Cardiac MRI Findings in Patients Clinically Referred for Evaluation of Post-Acute Sequelae of SARS-CoV-2 Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Cardiac MRI
2.3. Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Raman, B.; Bluemke, D.A.; Lüscher, T.F.; Neubauer, S. Long COVID: Post-acute sequelae of COVID-19 with a cardiovascular focus. Eur. Heart J. 2022, 11, 1157–1172. [Google Scholar] [CrossRef] [PubMed]
- Soriano, J.B.; Murthy, S.; Marshall, J.C.; Relan, P.; Diaz, J.V. A clinical case definition of post-COVID-19 condition by a Delphi consensus. Lancet Infect. Dis. 2022, 22, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, M.G.; Cooper, L.T. What we (don’t) know about myocardial injury after COVID-19. Eur. Heart J. 2021, 19, 1879–1882. [Google Scholar] [CrossRef] [PubMed]
- Gluckman, T.J.; Bhave, N.M.; Allen, L.A.; Chung, E.H.; Spatz, E.S.; Ammirati, E.; Baggish, A.L.; Bozkurt, B.; Cornwell, W.K.; Harmon, K.G.; et al. 2022 ACC Expert Consensus Decision Pathway on Cardiovascular Sequelae of COVID-19 in Adults: Myocarditis and Other Myocardial Involvement, Post-Acute Sequelae of SARS-CoV-2 Infection, and Return to Play: A Report of the American College of Cardiology Solution Set Oversight Committee. J. Am. Coll. Cardiol. 2022, 17, 1717–1756. [Google Scholar]
- Kelle, S.; Bucciarelli-Ducci, C.; Judd, R.M.; Kwong, R.Y.; Simonetti, O.; Plein, S.; Raimondi, F.; Weinsaft, J.W.; Wong, T.C.; Carr, J. Society for Cardiovascular Magnetic Resonance (SCMR) recommended CMR protocols for scanning patients with active or convalescent phase COVID-19 infection. J. Cardiovasc. Magn. Reson. 2020, 1, 61. [Google Scholar] [CrossRef]
- Messroghli, D.R.; Moon, J.C.; Ferreira, V.M.; Grosse-Wortmann, L.; He, T.; Kellman, P.; Mascherbauer, J.; Nezafat, R.; Salerno, M.; Schelbert, E.B.; et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J. Cardiovasc. Magn. Reson. 2017, 1, 75. [Google Scholar]
- Puntmann, V.O.; Martin, S.; Shchendrygina, A.; Hoffmann, J.; Ka, M.M.; Giokoglu, E.; Vanchin, B.; Holm, N.; Karyou, A.; Laux, G.S.; et al. Long-term cardiac pathology in individuals with mild initial COVID-19 illness. Nat. Med. 2022, 10, 2117–2123. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, E.B.; Nacif, M.S.; Guo, M.; McClelland, R.L.; Teixeira, P.B.; Bild, D.E.; Barr, R.G.; Shea, S.; Post, W.; Burk, G.; et al. Prevalence and Correlates of Myocardial Scar in a US Cohort. JAMA 2015, 18, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Halushka, M.K.; Vander Heide, R.S. Myocarditis is rare in COVID-19 autopsies: Cardiovascular findings across 277 postmortem examinations. Cardiovasc. Pathol. 2021, 50, 107300. [Google Scholar] [CrossRef] [PubMed]
| General parameters | |
| Total cohort (n) | 129 (100%) |
| Females | 63 (51%) |
