Vaccines in Breast Cancer: Challenges and Breakthroughs
Abstract
:1. Introduction
2. Diagnostics and Treatments in BC
3. Vaccine Therapy in BC
3.1. Introduction
3.2. Concepts in Designing of a Breast Cancer Vaccine (BCV)
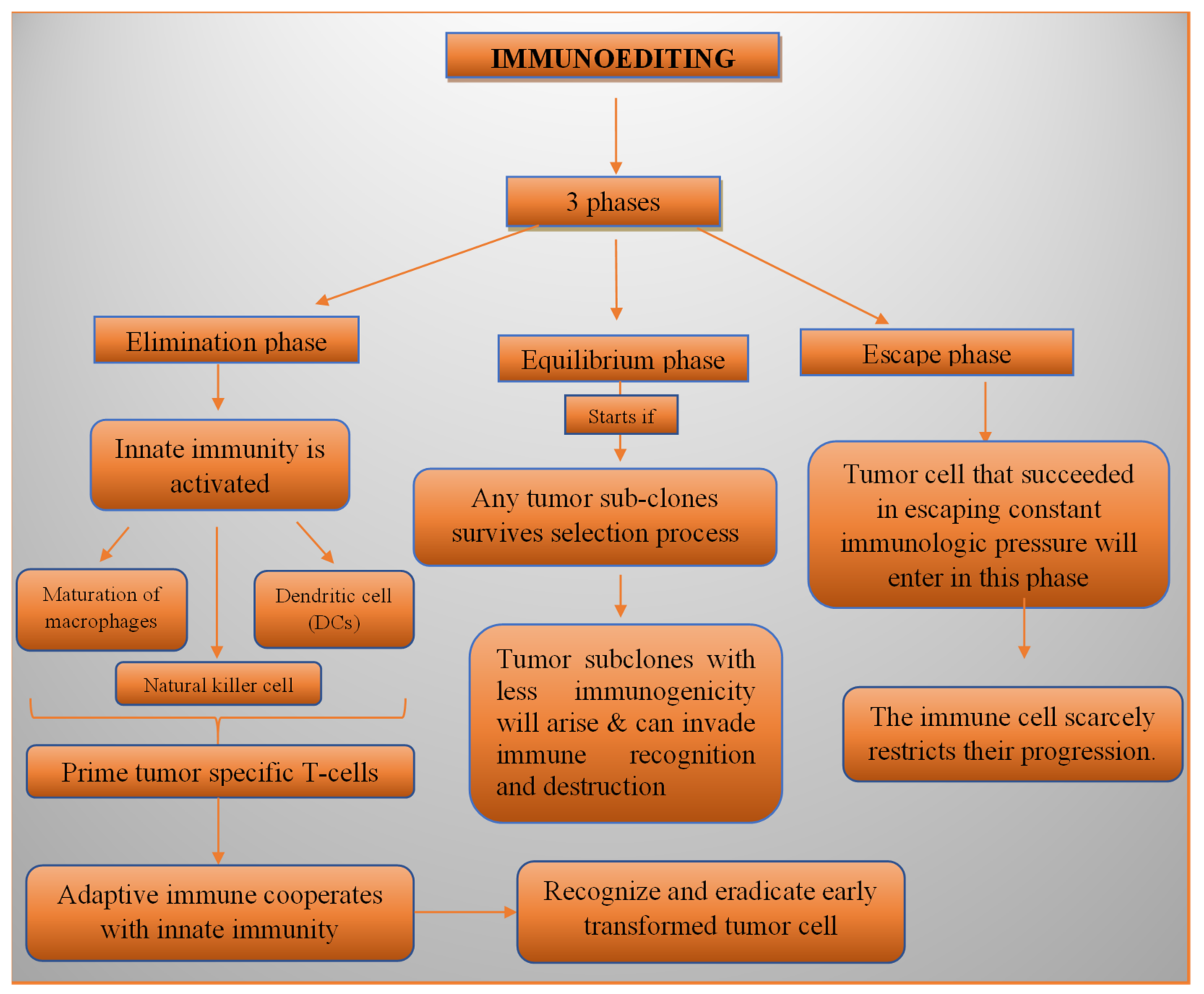
3.2.1. Immunoediting
3.2.2. Immune Surveillance
3.2.3. Immune Suppression
3.2.4. Identification of the Antigen for BC Immunotherapy
Human Epidermal Growth (HER2) Receptor 2
Tumour Suppressor p53 Protein (p53)
Mucin1 (MUC1)
Carcinoembryonic Antigen (CEA)
Human Telomerase Reverse Transcriptase (h-TERT)
3.3. Design Approaches of a BCV
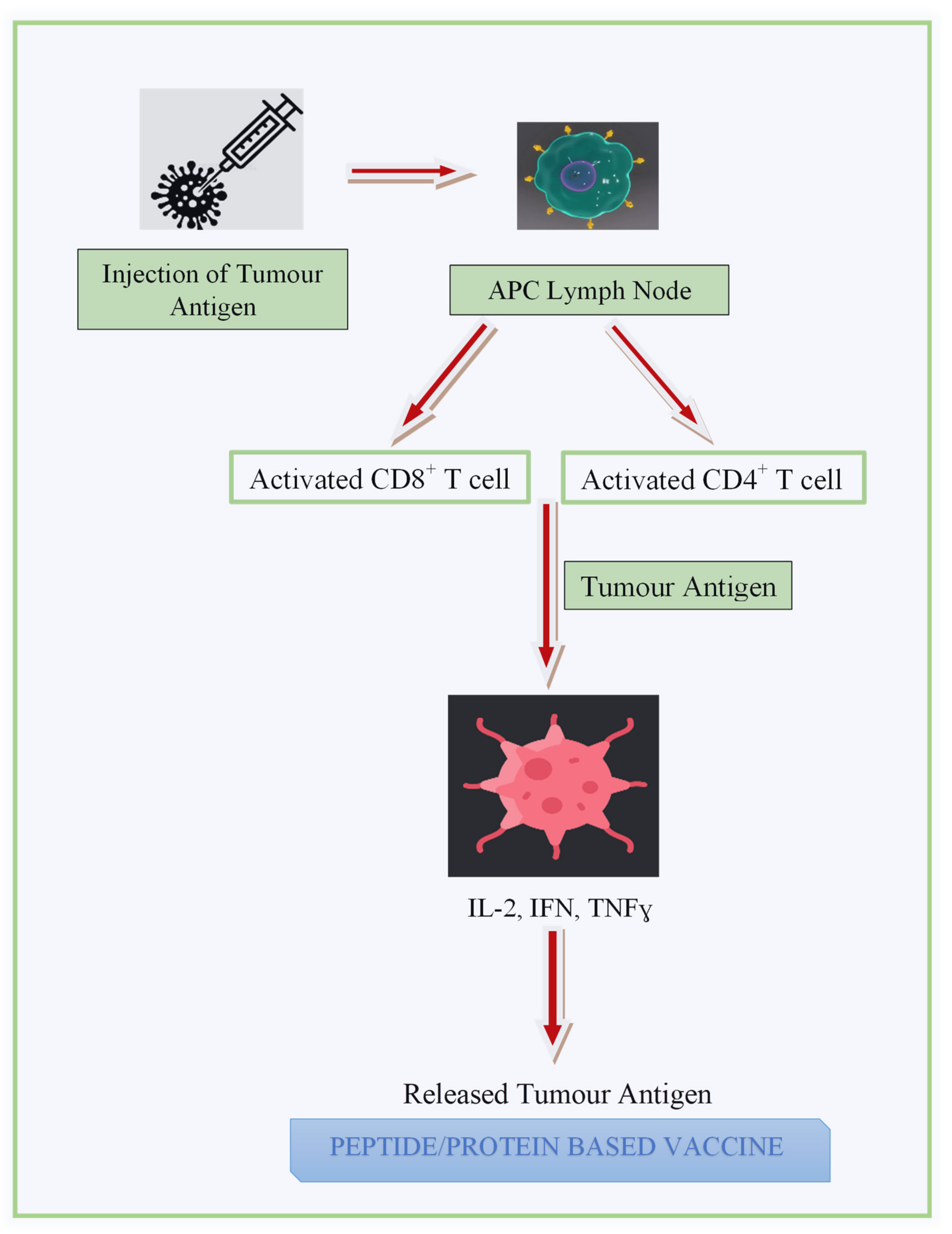
3.3.1. Peptide- and Protein-Based Vaccine (PV)
3.3.2. Carbohydrate Antigen-Based Vaccine (CAV)
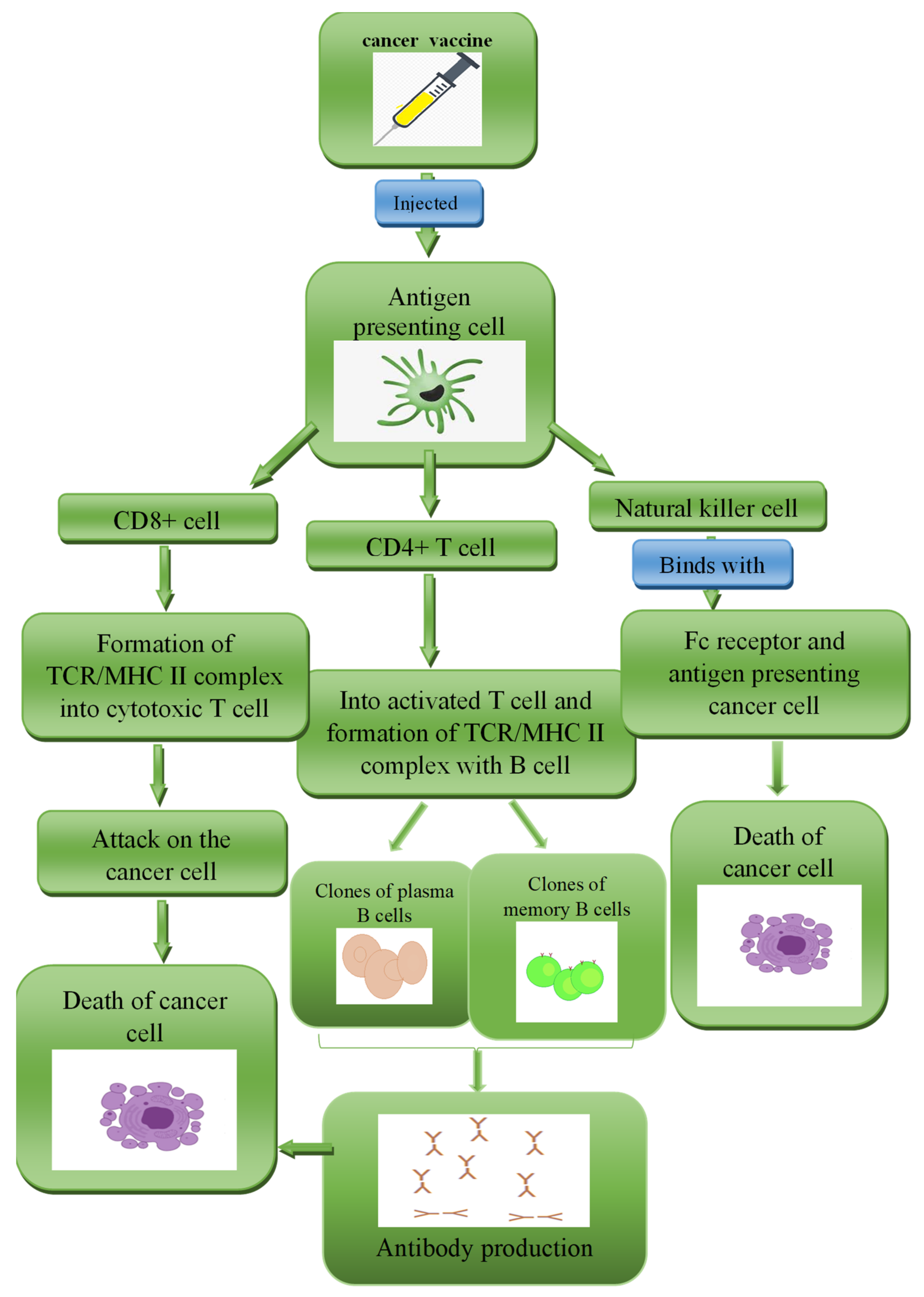
3.3.3. Whole Tumour Cell-Based Vaccine (WTCV)
3.3.4. Dendritic Cell-Based Vaccine (DCV)
3.3.5. Gene-Based Vaccine
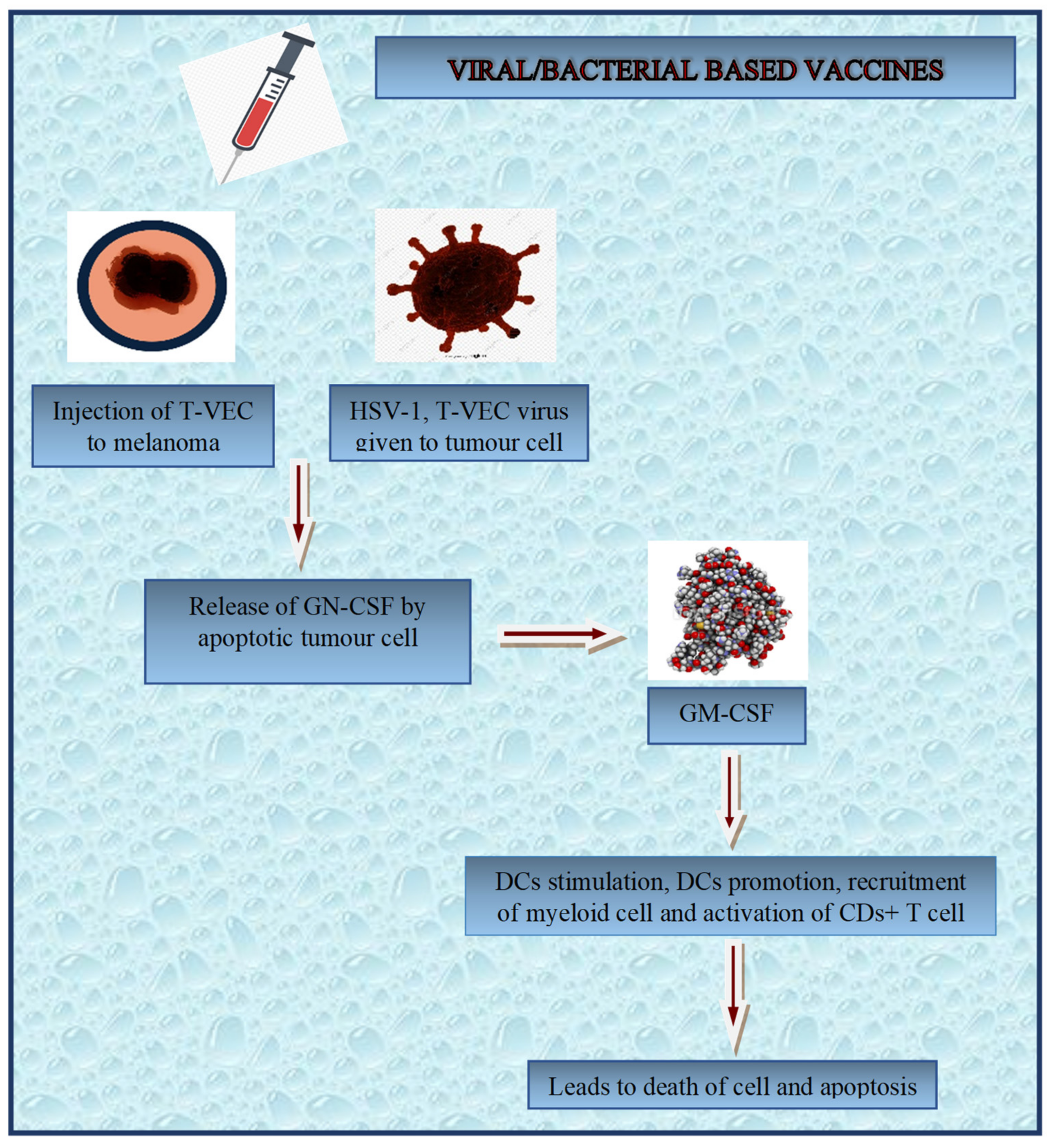
3.3.6. Fusion Vaccine
3.4. Adjuvants Used in Design of BCVS
3.5. Routes of BCV Administration
3.6. Clinical Trials of BCVs
3.7. Combinational Therapy of BCVs
3.8. Challenges Faced during the Course of Development of BCVs
4. Recent Patents Filed/Granted on Cancer Treatment and Diagnostics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| AI | artificial intelligence |
| APCs | antigen-presenting cells |
| BC | breast cancer |
| BCV | breast cancer vaccine |
| CAD | computer aided diagnosis |
| CAV | carbohydrate antigen-based vaccine |
| CEA | carcino-embryonic antigen |
| CT | computed tomography |
| CTLA-4 | cytotoxic T-lymphocyte antigen-4 |
| CTLs | cytotoxic lymphocytes |
| DCIS | ductal carcinoma in situ |
| DCs | dendritic cells |
| DCV | dendritic cell-based vaccine |
| ER | estrogen receptor |
| FDGePET/CT | 18F-fluorodeoxyglucose positron-emission tomography |
| FZD receptors | human Frizzled |
| GM-CSF | granulocyte-macrophage colony-stimulating factor |
| HER2 | human epidermal growth receptor2 |
| h-TERT | telomerase reverse transcriptase |
| ICB | immune checkpoint blockers |
| IDC | infiltrating ductal carcinoma |
| IFN-γ | interferon-γ |
| ILC | invasive lobular carcinoma |
| IORT | intraoperative radiation therapy |
| IT | immunotherapy |
| LCIS | lobular carcinoma in situ |
| MANPs | magnetic alloy nanoparticles |
| MDSCs | myeloid-derived suppressor cells |
| MHC | histocompatibility complex |
| MIAS | Mammographic Image Analysis Society |
| miRNAs | exosomal microRNAs |
| MMONPs | magnetic metal oxide nanoparticles |
| MNPs | magnetic nanoparticles |
| MRI | magnetic resonance imaging |
| MRM | multiple reaction monitoring |
| mTOR kinase | mammalian target of rapamycin kinase |
| mtp53 | mutation in the p53 gene |
| MUC-1 | mucin-1 |
| MW | microwave |
| NK-cells | natural killer cells |
| NPs | nanoparticles |
| p53 | tumour protein 53 |
| PALP | placental alkaline phosphatase |
| PCD | programmed cell death |
| PCDL-1 | programmed cell death ligand-1 |
| PCDR-1 | programmed cell death receptor-1 |
| PET | positron emission tomography |
| PR | progesterone receptor |
| PRR | pattern recognition receptors |
| PV | peptide and protein-based vaccine |
| RFA | radiofrequency ablation |
| ROR1-antibodies | receptor tyrosine kinase-like orphan receptor1 antibodies |
| siRNAs | small interfering RNAs |
| STn | Sialy-Tn |
| TA | thermal ablation |
| TAMs | tumour-associated macrophages |
| TCR | T-cell receptor |
| TGF-β | transforming growth factor-β |
| TILs | tumour-infiltration lymphocytes |
| TLR | toll-like receptors |
| TME | tumour microenvironment |
| TNBC | triple-negative breast cancer |
| Treg cells | regulatory T-cells |
| TSA | tumour-specific antigens |
| WTCV | whole tumour cell-based vaccine |
References
- Dittmer, J. Breast Cancer Stem Cells: Features, Key Drivers and Treatment Options. Semin. Cancer Biol. 2018, 53, 59–74. [Google Scholar] [CrossRef] [PubMed]
- Fisusi, F.A.; Akala, E.O. Drug Combinations in Breast Cancer Therapy. Pharm. Nanotechnol. 2019, 7, 3–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Yang, J.; Fong, S.; Zhao, Q.J. Artificial Intelligence in Cancer Diagnosis and Prognosis: Opportunities and Challenges. Cancer Lett. 2020, 471, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Hylton, N.M.; Gatsonis, C.A.; Rosen, M.A.; Lehman, C.D.; Newitt, D.C.; Partridge, S.C.; Bernreuter, W.K.; Pisano, E.D.; Morris, E.A.; Weatherall, P.T.; et al. Neoadjuvant Chemotherapy for Breast Cancer: Functional Tumor Volume by Mr Imaging Predicts Recurrence-Free Survival-Results from the Acrin 6657/Calgb 150007 I-Spy 1 Trial. Radiology 2016, 279, 44–55. [Google Scholar] [CrossRef] [Green Version]
- Da Costa, B.R.B.; De Martinis, B.S. Analysis of Urinary Vocs Using Mass Spectrometric Methods to Diagnose Cancer: A Review. Clin. Mass Spectrom. 2020, 18, 27–37. [Google Scholar] [CrossRef]
- Hasan, M.; Büyüktahtakın, İ.E.; Elamin, E. A Multi-Criteria Ranking Algorithm (Mcra) for Determining Breast Cancer Therapy. Omega 2019, 82, 83–101. [Google Scholar] [CrossRef]
- Han, H.J.; Ekweremadu, C.; Patel, N. Advanced Drug Delivery System with Nanomaterials for Personalised Medicine to Treat Breast Cancer. J. Drug Deliv. Sci. Technol. 2019, 52, 1051–1060. [Google Scholar] [CrossRef]
- Claessens, A.K.M.; Ibragimova, K.I.E.; Geurts, S.M.E.; Bos, M.E.M.M.; Erdkamp, F.L.G.; Tjan-Heijnen, V.C.G. The Role of Chemotherapy in Treatment of Advanced Breast Cancer: An Overview for Clinical Practice. Crit. Rev. Oncol. Hematol. 2020, 153, 102988. [Google Scholar] [CrossRef]
- De Cicco, P.; Catani, M.V.; Gasperi, V.; Sibilano, M.; Quaglietta, M.; Savini, I. Nutrition and Breast Cancer: A Literature Review on Prevention, Treatment and Recurrence. Nutrients 2019, 11, 1514. [Google Scholar] [CrossRef] [Green Version]
- Subramani, R.; Lakshmanaswamy, R. Chapter Three—Pregnancy and Breast Cancer. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 151, pp. 81–111. [Google Scholar] [CrossRef]
- Barzaman, K.; Karami, J.; Zarei, Z.; Hosseinzadeh, A.; Kazemi, M.H.; Moradi-Kalbolandi, S.; Safari, E.; Farahmand, L. Breast Cancer: Biology, Biomarkers, and Treatments. Int. Immunopharmacol. 2020, 84, 106535. [Google Scholar] [CrossRef]
- Bai, X.; Ni, J.; Beretov, J.; Graham, P.; Li, Y. Immunotherapy for Triple-Negative Breast Cancer: A Molecular Insight Into the Microenvironment, Treatment, and Resistance. J. Natl. Cancer Center 2021, 1, 75–87. [Google Scholar] [CrossRef]
- Huang, M.; Zhang, J.; Yan, C.; Li, X.; Zhang, J.; Ling, R. Small Molecule Hdac Inhibitors: Promising Agents for Breast Cancer Treatment. Bioorg. Chem. 2019, 91, 103184. [Google Scholar] [CrossRef] [PubMed]
- Swamy, N.; Rohilla, M.; Raichandani, S.; Bryant-Smith, G.J. Epidemiology of Male Breast Diseases: A 10-Year Institutional Review. Clin. Imaging 2021, 72, 142–150. [Google Scholar] [CrossRef]
