post-stroke hemiparesis. J. Biomech. 2006, 39, 1769–1777. [Google Scholar] [CrossRef]
- Frigo, C.; Crenna, P. Multichannel SEMG in clinical gait analysis: A review and state-of-the-art. Clin. Biomech. 2009, 24, 236–245. [Google Scholar] [CrossRef]
- Silva, A.; Sousa, A.S.; Tavares, J.M.R.; Tinoco, A.; Santos, R.; Sousa, F. Ankle dynamic in stroke patients: Agonist vs. antagonist muscle relations. Somatosens. Mot. Res. 2012, 29, 111–116. [Google Scholar] [CrossRef]
- Janshen, L.; Santuz, A.; Arampatzis, A. Muscle Synergies in Patients with Multiple Sclerosis Reveal Demand-Specific Alterations in the Modular Organization of Locomotion. Front. Hum. Neurosci. 2021, 14, 593365. [Google Scholar] [CrossRef] [PubMed]
- Socie, M.J.; Motl, R.W.; Pula, J.H.; Sandroff, B.M.; Sosnoff, J.J. Gait variability and disability in multiple sclerosis. Gait Posture 2013, 38, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Flegel, M.; Knox, K.; Nickel, D. Step-length variability in minimally disabled women with multiple sclerosis or clinically isolated syndrome. Int. J. MS Care 2012, 14, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, A.; Malouin, F.; Richards, C.L. Locomotor-specific measure of spasticity of plantarflexor muscles after stroke. Arch. Phys. Med. Rehabil. 2001, 82, 1696–1704. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, M.; Ranavolo, A.; Conforto, S.; Martino, G.; Draicchio, F.; Conte, C.; Varrecchia, T.; Bini, F.; Casali, C.; Pierelli, F.; et al. Increased lower limb muscle coactivation reduces gait performance and increases metabolic cost in patients with hereditary spastic paraparesis. Clin. Biomech. 2017, 48, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Lamontagne, A.; Richards, C.L.; Malouin, F. Coactivation during gait as an adaptive behavior after stroke. J. Electromyogr. Kinesiol. 2000, 10, 407–415. [Google Scholar] [CrossRef]
- Sebastião, E.; Bollaert, R.E.; Hubbard, E.A.; Motl, R.W. Gait variability and energy cost of overground walking in persons with multiple sclerosis: A cross-sectional study. Am. J. Phys. Med. Rehabil. 2018, 97, 646–650. [Google Scholar] [CrossRef] [PubMed]