| Age (years) | 41 ± 16 |
| Body mass index (kg/m²) | 26.6 ± 10.6 |
| Heart rate (bpm) | 71 ± 13 |
| Indication for cardiac MRI | |
| Exertion dyspnea | 30 (23%) |
| Tachycardia/palpitations | 29 (22%) |
| Exercise intolerance | 26 (20%) |
| Fatigue | 19 (15%) |
| Chest pain | 18 (14%) |
| Left ventricular ejection fraction (%) | 58.9 ± 9.2 |
| ≥55% | 102 (79%) |
| <55% | 27 (21%) |
| Left ventricular end-diastolic volume index (mL/m2) | 85.5 ± 24.8 |
| <100 mL/m2 | 104 (81%) |
| ≥100 mL/m2 | 25 (19%) |
| Left ventricular segmental hypokinesia | 1 (1%) |
| Late gadolinium enhancement | 49 (38%) |
| Subepicardial | 40 (82%) |
| Midmyocardial | 3 (6%) |
| Subendocardial | 3 (6%) |
| Transmural | 1 (2%) |
| RV insertion | 2 (4%) |
| Myocardial edema on T2-weighted imaging | 0 (0%) |
| Abnormal T1 relaxation times | 18 (14%) |
| Abnormal T2 relaxation times | 3 (2%) |
| Pericardial effusion (>5 mm) | 18 (14%) |
| Pleural effusion (>20 mm) | 6 (5%) |
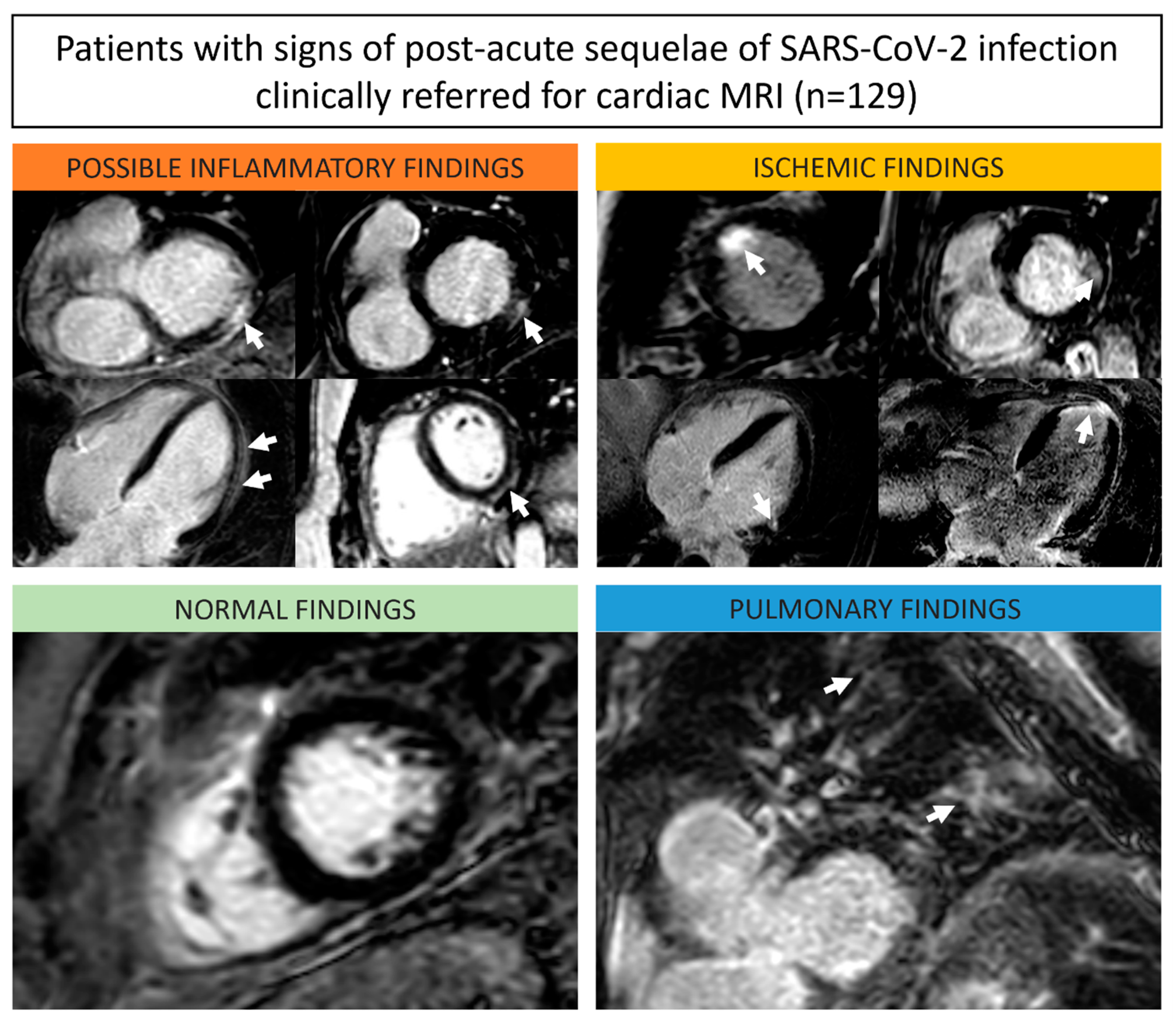
| Non-ischemic cardiac findings | 51 (40%) |
| Possible inflammatory | 39 (76%) |
| Post-inflammation | 39 (100%) |
| Positive for 2018 Lake Louise Criteria | 0 (0%) |
| Structural and Morphological | 12 (24%) |
| Valvular cardiomyopathy | 6 (50%) |
| Dilated cardiomyopathy | 3 (25%) |
| Hypertensive heart disease | 3 (25%) |
| Ischemic cardiac findings | 5 (4%) |
| No cardiac findings | 73 (57%) |
| Pulmonary findings | 6 (8%) |
| No extracardiac findings | 67 (92%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halfmann, M.C.; Luetkens, J.A.; Langenbach, I.L.; Kravchenko, D.; Wenzel, P.; Emrich, T.; Isaak, A. Cardiac MRI Findings in Patients Clinically Referred for Evaluation of Post-Acute Sequelae of SARS-CoV-2 Infection. Diagnostics 2023, 13, 2172. https://doi.org/10.3390/diagnostics13132172
Halfmann MC, Luetkens JA, Langenbach IL, Kravchenko D, Wenzel P, Emrich T, Isaak A. Cardiac MRI Findings in Patients Clinically Referred for Evaluation of Post-Acute Sequelae of SARS-CoV-2 Infection. Diagnostics. 2023; 13(13):2172. https://doi.org/10.3390/diagnostics13132172
Chicago/Turabian StyleHalfmann, Moritz C., Julian A. Luetkens, Isabel L. Langenbach, Dmitrij Kravchenko, Philip Wenzel, Tilman Emrich, and Alexander Isaak. 2023. "Cardiac MRI Findings in Patients Clinically Referred for Evaluation of Post-Acute Sequelae of SARS-CoV-2 Infection" Diagnostics 13, no. 13: 2172. https://doi.org/10.3390/diagnostics13132172
APA StyleHalfmann, M. C., Luetkens, J. A., Langenbach, I. L., Kravchenko, D., Wenzel, P., Emrich, T., & Isaak, A. (2023). Cardiac MRI Findings in Patients Clinically Referred for Evaluation of Post-Acute Sequelae of SARS-CoV-2 Infection. Diagnostics, 13(13), 2172. https://doi.org/10.3390/diagnostics13132172