- Konduri, S.; Singh, M.; Bobustuc, G.; Rovin, R.; Kassam, A. Epidemiology of Male Breast Cancer. Breast 2020, 54, 8–14. [Google Scholar] [CrossRef]
- Sheth, D.; Giger, M.L. Artificial Intelligence in the Interpretation of Breast Cancer on Mri. J. Magn. Reson. Imaging 2020, 51, 1310–1324. [Google Scholar] [CrossRef] [PubMed]
- Chesebro, A.L.; Rives, A.F.; Shaffer, K. Male Breast Disease: What the Radiologist Needs to Know. Curr. Probl. Diagn. Radiol. 2019, 48, 482–493. [Google Scholar] [CrossRef]
- Shaaban, A.M. Pathology of the Male Breast. Diagn. Histopathol. 2019, 25, 138–142. [Google Scholar] [CrossRef]
- Shin, K.; Whitman, G.J. Clinical Indications for Mammography in Men and Correlation with Breast Cancer. Curr. Probl. Diagn. Radiol. 2021, 50, 792–798. [Google Scholar] [CrossRef]
- Jalalian, A.; Mashohor, S.; Mahmud, R.; Karasfi, B.; Saripan, M.I.B.; Ramli, A.R.B. Foundation and Methodologies in Computer-Aided Diagnosis Systems for Breast Cancer Detection. EXCLI J. 2017, 16, 113–137. [Google Scholar] [CrossRef]
- Liew, X.Y.; Hameed, N.; Clos, J.J.C. A Review of Computer-Aided Expert Systems for Breast Cancer Diagnosis. Cancers 2021, 13, 2764. [Google Scholar] [CrossRef]
- Alkhateeb, A.J.J. Breast Cancer Computer-Aided Diagnosis System from Digital Mammograms. J. Adv. Med. 2019, 30, 530197. [Google Scholar] [CrossRef] [Green Version]
- Al-Shamlan, H.; El-Zaart, A. Breast Cancer Computer Aided Diagnosis (Cad) System. In Image Processing, Computer Vision, and Pattern Recognition; Kindle Direct Publishing: Seattle, WA, USA, 2011; p. 1. [Google Scholar]
- Chirita, A.J. Indications of the Magnetic Resonance Method in Breast Pathology. Chirurgia 2017, 112, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Guneyli, S.; Erdem, C.Z.; Erdem, L.O. Magnetic Resonance Imaging of Prostate Cancer. Clin. Imaging 2016, 40, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Rahbar, H.; Partridge, S.C. Multiparametric Mr Imaging of Breast Cancer. Magn. Reson. Imaging Clin. N. Am. 2016, 24, 223–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgeois, A.C.; Warren, L.A.; Chang, T.T.; Embry, S.; Hudson, K.; Bradley, Y.C. Role of Positron Emission Tomography/Computed Tomography in Breast Cancer. Radiol. Clin. N. Am. 2013, 51, 781–798. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Findikcioglu, A.; Guler, O.C.; Reyhan, M.J. The Use of 18f-Fdg Positron Emission Tomography to Detect Mediastinal Lymph Nodes in Metastatic Breast Cancer. Breast 2020, 54, 197–202. [Google Scholar] [CrossRef]
- Shang, L.; Liu, Z.; Rong, Y.; Zhou, T.; Zuo, L.J. Mri in Breast Cancer Radiotherapy in Prone and Supine Positions. Front. Biosci. 2017, 22, 570–579. [Google Scholar]
- Bahreini, M.; Hosseinzadegan, A.; Rashidi, A.; Miri, S.R.; Mirzaei, H.R.; Hajian, P.J. A Raman-Based Serum Constituents’ Analysis for Gastric Cancer Diagnosis: In Vitro Study. Talanta 2019, 204, 826–832. [Google Scholar] [CrossRef]
- Caldarone, A.; Piccotti, F.; Morasso, C.; Truffi, M.; Sottotetti, F.; Guerra, C.; Albasini, S.; Agozzino, M.; Villani, L.; Corsi, F.; et al. Raman Analysis of Microcalcifications in Male Breast Cancer. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 263, 120185. [Google Scholar] [CrossRef]
- Kopec, M.; Błaszczyk, M.; Radek, M.; Abramczyk, H.; Spectroscopy, B. Raman Imaging and Statistical Methods for Analysis Various Type of Human Brain Tumors and Breast Cancers. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 262, 120091. [Google Scholar] [CrossRef]
- Auner, G.W.; Koya, S.K.; Huang, C.; Broadbent, B.; Trexler, M.; Auner, Z.; Elias, A.; Mehne, K.C.; Brusatori, M.A. Applications of Raman Spectroscopy in Cancer Diagnosis. Cancer Metastasis Rev. 2018, 37, 691–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paidi, S.K.; Shah, V.; Raj, P.; Glunde, K.; Pandey, R.; Barman, I.J. Coarse Raman and Optical Diffraction Tomographic Imaging Enable Label-Free Phenotyping of Isogenic Breast Cancer Cells of Varying Metastatic Potential. Biosens. Bioelectron. 2021, 175, 112863. [Google Scholar] [CrossRef] [PubMed]
- Fiorica, J.V. Breast Cancer Screening, Mammography, and Other Modalities. Clin. Obstet. Gynecol. 2016, 59, 688–709. [Google Scholar] [CrossRef] [PubMed]
- Gartlehner, G.; Thaler, K.J.; Chapman, A.; Kaminski, A.; Berzaczy, D.; Van Noord, M.G.; Helbich, T.H. Adjunct Ultrasonography for Breast Cancer Screening in Women At Average Risk: A Systematic Review. Int. J. Evid. Based Healthc. 2013, 11, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.Z. Methods Used in Computer-Aided Diagnosis for Breast Cancer Detection Using Mammograms: A Review. J. Healthc. Eng. 2020, 2020, 9162464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sala, M.; Domingo, L.; Macià, F.; Comas, M.; Burón, A.; Castells, X. Does Digital Mammography Suppose An Advance in Early Diagnosis? Trends in Performance Indicators 6 Years After Digitalization. Eur. Radiol. 2015, 25, 850–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schettini, F.; Giuliano, M.; De Placido, S.; Arpino, G. Nab-Paclitaxel for the Treatment of Triple-Negative Breast Cancer: Rationale, Clinical Data and Future Perspectives. Cancer Treat. Rev. 2016, 50, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Saadatmand, S.; Geuzinge, H.A.; Rutgers, E.J.; Mann, R.M.; Van Zuidewijn, D.B.D.R.; Zonderland, H.M.; Tollenaar, R.A.; Lobbes, M.B.; Ausems, M.G.; Van’t Riet, M. Mri Versus Mammography for Breast Cancer Screening in Women with Familial Risk (Famrisc): A Multicentre, Randomised, Controlled Trial. Lancet Oncol. 2019, 20, 1136–1147. [Google Scholar] [CrossRef] [Green Version]
- Jochelson, M. Contrast-Enhanced Digital Mammography. Radiol. Clin. 2014, 52, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Lee-Felker, S.A.; Tekchandani, L.; Thomas, M.; Gupta, E.; Andrews-Tang, D.; Roth, A.; Sayre, J.; Rahbar, G. Newly Diagnosed Breast Cancer: Comparison of Contrast-Enhanced Spectral Mammography and Breast Mr Imaging in the Evaluation of Extent of Disease. Radiology 2017, 285, 389–400. [Google Scholar] [CrossRef] [Green Version]
- Mann, R.M.; Hooley, R.; Barr, R.G.; Moy, L. Novel Approaches to Screening for Breast Cancer. Radiology 2020, 297, 266–285. [Google Scholar] [CrossRef] [PubMed]
- Niikura, N.; Odisio, B.C.; Tokuda, Y.; Symmans, F.W.; Hortobagyi, G.N.; Ueno, N.T. Latest Biopsy Approach for Suspected Metastases in Patients with Breast Cancer. Nat. Rev. Clin. Oncol. 2013, 10, 711–719. [Google Scholar] [CrossRef] [PubMed]