- Marvi-Esfahani, M.; Karimi, M.T.; Etemadifar, M.; Fatoye, F. Comparison of energy consumption in different clinical forms of multiple sclerosis with normal subjects (cohort study). Mult. Scler. Relat. Disord. 2016, 6, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Buoite Stella, A.; Morelli, M.E.; Giudici, F.; Sartori, A.; Manganotti, P.; di Prampero, P.E. Comfortable Walking Speed and Energy Cost of Locomotion in Patients with Multiple Sclerosis. Eur. J. Appl. Physiol. 2020, 120, 551–566. [Google Scholar] [CrossRef] [PubMed]
- Cofré Lizama, L.E.; Strik, M.; Van der Walt, A.; Kilpatrick, T.J.; Kolbe, S.C.; Galea, M.P. Gait stability reflects motor tracts damage at early stages of multiple sclerosis. Mult. Scler. J. 2022, 28, 1773–1782. [Google Scholar] [CrossRef]
| Parameters | Group | Mean (SD) | p Values * |
|---|---|---|---|
| Age (years) | MS | 34.44 (8.90) | 0.958 |
| Control | 34.67 (8.80) | ||
| Sex (female %) | MS | 77.78 | 1.000 |
| Control | 77.78 | ||
| Weight (Kg) | MS | 68.63 (9.15) | 0.278 |
| Control | 63.21 (11.21) | ||
| Height (cm) | MS | 169.60 (0.09) | 0.448 |
| Control | 172.50 (0.81) | ||
| Cadence (steps/min) | MS | 113.85 (8.33) | 0.621 |
| Control | 112.12 (6.07) | ||
| Walking speed (m/s) | MS | 1.19 (0.15) | 0.417 |
| Control | 1.25 (0.12) | ||
| Foot off (%) | MS | 61.43 (2.12) | 0.275 |
| Control | 60.38 (1.81) | ||
| Stride length (m) | MS | 1.26 (0.10) | 0.108 |
| Control | 1.35 (0.10) | ||
| ME type (RR) | MS | 100% | |
| EDSS | MS | 1.83 (1.20) | |
| Years since diagnosis | MS | 6.9 (5.7) |
| Lower Limb | AUC BF | AUC RF | AUC GastrocL | AUC TA | AUC Gmed | AUC Gmax |
|---|---|---|---|---|---|---|
| Control | 17.08 (4.67) | 24.83 (7.13) | 18.22 (1.24) | 21.64 (1.48) | 14.14 (0.38) | 15.65 (3.06) |
| MS MALL | 18.37 (5.93) | 23 (4.18) | 22.42 (1.43) | 28.12 (1.25) | 17.84 (4.26) | 15.71 (2.17) |
| MS LALL | 19.83 (3.57) | 15.06 (6.26) | 18.6 (3.08) | 24.13 (4.41) | 15.99 (3.64) | 14.76 (4.43) |
| Lower Limb | CI (%) BF-RF | CI (%) GastrocL–TA | CI (%) Gmax–RF | CI (%) Gmed–Gmax | CI (%) Gmax–GastrocL | |