- Tagliafico, A.S.; Piana, M.; Schenone, D.; Lai, R.; Massone, A.M.; Houssami, N.J. Overview of Radiomics in Breast Cancer Diagnosis and Prognostication. Breast 2020, 49, 74–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tran, W.T.; Jerzak, K.; Lu, F.-I.; Klein, J.; Tabbarah, S.; Lagree, A.; Wu, T.; Rosado-Mendez, I.; Law, E.; Saednia, K.; et al. Personalized Breast Cancer Treatments Using Artificial Intelligence in Radiomics and Pathomics. J. Med. Imaging Radiat. Sci. 2019, 50, S32–S41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forghani, R.; Savadjiev, P.; Chatterjee, A.; Muthukrishnan, N.; Reinhold, C.; Forghani, B. Radiomics and Artificial Intelligence for Biomarker and Prediction Model Development in Oncology. Comput. Struct. Biotechnol. J. 2019, 17, 995. [Google Scholar] [CrossRef]
- Gupta, R.; Kurc, T.; Sharma, A.; Almeida, J.S.; Saltz, J. The Emergence of Pathomics. Curr. Pathobiol. Rep. 2019, 7, 73–84. [Google Scholar] [CrossRef] [Green Version]
- Hendrix, N.; Hauber, B.; Lee, C.I.; Bansal, A.; Veenstra, D.L. Artificial Intelligence in Breast Cancer Screening: Primary Care Provider Preferences. J. Am. Med. Inform. Assoc. 2021, 28, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, A.; Lång, K.; Gubern-Merida, A.; Broeders, M.; Gennaro, G.; Clauser, P.; Helbich, T.H.; Chevalier, M.; Tan, T.; Mertelmeier, T. Stand-Alone Artificial Intelligence for Breast Cancer Detection in Mammography: Comparison with 101 Radiologists. J. Nat. Cancer Inst. 2019, 111, 916–922. [Google Scholar] [CrossRef]
- Scimeca, M.; Urbano, N.; Toschi, N.; Bonanno, E.; Schillaci, O. Precision Medicine in Breast Cancer: From Biological Imaging to Artificial Intelligence. Semin. Cancer Biol. 2021, 72, 19. [Google Scholar] [CrossRef]
- Sechopoulos, I.; Teuwen, J.; Mann, R. Artificial Intelligence for Breast Cancer Detection in Mammography and Digital Breast Tomosynthesis: State of the Art. Semin. Cancer Biol. 2021, 72, 214–225. [Google Scholar] [CrossRef]
- Masaoutis, C.; Mihailidou, C.; Tsourouflis, G.; Theocharis, S. Exosomes in Lung Cancer Diagnosis and Treatment. From the Translating Research Into Future Clinical Practice. Biochimie 2018, 151, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Y.; Du, S.L.; Zhang, J.; Liang, A.L.; Liu, Y.J. Exosomes and Breast Cancer: A Comprehensive Review of Novel Therapeutic Strategies from Diagnosis to Treatment. Cancer Gene Ther. 2017, 24, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Hasan, A.; Nejadi Babadaei, M.M.; Behzadi, E.; Nouri, M.; Sharifi, M.; Falahati, M. Exosomes: Multiple-Targeted Multifunctional Biological Nanoparticles in the Diagnosis, Drug Delivery, and Imaging of Cancer Cells. Biomed. Pharmacother. 2020, 129, 110442. [Google Scholar] [CrossRef] [PubMed]
- Hanjani, N.A.; Esmaelizad, N.; Zanganeh, S.; Gharavi, A.T.; Heidarizadeh, P.; Radfar, M.; Omidi, F.; Macloughlin, R.; Doroudian, M. Emerging Role of Exosomes As Biomarkers in Cancer Treatment and Diagnosis. Crit. Rev. Oncol. Hematol. 2022, 169, 103565. [Google Scholar] [CrossRef] [PubMed]
- Jafari, S.H.; Saadatpour, Z.; Salmaninejad, A.; Momeni, F.; Mokhtari, M.; Nahand, J.S.; Rahmati, M.; Mirzaei, H.; Kianmehr, M. Breast Cancer Diagnosis: Imaging Techniques and Biochemical Markers. J. Cell. Physiol. 2018, 233, 5200–5213. [Google Scholar] [CrossRef]
- Jia, Y.; Chen, Y.; Wang, Q.; Jayasinghe, U.; Luo, X.; Wei, Q.; Wang, J.; Xiong, H.; Chen, C.; Xu, B. Exosome: Emerging Biomarker in Breast Cancer. Oncotarget 2017, 8, 41717. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Hu, J.; Hu, G. Biomarker Studies in Early Detection and Prognosis of Breast Cancer. In Translational Research in Breast Cancer: Biomarker Diagnosis, Targeted Therapies and Approaches to Precision Medicine; Springer International Publishing: Cham, Switzerland, 2017; pp. 27–39. [Google Scholar] [CrossRef]
- Barzaman, K.; Moradi-Kalbolandi, S.; Hosseinzadeh, A.; Kazemi, M.H.; Khorramdelazad, H.; Safari, E.; Farahmand, L.J.I.I. Breast Cancer Immunotherapy: Current and Novel Approaches. Int. Immunopharmacol. 2021, 98, 107886. [Google Scholar] [CrossRef]
- Hristova, V.A.; Chan, D.W. Cancer Biomarker Discovery and Translation: Proteomics and Beyond. Expert Rev. Proteom. 2019, 16, 93–103. [Google Scholar] [CrossRef]
- Afzal, M.; Alharbi, K.S.; Alruwaili, N.K.; Al-Abassi, F.A.; Al-Malki, A.A.L.; Kazmi, I.; Kumar, V.; Kamal, M.A.; Nadeem, M.S.; Aslam, M.; et al. Nanomedicine in Treatment of Breast Cancer—A Challenge to Conventional Therapy. Semin. Cancer Biol. 2021, 69, 279–292. [Google Scholar] [CrossRef]
- Hashemzadeh, N.; Dolatkhah, M.; Adibkia, K.; Aghanejad, A.; Barzegar-Jalali, M.; Omidi, Y.; Barar, J. Recent Advances in Breast Cancer Immunotherapy: The Promising Impact of Nanomedicines. Life Sci. 2021, 271, 119110. [Google Scholar] [CrossRef]
- Boix-Montesinos, P.; Soriano-Teruel, P.M.; Armiñán, A.; Orzáez, M.; Vicent, M. The Past, Present, and Future of Breast Cancer Models for Nanomedicine Development. Adv. Drug Deliv. Rev. 2021, 173, 306–330. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Dong, Z.; Chen, M.; Feng, L. Phenolic Molecules Constructed Nanomedicine for Innovative Cancer Treatment. Coord. Chem. Rev. 2021, 439, 213912. [Google Scholar] [CrossRef]
- Zhang, C.; Yan, L.; Wang, X.; Zhu, S.; Chen, C.; Gu, Z.; Zhao, Y. Progress, Challenges, and Future of Nanomedicine. Nano Today 2020, 35, 101008. [Google Scholar] [CrossRef]
- Nuzhina, J.V.; Shtil, A.A.; Prilepskii, A.Y.; Vinogradov, V.V. Preclinical Evaluation and Clinical Translation of Magnetite-Based Nanomedicines. J. Drug Deliv. Sci. Technol. 2019, 54, 101282. [Google Scholar] [CrossRef]
- Sousa, C.; Cruz, M.; Neto, A.; Pereira, K.; Peixoto, M.; Bastos, J.; Henriques, M.; Roda, D.; Marques, R.; Miranda, C.; et al. Neoadjuvant Radiotherapy in the Approach of Locally Advanced Breast Cancer. Ann. Oncol. 2020, 5, E000640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montagna, G.; Ferraro, E.; Pilewskie, M.L. Neoadjuvant Chemotherapy for Nonmetastatic Breast Cancer: How Response Impacts Locoregional and Adjuvant Systemic Therapy Decision Making. Adv. Oncol. 2022, 2, 47–61. [Google Scholar] [CrossRef]
- Ataseven, B.; Von Minckwitz, G. The Impact of Neoadjuvant Treatment on Surgical Options and Outcomes. Ann. Surg. Oncol. 2016, 23, 3093–3099. [Google Scholar] [CrossRef]
- Dastjerd, N.T.; Valibeik, A.; Rahimi Monfared, S.; Goodarzi, G.; Moradi Sarabi, M.; Hajabdollahi, F.; Maniati, M.; Amri, J.; Tehrani, S.S. Gene Therapy: A Promising Approach for Breast Cancer Treatment. Cell Biochem. 2022, 40, 28–48. [Google Scholar] [CrossRef]
- Cao, K.; Abbassi, L.; Romano, E.; Kirova, Y. Radiation Therapy and Immunotherapy in Breast Cancer Treatment: Preliminary Data and Perspectives. Expert Rev. Anticancer Ther. 2021, 21, 501–510. [Google Scholar] [CrossRef]
- Orecchia, R. Radiation Therapy for Inflammatory Breast Cancer. Eur. J. Surg. Oncol. 2018, 44, 1148–1150. [Google Scholar] [CrossRef]