| Control | 53.55 (8.80) | 36.44 (7.46) | 54.59 (9.34) | 55.8 (12.2) | 40.19 (6.64) | |
| MS MALL | 51.63 (5.64) | 54.05 (13.73) | 58.04 (6.38) | 68.29 (5.94) | 51.53 (8.39) | |
| MS LALL | 57.54 (12.16) | 41.57 (10.10) | 66.44 (12.6) | 69.97 (9.69) | 40.81 (12.27) | |
| Dependent Variable | ANOVA | Post Hoc Analysis (Bonferroni) | |||||
|---|---|---|---|---|---|---|---|
| F | p Value | η² | Group | DM | p Value | 95%CI | |
| GM | 0.875 | 0.430 | Control vs. MS MALL | −0.21 | 1.000 | −5.50 to 5.08 | |
| 0.071 | Control vs. MS LALL | 2.19 | 0.844 | −2.94 to 7.33 | |||
| MS LALL vs. MS MALL | 2.40 | 0.758 | −2.88 to 7.70 | ||||
| GMED | 0.761 | 0.478 | Control vs. MS MALL | −0.75 | 1.000 | −4.94 to 3.43 | |
| 0.062 | Control vs. MS LALL | 1.22 | 1.000 | −2.84 to 5.28 | |||
| MS LALL vs. MS MALL | 1.97 | 0.709 | −2.21 to 6.16 | ||||
| BF | 0.514 | 0.605 | Control vs. MS MALL | −1.10 | 1.000 | −6.33 to 4.12 | |
| 0.045 | Control vs. MS LALL | −2.04 | 0.966 | −7.27 to 3.18 | |||
| MS LALL vs. MS MALL | −0.93 | 1.000 | −5.82 to 3.95 | ||||
| RF | 1.708 | 0.203 | Control vs. MS MALL | −0.71 | 1.000 | −8.70 to 7.27 | |
| 0.129 | Control vs. MS LALL | 4.47 | 0.449 | −3.27 to 12.23 | |||
| MS LALL vs. MS MALL | 5.19 | 0.321 | −2.79 to 13.18 | ||||
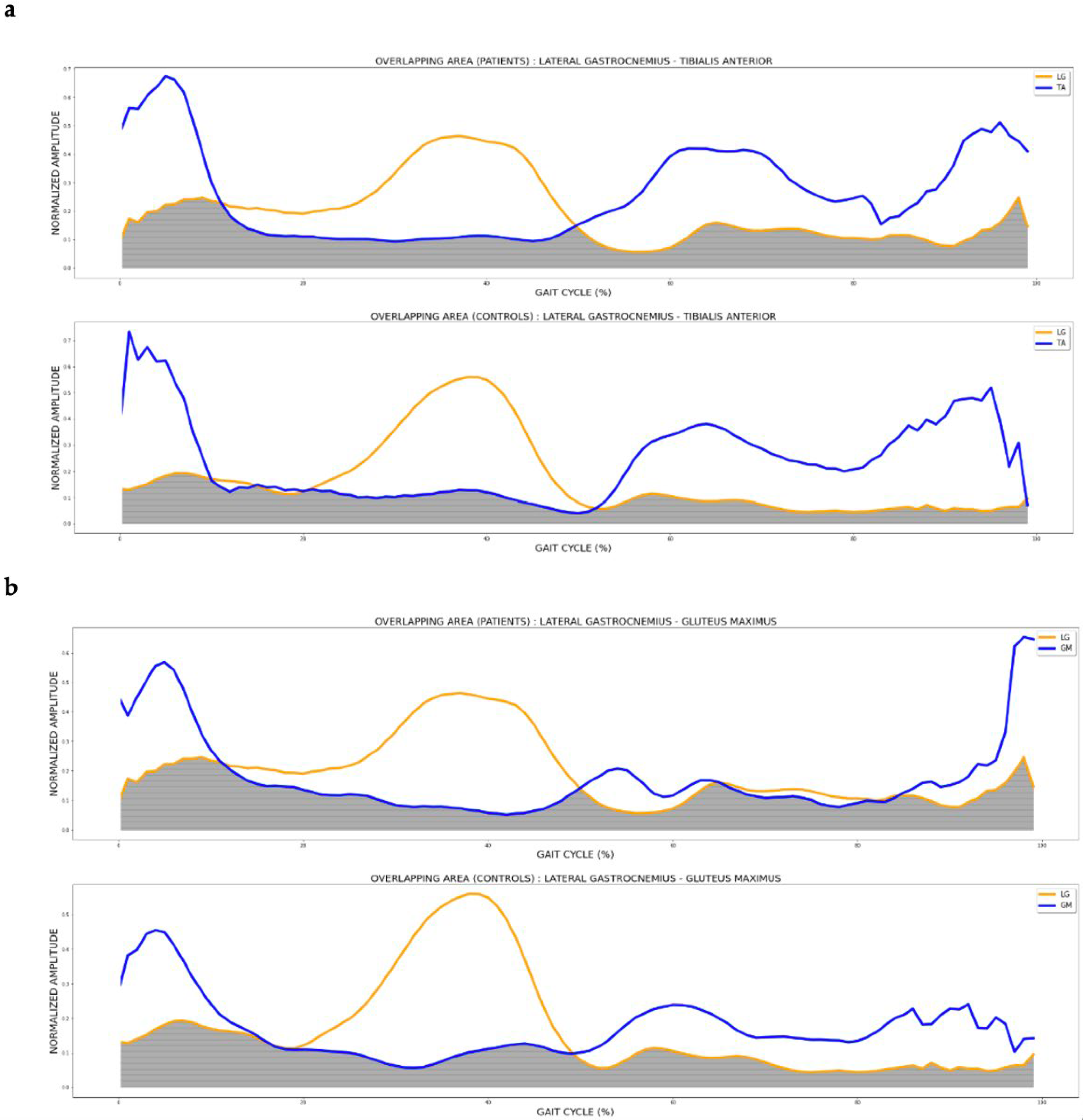
| LG | 4.520 | 0.023 * | Control vs. MS MALL | −4.73 | 0.020 * | −8.83 to −0.62 | |
| 0.191 | Control vs. MS LALL | −1.91 | 0.677 | −5.90 to 2.06 | |||
| MS LALL vs. MS MALL | 2.81 | 0.243 | −1.17 to 6.79 | ||||
| TA | 1.516 | 0.241 | Control vs. MS MALL | −3.19 | 0.291 | −7.95 to 1.57 | |
| 0.117 | Control vs. MS LALL | −1.99 | 0.869 | −6.76 to 2.76 | |||
| MS LALL vs. MS MALL | 1.19 | 1.000 | −3.42 to 5.81 | ||||
| Dependent Variable | ANOVA | Post Hoc Analysis (Bonferroni) | |||||
|---|---|---|---|---|---|---|---|
| F | p Value | η² | Group | DM | p Value | 95%CI | |
| GM–GMED | 1.640 | 0.218 | Control vs. MS MALL | −6.60 | 0.625 | −19.85 to 6.63 | |
| 0.135 | Control vs. MS LALL | −8.76 | 0.275 | −21.66 to 4.13 | |||
| MS LALL vs. MS MALL | −2.15 | 1.000 | −14.59 to 10.27 | ||||
| BF–RF | 0.707 | 0.503 | Control vs. MS MALL | −2.28 | 1.000 | −14.52 to 9.94 | |
| 0.058 | Control vs. MS LALL | −5.44 | 0.745 | −17.31 to 6.42 | |||
| MS LALL vs. MS MALL | −3.15 | 1.000 | −15.39 to 9.07 | ||||
| LG–TA | 4.460 | 0.024 * | Control vs. MS MALL | −16.82 | 0.022 * | −31.56 to −2.09 | |
| 0.298 | Control vs. MS LALL | −7.57 | 0.552 | −21.92 to 6.76 | |||
| MS LALL vs. MS MALL | 9.25 | 0.290 | −4.58 to 23.08 | ||||
| GM–RF | 1.228 | 0.313 | Control vs. MS MALL | 2.34 | 1.000 | −12.03 to 16.71 | |
| 0.105 | Control vs. MS LALL | −5.54 | 0.944 | −19.53 to 8.45 | |||
| MS LALL vs. MS MALL | −7.88 | 0.431 | −21.37 to 5.61 | ||||
| GM–LG | 4.165 | 0.031 * | Control vs. MS MALL | −15.70 | 0.047 * | −31.78 to −0.38 | |
| 0.294 | Control vs. MS LALL | −1.20 | 1.000 | −16.36 to 13.95 | |||
| MS LALL vs. MS MALL | 14.49 | 0.064 | −0.66 to 29.65 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina-Rueda, F.; Fernández-Vázquez, D.; Navarro-López, V.; López-González, R.; Carratalá-Tejada, M. Muscle Coactivation Index during Walking in People with Multiple Sclerosis with Mild Disability, a Cross-Sectional Study. Diagnostics 2023, 13, 2169. https://doi.org/10.3390/diagnostics13132169
Molina-Rueda F, Fernández-Vázquez D, Navarro-López V, López-González R, Carratalá-Tejada M. Muscle Coactivation Index during Walking in People with Multiple Sclerosis with Mild Disability, a Cross-Sectional Study. Diagnostics. 2023; 13(13):2169. https://doi.org/10.3390/diagnostics13132169
Chicago/Turabian StyleMolina-Rueda, Francisco, Diego Fernández-Vázquez, Víctor Navarro-López, Raúl López-González, and María Carratalá-Tejada. 2023. "Muscle Coactivation Index during Walking in People with Multiple Sclerosis with Mild Disability, a Cross-Sectional Study" Diagnostics 13, no. 13: 2169. https://doi.org/10.3390/diagnostics13132169
APA StyleMolina-Rueda, F., Fernández-Vázquez, D., Navarro-López, V., López-González, R., & Carratalá-Tejada, M. (2023). Muscle Coactivation Index during Walking in People with Multiple Sclerosis with Mild Disability, a Cross-Sectional Study. Diagnostics, 13(13), 2169. https://doi.org/10.3390/diagnostics13132169