- García-Aranda, M.; Redondo, M. Immunotherapy: A Challenge of Breast Cancer Treatment. Cancers 2019, 11, 1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexander, C.A.; Yang, Y.Y. Harnessing the Combined Potential of Cancer Immunotherapy and Nanomedicine: A New Paradigm in Cancer Treatment. Nanomed. Nanotechnol. Biol. Med. 2022, 40, 102492. [Google Scholar] [CrossRef] [PubMed]
- Amaoui, B.; Lalya, I.; Safini, F.; Semghouli, S. Combination of Immunotherapy-Radiotherapy in Non-Small Cell Lung Cancer: Reality and Perspective. Radiat. Med. Prot. 2021, 2, 160–164. [Google Scholar] [CrossRef]
- Jafarzadeh, L.; Khakpoor-Koosheh, M.; Mirzaei, H.; Mirzaei, H.R. Biomarkers for Predicting the Outcome of Various Cancer Immunotherapies. Crit. Rev. Oncol. Hematol. 2021, 157, 103161. [Google Scholar] [CrossRef] [PubMed]
- Disis, M.L.; Stanton, S.E. Immunotherapy in Breast Cancer: An Introduction. Breast 2018, 37, 196–199. [Google Scholar] [CrossRef] [Green Version]
- Skala, M.C.; Ayuso, J.M.; Burkard, M.E.; Deming, D.A. Breast Cancer Immunotherapy: Current Biomarkers and the Potential of in Vitro Assays. Curr. Opin. Biomed. Eng. 2022, 21, 100348. [Google Scholar] [CrossRef]
- Borgers, J.S.W.; Heimovaara, J.H.; Cardonick, E.; Dierickx, D.; Lambertini, M.; Haanen, J.B.A.G.; Amant, F. Immunotherapy for Cancer Treatment During Pregnancy. Lancet Oncol. 2021, 22, E550–E561. [Google Scholar] [CrossRef]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Combating Breast Cancer Using Combination Therapy with 3 Phytochemicals: Piperine, Sulforaphane, and Thymoquinone. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef]
- Morales, M.A.G.; Rodríguez, R.B.; Cruz, J.R.S.; Teran, L.M. Overview of New Treatments with Immunotherapy for Breast Cancer and A Proposal of A Combination Therapy. Molecules 2020, 25, 5686. [Google Scholar] [CrossRef]
- Núñez, C.; Capelo, J.L.; Igrejas, G.; Alfonso, A.; Botana, L.M.; Lodeiro, C. An Overview of the Effective Combination Therapies for the Treatment of Breast Cancer. Biomaterials 2016, 97, 34–50. [Google Scholar] [CrossRef]
- Ma, J.; Li, X.; Zhang, Q.; Li, N.; Sun, S.; Zhao, S.; Zhao, Z.; Li, M. A Novel Treatment Strategy of Her2-Targeted Therapy in Combination with Everolimus for Hr+/Her2- Advanced Breast Cancer Patients with Her2 Mutations. Transl. Oncol. 2022, 21, 101444. [Google Scholar] [CrossRef]
- Ahmed, M.; Moussa, M.; Goldberg, S.N. Synergy in Cancer Treatment Between Liposomal Chemotherapeutics and Thermal Ablation. Chem. Phy. Lipids 2012, 165, 424–437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poel, H.G.; Bergh, R.C.N.; Briers, E.; Cornford, P.; Govorov, A.; Henry, A.M.; Lam, T.B.; Mason, M.D.; Rouvière, O.; Santis, M.D.; et al. Focal Therapy in Primary Localised Prostate Cancer: The European Association of Urology Position in 2018. Eur. Urol. 2018, 74, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Vogl, T.J.; Farshid, P.; Naguib, N.N.N.; Zangos, S. Thermal Ablation Therapies in Patients with Breast Cancer Liver Metastases: A Review. Eur. Radiol. 2013, 23, 797–804. [Google Scholar] [CrossRef] [PubMed]
- Voort, E.M.; Struik, G.M.; Birnie, E.; Moelker, A.; Verhoef, C.; Klem, T.M. Thermal Ablation As An Alternative for Surgical Resection of Small (≤2 cm) Breast Cancers: A Meta-Analysis. Clin. Breast Cancer 2021, 21, E715–E730. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; Auperin, A.; Hakime, A.; Cartier, V.; Tacher, V.; Otmezguine, Y.; Tselikas, L.; De Baere, T.; Deschamps, F. Percutaneous Thermal Ablation of Breast Cancer Metastases in Oligometastatic Patients. Cardiovasc. Interv. Radiol. 2016, 39, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Pusceddu, C.; Paliogiannis, P.; Nigri, G.; Fancellu, A. Cryoablation in the Management of Breast Cancer: Evidence to Date. Breast Cancer: Targets Ther. 2019, 11, 283. [Google Scholar] [CrossRef] [Green Version]
- Roubidoux, M.A.; Yang, W.; Stafford, R. Image-Guided Ablation in Breast Cancer Treatment. Tech. Vasc. Interv. Radiol. 2014, 17, 49–54. [Google Scholar] [CrossRef]
- Webb, H.; Lubner, M.G.; Hinshaw, J.L. Thermal Ablation. Semin. Roentgenol. 2011, 46, 133–141. [Google Scholar] [CrossRef]
- Chatterjee, A.; He, D.; Fan, X.; Wang, S.; Szasz, T.; Yousuf, A.; Pineda, F.; Antic, T.; Mathew, M.; Karczmar, G.S.; et al. Performance of Ultrafast Dce-Mri for Diagnosis of Prostate Cancer. Acad. Radiol. 2018, 25, 349–358. [Google Scholar] [CrossRef]
- Peiravi, M.; Eslami, H.; Ansari, M.; Zare-Zardini, H. Magnetic Hyperthermia: Potentials and Limitations. J. Ind. Chem. Soc. 2022, 99, 100269. [Google Scholar] [CrossRef]
- Jose, J.; Kumar, R.; Harilal, S.; Mathew, G.E.; Parambi, D.G.T.; Prabhu, A.; Uddin, M.; Aleya, L.; Kim, H.; Mathew, B. Magnetic Nanoparticles for Hyperthermia in Cancer Treatment: An Emerging Tool. Environ. Sci. Pollut. Res. 2020, 27, 19214–19225. [Google Scholar] [CrossRef] [PubMed]
- Ezzati, M.; Yousefi, B.; Velaei, K.; Safa, A. A Review on Anti-Cancer Properties of Quercetin in Breast Cancer. Life Sci. 2020, 248, 117463. [Google Scholar] [CrossRef] [PubMed]
- Binienda, A.; Ziolkowska, S.; Pluciennik, E. The Anticancer Properties of Silibinin: Its Molecular Mechanism and Therapeutic Effect in Breast Cancer. Anticancer Agents Med. Chem. 2020, 20, 1787–1796. [Google Scholar] [CrossRef]
- Bonofiglio, D.; Giordano, C.; Francesca, A.; Lanzino, M.; Sebastiano, A. Natural Products As Promising Antitumoral Agents in Breast Cancer: Mechanisms of Action and Molecular Targets. Mini Rev. Med. Chem. 2016, 16, 596–604. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Koutras, A.; Syllaios, A.; Schizas, D.; Garmpis, N.; Diakosavvas, M.; Angelou, K.; Tsatsaris, G.; Pagkalos, A.; Ntounis, T.; et al. Breast Cancer Apoptosis and the Therapeutic Role of Luteolin. Chirurgia 2021, 116, 170–177. [Google Scholar] [CrossRef]
- Cheuk, I.W.; Chen, J.; Siu, M.; Ho, J.C.; Lam, S.S.; Shin, V.Y.; Kwong, A.A. Resveratrol Enhanced Chemosensitivity by Reversing Macrophage Polarization in Breast Cancer. Clin. Transl. Oncol. 2022, 24, 854–863. [Google Scholar] [CrossRef]
- Sharma, P.; Mcclees, S.F.; Afaq, F. Pomegranate for Prevention and Treatment of Cancer: An Update. Molecules 2017, 22, 177. [Google Scholar] [CrossRef] [Green Version]
- Rappuoli, R.; Pizza, M.; Del Giudice, G.; De Gregorio, E. Vaccines, New Opportunities for A New Society. Proc. Natl. Acad. Sci. USA 2014, 111, 12288–12293. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, Y.; Sasada, T. Cancer Vaccines: Toward the Next Breakthrough in Cancer Immunotherapy. J. Immunol. Res. 2020, 2020, 5825401. [Google Scholar] [CrossRef]
- Smith, R.T. Immune Surveillance; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Bitton, R.J. Cancer Vaccines: A Critical Review on Clinical Impact. Curr. Opin. Mol. Ther. 2004, 6, 17–26. [Google Scholar] [PubMed]
- Starling, S. Immune Editing Shapes the Cancer Landscape. Nat. Rev. Immunol. 2017, 17, 729. [Google Scholar] [CrossRef] [PubMed]
- Hegde, P.S.; Chen, D.S. Top 10 Challenges in Cancer Immunotherapy. Immunity 2020, 52, 17–35. [Google Scholar] [CrossRef]
- Waldman, A.D.; Fritz, J.M.; Lenardo, M.J. A Guide to Cancer Immunotherapy: From T Cell Basic Science to Clinical Practice. Nat. Rev. Immunol. 2020, 20, 651–668. [Google Scholar] [CrossRef] [PubMed]
- Mccarthy, E.F. The Toxins of William B. Coley and the Treatment of Bone and Soft-Tissue Sarcomas. Iowa Orthop. J. 2006, 26, 154–158. [Google Scholar]
- Grimmett, E.; Al-Share, B.; Alkassab, M.B.; Zhou, R.W.; Desai, A.; Rahim, M.M.A.; Woldie, I. Cancer Vaccines: Past, Present and Future; A Review Article. Discov. Oncol. 2022, 13, 31. [Google Scholar] [CrossRef]
- Sela, M.; Mozes, E. Therapeutic Vaccines in Autoimmunity. Proc. Natl. Acad. Sci. USA 2004, 101 (Suppl. S2), 14586–14592. [Google Scholar] [CrossRef] [Green Version]
- Guo, C.; Manjili, M.H.; Subjeck, J.R.; Sarkar, D.; Fisher, P.B.; Wang, X.Y. Therapeutic Cancer Vaccines: Past, Present, and Future. Adv. Cancer Res. 2013, 119, 421–475. [Google Scholar] [CrossRef] [Green Version]
- Pan, C.; Yue, H.; Zhu, L.; Ma, G.-H.; Wang, H.-L. Prophylactic Vaccine Delivery Systems Against Epidemic Infectious Diseases. Adv. Drug Deliv. Rev. 2021, 176, 113867. [Google Scholar] [CrossRef]
- Burke, E.E.; Kodumudi, K.; Ramamoorthi, G.; Czerniecki, B.J. Vaccine Therapies for Breast Cancer. Surg. Oncol. Clin. 2019, 28, 353–367. [Google Scholar] [CrossRef]
- Stern, P.L. Key Steps in Vaccine Development. Ann. Allergy Asthma Immunol. 2020, 125, 17–27. [Google Scholar] [CrossRef]
- Desai, R.; Coxon, A.T.; Dunn, G.P. Therapeutic Applications of the Cancer Immunoediting Hypothesis. Semin. Cancer Biol. 2022, 78, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Alves-Fernandes, D.K.; Jasiulionis, M.G. The Role of Sirt1 on Dna Damage Response and Epigenetic Alterations in Cancer. Int. J. Mol. Sci. 2019, 20, 3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saito, E.; Kuo, R.; Kramer, K.R.; Gohel, N.; Giles, D.A.; Moore, B.B.; Miller, S.D.; Shea, L.D. Design of Biodegradable Nanoparticles to Modulate Phenotypes of Antigen-Presenting Cells for Antigen-Specific Treatment of Autoimmune Disease. Biomaterials 2019, 222, 119432. [Google Scholar] [CrossRef] [PubMed]
- Shaaban, M.; Othman, H.; Ibrahim, T.; Ali, M.; Abdelmoaty, M.; Abdel-Kawi, A.-R.; Mostafa, A.; El Nakeeb, A.; Emam, H.; Refaat, A. Immune Checkpoint Regulators: A New Era Toward Promising Cancer Therapy. Curr. Cancer Drug Targets 2020, 20, 429–460. [Google Scholar] [CrossRef]
- Dersh, D.; Hollý, J.; Yewdell, J.W. A Few Good Peptides: Mhc Class I-Based Cancer Immunosurveillance and Immunoevasion. Nat. Rev. Immunol. 2021, 21, 116–128. [Google Scholar] [CrossRef]
- Johnson, A.M.; Bullock, B.L.; Neuwelt, A.J.; Poczobutt, J.M.; Kaspar, R.E.; Li, H.Y.; Kwak, J.W.; Hopp, K.; Weiser-Evans, M.C.; Heasley, L.E. Cancer Cell–Intrinsic Expression of Mhc Class Ii Regulates the Immune Microenvironment and Response to Anti–Pd-1 Therapy in Lung Adenocarcinoma. J. Immunol. 2020, 204, 2295–2307. [Google Scholar] [CrossRef]
- Criscitiello, C. Tumor-Associated Antigens in Breast Cancer. Breast Care 2012, 7, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Fracol, M.; Shah, N.; Dolivo, D.; Hong, S.; Giragosian, L.; Galiano, R.; Mustoe, T.; Kim, J. Can Breast Implants Induce Breast Cancer Immunosurveillance? An Analysis of Antibody Response to Breast Cancer Antigen Following Implant Placement. Plast. Reconstr. Surg. 2021, 148, 287–298. [Google Scholar] [CrossRef]
- Huber, V.; Camisaschi, C.; Berzi, A.; Ferro, S.; Lugini, L.; Triulzi, T.; Tuccitto, A.; Tagliabue, E.; Castelli, C.; Rivoltini, L. Cancer Acidity: An Ultimate Frontier of Tumor Immune Escape and A Novel Target of Immunomodulation. Semin. Cancer Biol. 2017, 43, 74–89. [Google Scholar] [CrossRef]
- Shimizu, K.; Iyoda, T.; Okada, M.; Yamasaki, S.; Fujii, S.I. Immune Suppression and Reversal of the Suppressive Tumor Microenvironment. Int. Immunol. 2018, 30, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.Y.; Flavell, R.A. ‘Yin-Yang’ Functions of Transforming Growth Factor-Beta and T Regulatory Cells in Immune Regulation. Immunol. Rev. 2007, 220, 199–213. [Google Scholar] [CrossRef] [PubMed]
- Cornel, A.M.; Mimpen, I.L.; Nierkens, S. Mhc Class I Downregulation in Cancer: Underlying Mechanisms and Potential Targets for Cancer Immunotherapy. Cancers 2020, 12, 1760. [Google Scholar] [CrossRef] [PubMed]
- Guerrouahen, B.S.; Maccalli, C.; Cugno, C.; Rutella, S.; Akporiaye, E.T. Reverting Immune Suppression to Enhance Cancer Immunotherapy. Front. Oncol. 2020, 9, 1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oshi, M.; Asaoka, M.; Tokumaru, Y.; Angarita, F.A.; Yan, L.; Matsuyama, R.; Zsiros, E.; Ishikawa, T.; Endo, I.; Takabe, K. Abundance of Regulatory T Cell (Treg) As A Predictive Biomarker for Neoadjuvant Chemotherapy in Triple-Negative Breast Cancer. Cancers 2020, 12, 3038. [Google Scholar] [CrossRef]
- Fasoulakis, Z.; Kolios, G.; Papamanolis, V.; Kontomanolis, E.N. Interleukins Associated with Breast Cancer. Cureus 2018, 10, E3549. [Google Scholar] [CrossRef] [Green Version]
- Van Den Hove, L.E.; Vandenberghe, P.; Van Gool, S.W.; Ceuppens, J.L.; Demuynck, H.; Verhoef, G.E.; Boogaerts, M.A. Peripheral Blood Lymphocyte Subset Shifts in Patients with Untreated Hematological Tumors: Evidence for Systemic Activation of the T Cell Compartment. Leuk. Res. 1998, 22, 175–184. [Google Scholar] [CrossRef]
- Schütz, F.; Stefanovic, S.; Mayer, L.; Von Au, A.; Domschke, C.; Sohn, C. Pd-1/Pd-L1 Pathway in Breast Cancer. Oncol. Res. Treat. 2017, 40, 294–297. [Google Scholar] [CrossRef]
- Planes-Laine, G.; Rochigneux, P.; Bertucci, F.; Chrétien, A.S.; Viens, P.; Sabatier, R.; Gonçalves, A. Pd-1/Pd-L1 Targeting in Breast Cancer: The First Clinical Evidences Are Emerging. A Literature Review. Cancers 2019, 11, 1033. [Google Scholar] [CrossRef] [Green Version]
- Lü, M.-H.; Liao, Z.-L.; Zhao, X.-Y.; Fan, Y.-H.; Lin, X.-L.; Fang, D.-C.; Guo, H.; Yang, S.-M. Htert-Based Therapy: A Universal Anticancer Approach. Oncol. Rep. 2012, 28, 1945–1952. [Google Scholar] [CrossRef] [Green Version]
- Krishnamurti, U.; Silverman, J.F. Her2 in Breast Cancer: A Review and Update. Adv. Anat. Pathol. 2014, 21, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Shastry, M.; Hamilton, E. Targeting Her2-Positive Breast Cancer: Advances and Future Directions. Nat. Rev. Drug Discov. 2022, 22, 101–126. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sáez, O.; Prat, A. Current and Future Management of Her2-Positive Metastatic Breast Cancer. J. Oncol. Pract. 2021, 17, 594–604. [Google Scholar] [CrossRef] [PubMed]
- Mitri, Z.; Constantine, T.; O’regan, R. The Her2 Receptor in Breast Cancer: Pathophysiology, Clinical Use, and New Advances in Therapy. Chemother. Res. Pract. 2012, 2012, 743193. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Du, C.W.; Kwan, M.; Liang, S.X.; Zhang, G.J. The Impact of P53 in Predicting Clinical Outcome of Breast Cancer Patients with Visceral Metastasis. Sci. Rep. 2013, 3, 2246. [Google Scholar] [CrossRef] [Green Version]
- Berke, T.P.; Slight, S.H.; Hyder, S.M. Role of Reactivating Mutant P53 Protein in Suppressing Growth and Metastasis of Triple-Negative Breast Cancer. Onco Targets Ther. 2022, 15, 23. [Google Scholar] [CrossRef]
- Gasco, M.; Shami, S.; Crook, T. The P53 Pathway in Breast Cancer. Breast Cancer Res. 2002, 4, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Duffy, M.J.; Synnott, N.C.; Crown, J. Mutant P53 in Breast Cancer: Potential As A Therapeutic Target and Biomarker. Breast Cancer Res. Treat. 2018, 170, 213–219. [Google Scholar] [CrossRef]
- Kufe, D.W. Muc1-C Oncoprotein As A Target in Breast Cancer: Activation of Signaling Pathways and Therapeutic Approaches. Oncogene 2013, 32, 1073–1081. [Google Scholar] [CrossRef] [Green Version]
- Zaretsky, J.Z.; Barnea, I.; Aylon, Y.; Gorivodsky, M.; Wreschner, D.H.; Keydar, I. Muc1 Gene Overexpressed in Breast Cancer: Structure and Transcriptional Activity of the Muc1 Promoter and Role of Estrogen Receptor Alpha (Erα) in Regulation of the Muc1 Gene Expression. Mol. Cancer 2006, 5, 57. [Google Scholar] [CrossRef] [Green Version]
- Taylor-Papadimitriou, J.; Burchell, J.; Miles, D.W.; Dalziel, M. Muc1 and Cancer. Biochim. Biophys. Acta Mol. Basis Dis. 1999, 1455, 301–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guillen-Poza, P.A.; Sánchez-Fernández, E.M.; Artigas, G.; García Fernández, J.M.; Hinou, H.; Ortiz Mellet, C.; Nishimura, S.-I.; Garcia-Martin, F. Amplified Detection of Breast Cancer Autoantibodies Using Muc1-Based Tn Antigen Mimics. J. Med. Chem. 2020, 63, 8524–8533. [Google Scholar] [CrossRef] [PubMed]
- Anoop, T.M.; Joseph, P.R.; Soman, S.; Chacko, S.; Mathew, M. Significance of Serum Carcinoembryonic Antigen in Metastatic Breast Cancer Patients: A Prospective Study. World J. Clin. Oncol. 2022, 13, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Kabel, A.M. Tumor Markers of Breast Cancer: New Prospectives. J. Oncol. Sci. 2017, 3, 5–11. [Google Scholar] [CrossRef]
- Imamura, M.; Morimoto, T.; Nomura, T.; Michishita, S.; Nishimukai, A.; Higuchi, T.; Fujimoto, Y.; Miyagawa, Y.; Kira, A.; Murase, K.; et al. Independent Prognostic Impact of Preoperative Serum Carcinoembryonic Antigen and Cancer Antigen 15-3 Levels for Early Breast Cancer Subtypes. World J. Surg. Oncol. 2018, 16, 26. [Google Scholar] [CrossRef] [Green Version]
- Kirkpatrick, K.L.; Ogunkolade, W.; Elkak, A.E.; Bustin, S.; Jenkins, P.; Ghilchick, M.; Newbold, R.F.; Mokbel, K. Htert Expression in Human Breast Cancer and Non-Cancerous Breast Tissue: Correlation with Tumour Stage and C-Myc Expression. Breast Cancer Res. Treat. 2003, 77, 277–284. [Google Scholar] [CrossRef]
- Dratwa, M.; Wysoczańska, B.; Łacina, P.; Kubik, T.; Bogunia-Kubik, K. Tert—Regulation and Roles in Cancer Formation. Front. Immunol. 2020, 11, 589929. [Google Scholar] [CrossRef]
- Kirkpatrick, K.L.; Clark, G.; Ghilchick, M.; Newbold, R.F.; Mokbel, K. Htert Mrna Expression Correlates with Telomerase Activity in Human Breast Cancer. Eur. J. Surg. Oncol. 2003, 29, 321–326. [Google Scholar] [CrossRef] [Green Version]
- Davodabadi, F.; Sarhadi, M.; Arabpour, J.; Sargazi, S.; Rahdar, A.; Díez-Pascual, A.M. Breast Cancer Vaccines: New Insights Into Immunomodulatory and Nano-Therapeutic Approaches. J. Control. Release 2022, 349, 844–875. [Google Scholar] [CrossRef]
- Pallerla, S.; Abdul, A.U.R.M.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on Her2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Koido, S. Dendritic-Tumor Fusion Cell-Based Cancer Vaccines. Int. J. Mol. Sci. 2016, 17, 828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzzubbo, S.; Mangsbo, S.; Nagarajan, D.; Habra, K.; Pockley, A.G.; Mcardle, S.E.B. Cancer Vaccines: Adjuvant Potency, Importance of Age, Lifestyle, and Treatments. Front. Immunol. 2021, 11, 615240. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.-Y.; Yu, K.-D. Breast Cancer Vaccines: Disappointing Or Promising? Front. Immunol. 2022, 13, 828386. [Google Scholar] [CrossRef]
- De Paula Peres, L.; Da Luz, F.A.C.; Dos Anjos Pultz, B.; Brígido, P.C.; De Araújo, R.A.; Goulart, L.R.; Silva, M.J.B. Peptide Vaccines in Breast Cancer: The Immunological Basis for Clinical Response. Biotechnol. Adv. 2015, 33, 1868–1877. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, N.; Zhang, W.; Cheng, X.; Yan, Z.; Shao, G.; Wang, X.; Wang, R.; Fu, C. Therapeutic Peptides: Current Applications and Future Directions. Signal Transduct. Target. Ther. 2022, 7, 48. [Google Scholar] [CrossRef]
- Nicolás-Morales, M.L.; Luisa-Sanjuan, A.; Gutiérrez-Torres, M.; Vences-Velázquez, A.; Ortuño-Pineda, C.; Espinoza-Rojo, M.; Navarro-Tito, N.; Cortés-Sarabia, K. Peptide-Based Vaccines in Clinical Phases and New Potential Therapeutic Targets As A New Approach for Breast Cancer: A Review. Vaccines 2022, 10, 1249. [Google Scholar] [CrossRef]
- Liu, J.; Fu, M.; Wang, M.; Wan, D.; Wei, Y.; Wei, X. Cancer Vaccines As Promising Immuno-Therapeutics: Platforms and Current Progress. J. Hematol. Oncol. 2022, 15, 28. [Google Scholar] [CrossRef]
- Corti, C.; Giachetti, P.P.M.B.; Eggermont, A.M.M.; Delaloge, S.; Curigliano, G. Therapeutic Vaccines for Breast Cancer: Has the Time Finally Come? Eur. J. Cancer 2022, 160, 150–174. [Google Scholar] [CrossRef]
- Kang, J.; Lee, H.-J.; Lee, J.; Hong, J.; Hong Kim, Y.; Disis, M.L.; Gim, J.-A.; Park, K.H. Novel Peptide-Based Vaccine Targeting Heat Shock Protein 90 Induces Effective Antitumor Immunity in A Her2+ Breast Cancer Murine Model. J. Immunother. Cancer 2022, 10, E004702. [Google Scholar] [CrossRef]
- Eavarone, D.A.; Al-Alem, L.; Lugovskoy, A.; Prendergast, J.M.; Nazer, R.I.; Stein, J.N.; Dransfield, D.T.; Behrens, J.; Rueda, B.R. Humanized Anti-Sialyl-Tn Antibodies for the Treatment of Ovarian Carcinoma. PLoS ONE 2018, 13, E0201314. [Google Scholar] [CrossRef]
- Munkley, J. The Role of Sialyl-Tn in Cancer. Int. Mol. Sci. 2016, 17, 275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorup-Jensen, T. On the Roles of Polyvalent Binding in Immune Recognition: Perspectives in the Nanoscience of Immunology and the Immune Response to Nanomedicines. Adv. Drug Deliv. Rev. 2012, 64, 1759–1781. [Google Scholar] [CrossRef] [PubMed]
- Ward, S.; Casey, D.; Labarthe, M.-C.; Whelan, M.; Dalgleish, A.; Pandha, H.; Todryk, S. Immunotherapeutic Potential of Whole Tumour Cells. Cancer Immunol. Immunother. 2002, 51, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Al-Awadhi, A.; Lee Murray, J.; Ibrahim, N.K. Developing Anti-Her2 Vaccines: Breast Cancer Experience. Int. J. Cancer 2018, 143, 2126–2132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitner, W.W.; Ying, H.; Restifo, N.P. Dna and Rna-Based Vaccines: Principles, Progress and Prospects. Vaccine 1999, 18, 765–777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fritah, H.; Rovelli, R.; Chiang, C.L.-L.; Kandalaft, L.E. The Current Clinical Landscape of Personalized Cancer Vaccines. Cancer Treat. Rev. 2022, 106, 102383. [Google Scholar] [CrossRef]
- Bird, R.C.; Deinnocentes, P.; Church Bird, A.E.; Lutful Kabir, F.M.; Martinez-Romero, E.G.; Smith, A.N.; Smith, B.F. Autologous Hybrid Cell Fusion Vaccine in A Spontaneous Intermediate Model of Breast Carcinoma. J. Vet. Sci. 2019, 20, E48. [Google Scholar] [CrossRef]
- Paston, S.J.; Brentville, V.A.; Symonds, P.; Durrant, L.G. Cancer Vaccines, Adjuvants, and Delivery Systems. Front. Immunol. 2021, 12, 627932. [Google Scholar] [CrossRef]
- Khong, H.; Overwijk, W.W. Adjuvants for Peptide-Based Cancer Vaccines. J. Immunother. Cancer 2016, 4, 56. [Google Scholar] [CrossRef] [Green Version]
- Dubensky, T.W.; Reed, S.G. Adjuvants for Cancer Vaccines. Semin. Immunol. 2010, 22, 155–161. [Google Scholar] [CrossRef]
- He, X.; Zhou, S.; Huang, W.-C.; Seffouh, A.; Mabrouk, M.T.; Morgan, M.T.; Ortega, J.; Abrams, S.I.; Lovell, J.F. A Potent Cancer Vaccine Adjuvant System for Particleization of Short, Synthetic Cd8+ T Cell Epitopes. Acs Nano 2021, 15, 4357–4371. [Google Scholar] [CrossRef] [PubMed]
- Bobanga, I.D.; Petrosiute, A.; Huang, A.Y. Chemokines As Cancer Vaccine Adjuvants. Vaccines 2013, 1, 444–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knutson, K.L.; Schiffman, K.; Disis, M.L. Immunization with A Her-2/Neu Helper Peptide Vaccine Generates Her-2/Neu Cd8 T-Cell Immunity in Cancer Patients. J. Clin. Investig. 2001, 107, 477–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malonis, R.J.; Lai, J.R.; Vergnolle, O. Peptide-Based Vaccines: Current Progress and Future Challenges. Chem. Rev. 2020, 120, 3210–3229. [Google Scholar] [CrossRef] [Green Version]
- Tobias, J.; Garner-Spitzer, E.; Drinić, M.; Wiedermann, U. Vaccination Against Her-2/Neu, with Focus on Peptide-Based Vaccines. Ann. Oncol. 2022, 7, 100361. [Google Scholar] [CrossRef] [PubMed]
- Nordin, M.L.; Mohamad Norpi, A.S.; Ng, P.Y.; Yusoff, K.; Abu, N.; Lim, K.P.; Azmi, F. Her2/Neu-Based Peptide Vaccination-Pulsed with B-Cell Epitope Induced Efficient Prophylactic and Therapeutic Antitumor Activities in Tubo Breast Cancer Mice Model. Cancers 2021, 13, 4958. [Google Scholar] [CrossRef]
- Pierini, S.; Perales-Linares, R.; Uribe-Herranz, M.; Pol, J.G.; Zitvogel, L.; Kroemer, G.; Facciabene, A.; Galluzzi, L. Trial Watch: Dna-Based Vaccines for Oncological Indications. Oncoimmunology 2017, 6, E1398878. [Google Scholar] [CrossRef]
- Benedetti, R.; Dell’aversana, C.; Giorgio, C.; Astorri, R.; Altucci, L. Breast Cancer Vaccines: New Insights. Front. Endocrinol. 2017, 8, 270. [Google Scholar] [CrossRef] [Green Version]
- Holmberg, L.A.; Sandmaier, B.M. Theratope® Vaccine (Stn-Klh). Expert Opin. Biol. Ther. 2001, 1, 881–891. [Google Scholar] [CrossRef]
- Kwa, M.J.; Adams, S. Checkpoint Inhibitors in Triple-Negative Breast Cancer (Tnbc): Where to Go from Here. Cancer 2018, 124, 2086–2103. [Google Scholar] [CrossRef] [Green Version]
- Emens, L.A.; Esteva, F.J.; Beresford, M.; Saura, C.; De Laurentiis, M.; Kim, S.-B.; Im, S.-A.; Wang, Y.; Salgado, R.; Mani, A.; et al. Trastuzumab Emtansine Plus Atezolizumab Versus Trastuzumab Emtansine Plus Placebo in Previously Treated, Her2-Positive Advanced Breast Cancer (Kate2): A Phase 2, Multicentre, Randomised, Double-Blind Trial. Lancet Oncol. 2020, 21, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Fares, J.; Kanojia, D.; Rashidi, A.; Ulasov, I.; Lesniak, M.S. Landscape of Combination Therapy Trials in Breast Cancer Brain Metastasis. Int. J. Cancer 2020, 147, 1939–1952. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, Y.; Sun, Y.; Torphy, R.J.; He, J.; Yanaga, K.; Edil, B.H.; Schulick, R.D.; Zhu, Y. Pomalidomide Inhibits Pd-L1 Induction to Promote Antitumor Immunity. Cancer Res. 2018, 78, 6655–6665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.; Beavis, P.A.; Li, J.; Darcy, P.K. Augmenting Adoptive T-Cell Immunotherapy by Targeting the Pd-1/Pd-L1 Axis. Cancer Res. 2021, 81, 5803–5805. [Google Scholar] [CrossRef]
- Roy, S.; Sethi, T.K.; Taylor, D.; Kim, Y.J.; Johnson, D.B. Breakthrough Concepts in Immune-Oncology: Cancer Vaccines At the Bedside. J. Leuk. Biol. 2020, 108, 1455–1489. [Google Scholar] [CrossRef]
- Crosby, E.J.; Acharya, C.R.; Haddad, A.-F.; Rabiola, C.A.; Lei, G.; Wei, J.-P.; Yang, X.-Y.; Wang, T.; Liu, C.-X.; Wagner, K.U.; et al. Stimulation of Oncogene-Specific Tumor-Infiltrating T Cells Through Combined Vaccine and Apd-1 Enable Sustained Antitumor Responses Against Established Her2 Breast Cancer. Clin. Cancer Res. 2020, 26, 4670–4681. [Google Scholar] [CrossRef] [PubMed]
- Gall, V.A.; Philips, A.V.; Qiao, N.; Clise-Dwyer, K.; Perakis, A.A.; Zhang, M.; Clifton, G.T.; Sukhumalchandra, P.; Ma, Q.; Reddy, S.M.; et al. Trastuzumab Increases Her2 Uptake and Cross-Presentation by Dendritic Cells. Cancer Res. 2017, 77, 5374–5383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, A.S.; Gärtner, F.; Vale, N. Drug Combination and Repurposing for Cancer Therapy: The Example of Breast Cancer. Heliyon 2021, 7, E05948. [Google Scholar] [CrossRef]
- Hodge, J.W.; Ardiani, A.; Farsaci, B.; Kwilas, A.R.; Gameiro, S.R. The Tipping Point for Combination Therapy: Cancer Vaccines with Radiation, Chemotherapy, Or Targeted Small Molecule Inhibitors. Semin. Oncol. 2012, 39, 323–339. [Google Scholar] [CrossRef] [Green Version]
- Nicolini, A.; Carpi, A.; Ferrari, P.; Mario Biava, P.; Rossi, G. Immunotherapy and Hormone-Therapy in Metastatic Breast Cancer: A Review and An Update. Curr. Drug Targets 2016, 17, 1127–1139. [Google Scholar] [CrossRef]
- Sertoli, M.R.; Scarsi, P.G.; Rosso, R. Rationale for Combining Chemotherapy and Hormonal Therapy in Breast Cancer. J. Steroid Biochem. 1985, 23, 1097–1103. [Google Scholar] [CrossRef]
- Mohit, E.; Hashemi, A.; Allahyari, M. Breast Cancer Immunotherapy: Monoclonal Antibodies and Peptide-Based Vaccines. Expert Rev. Clin. Immunol. 2014, 10, 927–961. [Google Scholar] [CrossRef] [PubMed]
- Wolfson, B.; Franks, S.E.; Hodge, J.W. Stay on Target: Reengaging Cancer Vaccines in Combination Immunotherapy. Vaccines 2021, 9, 509. [Google Scholar] [CrossRef] [PubMed]
- Mirjolet, C.; Truc, G. [Abscopal Effect: Myth Or Reality?]. Cancer Radiother. 2021, 25, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Sindoni, A.; Minutoli, F.; Ascenti, G.; Pergolizzi, S. Combination of Immune Checkpoint Inhibitors and Radiotherapy: Review of the Literature. Crit. Rev. Oncol. Hematol. 2017, 113, 63–70. [Google Scholar] [CrossRef]
- Antonarelli, G.; Corti, C.; Tarantino, P.; Ascione, L.; Cortes, J.; Romero, P.; Mittendorf, E.A.; Disis, M.L.; Curigliano, G. Therapeutic Cancer Vaccines Revamping: Technology Advancements and Pitfalls. Ann. Oncol. 2021, 32, 1537–1551. [Google Scholar] [CrossRef]
- Li, W.; Joshi, M.D.; Singhania, S.; Ramsey, K.H.; Murthy, A.K. Peptide Vaccine: Progress and Challenges. Vaccines 2014, 2, 515–536. [Google Scholar] [CrossRef] [Green Version]
- Smith, P.L.; Piadel, K.; Dalgleish, A.G. Directing T-Cell Immune Responses for Cancer Vaccination and Immunotherapy. Vaccines 2021, 9, 1392. [Google Scholar] [CrossRef]
- Robbins, P.F.; Rosenberg, S.A.; Zhu, S.; Feldman, S.A.; Morgan, R.A. T cell Receptors Recognizing hla-a1-Restricted Mage-a3. U.S. Patent 2022/0195008 A1, 23 June 2022. [Google Scholar]
- Gey, A.; Tartour, E.; Bechard, D.U.S. Il-15 and il-15ralpha sushi Domain BASED Modulokines. U.S. Patent 2022/0193199 A1, 23 June 2022. [Google Scholar]
- Chen, Y.; Huang, X.; Kim, S. Combination Therapy for the Treatment of Cancer. U.S. Patent 2022/0193079 A1, 23 June 2022. [Google Scholar]
- June, C.H.; Zhao, Y.U.S. Rna Engineered t Cells for the Treatment of. Cancer. Patent 2022/0170012 A1, 22 June 2022. [Google Scholar]
- Roberts, D.D.; Pantoja, D.R.S. Methods for Modulating Chemotherapeutic Cytotoxicity. U.S. Patent 2022/0184111 A1, 16 June 2022. [Google Scholar]
- Korman, A.J.; Lonberg, N.; Fontana, D.J.; Gutierrez, A.A.; Selby, M.J.; Lewis, K. Combination of Anti-LAG-3 Antibodies and Anti-PD-1 Antibodies to Treat Tumors. U.S. Patent 2022/0185892 A1, 16 June 2022. [Google Scholar]
- Mortimer, S.A.W.; Talasaz, A.A.; Chudova, D.; Eltoukhy, H. Methods for Early Detection of Cancer. U.S. Patent 2022/0186323 A1, 16 June 2022. [Google Scholar]
- Clube, J. Selectively Altering Microbiota for Immune Modulation. U.S. Patent 11,291,723 A2, 5 April 2022. [Google Scholar]
- Mahr, A.; Weinschenk, T.; Schoor, O.; Fritsche, J.; Singh, H.; Song, C. Novel Peptides and Combination of Peptides and Scaffolds for Use in Immunotherapy Against Renal Cell Carcinoma (rcc) and Other Cancers. U.S. Patent 2022/0040278 A1, 10 February 2022. [Google Scholar]
- Manie, E.; Stern, M.-H.; Popova, T. Methods for Detecting Inactivation of the Homologous Recombination Pathway (brca1/2) in Human Tumors. U.S. Patent 2022/0010385 A1, 13 January 2022. [Google Scholar]
- Cobleigh, M.A.; Shak, S.; Baker, J.B. Gene Expression Markers for Breast Cancer Prognosis. U.S. Patent 11,220,715 B2, 11 January 2022. [Google Scholar]
- Fan, T.W.-M.; Lane, A.N.; Higashi, R.M.; Bousamra, M. Methods Related to Cancer. U.S. Patent 2022/0003792 A1, 6 January 2022. [Google Scholar]
- Diehn, M.; Alizadeh, A.A.; Newman, A.M. Identification and Use of Circulating Nucleic Acids. U.S. Patent 11,085,084 B2, 10 August 2021. [Google Scholar]
- Chen, J.; Buggy, J.J.; Elias, L. Methods for the Treatment of her2 Amplified Cancer. U.S. Patent 2021/0145835 A1, 20 May 2021. [Google Scholar]
- Kuntz, K.W.; Huang, K.-C.; Wook, H. Salt Form of a Human Histone Methyltransferase ezh2 Inhibitor. U.S. Patent 2021/0137936 A1, 13 May 2021. [Google Scholar]
- Vaske, C.J.; Benz, S.C.; Stuart, J.M. Pathway Recognition Algorithm Using Data Integration on Genomic Models (Paradigm). U.S. Patent 10,991,448 B2, 27 April 2021. [Google Scholar]
- Hoon, D.; Taback, B.; Shaolian, S. Method and Apparatus for in Vivo Surveillance of Circulating Biological Components. U.S. Patent 10,987,037 B2, 27 April 2021. [Google Scholar]
- Fyfe, G.; Phan, S.C.; Zhou, X. Anti-Angiogenesis Therapy for the Treatment of Breast Cancer. U.S. Patent 2021/0093715 A1, 1 April 2021. [Google Scholar]
- Paton, V.; Chirchir, A.B.; Klein, P. Treatment of Metastic Breast Cancer. U.S. Patent 2021/0047429 A1, 18 February 2021. [Google Scholar]
- Chui, S.; Smitt, M.; Patre, M. Methods of Treating her2-Positive Cancer. U.S. Patent 2021/0040216 A1, 11 February 2021. [Google Scholar]
- Knudsen, S. Methods for Predicting Drug Responsiveness in Cancer Patients. U.S. Patent 10,907,214 B2, 2 February 2021. [Google Scholar]
- Xiao, L.; Pu, C.; Cao, Z. Use of Chimeric Antigen Receptor Modified Cells to Treat Cancer. U.S. Patent 2020/0385484 A1, 10 December 2020. [Google Scholar]
- Doucey, M.-A.; Guex, N.; Crespo, I. Adoptive Immunotherapy for Treating Cancer. U.S. Patent 10,858,626b2, 8 December 2020. [Google Scholar]
- Pierce, D.; Carleton, M. Combination Therapy Comprising Nanoparticles of a Taxane and Albumin with abt-263 in Methods for Treating Cancer. U.S. Patent 2020/0246275 A1, 6 August 2020. [Google Scholar]
- Das-Young, L.; Wilner, K.D.; Nicholas, S. Combination of a PD-1 Antagonist and an ALK Inhibitor for Treating Cancer. U.S. Patent 10,695,426 B2, 30 June 2020. [Google Scholar]
- Mahr, A.; Weinschenk, T.; Schoor, O. Novel Peptides and Combination of Peptides for Use in Immunotherapy and Methods for Generating Scaffolds for the Use Against Pancreatic Cancer and Other Cancers. U.S. Patent 2020/0157177 A1, 21 May 2020. [Google Scholar]
- Adusumilli, P.S.; Sadelain, M.; Dimitrov, D.S. Mesothelin-Targeted Chimeric Antigen Receptors and Uses Thereof. U.S. Patent 10,633,441b2, 28 April 2020. [Google Scholar]
- Wang, D.; Jiang, W.; Agrawal, S. Treatment of Cancer Using tlr9 Agonist with Checkpoint Inhibitors. U.S. Patent 2020/0101102 A1, 2 April 2020. [Google Scholar]
- Markovic, S.N.; Nevala, W.K. Methods of Using Albumin-Antibody Nanoparticle Complex Compositions for Treating Cancer. U.S. Patent 10,596,112 B2, 24 March 2020. [Google Scholar]
- O’Donnell, J.; Bylesjo, M.; Patterson, F. Molecular Diagnostic Test for Cancer. U.S. Patent 10,378,066 B2, 13 August 2019. [Google Scholar]
- Themeli, M.; Sadelain, M.; Kloss, C.C. Effective Generation of Tumor-Targeted T Cells DERIVED from Pluripotent stem Cells. U.S. Patent 10,370,452 B2, 6 August 2019. [Google Scholar]
- Kipps, T.J.; Yu, J.; Cui, B. Antibodies and Vaccines for Use in Treating ROR1 Cancers and Inhibiting Metastasis. U.S. Patent 10,344,096 B2, 9 July 2019. [Google Scholar]
- Jensen, M.C. Drug Regulated Transgene Expression. U.S. Patent 10,266,592 B2, 23 April 2019. [Google Scholar]
- Mahr, A.; Weinschenk, T.; Hoerzer, H. Peptides and Combination of Peptides for Use in Immunotherapy Against BREAST Cancer and other Cancers. U.S. Patent 10,213,499 B2, 26 February 2019. [Google Scholar]
- Elsner, J.; Harris, R.L.; Lee, B.G. Substituted Pyrazino[2,3-b]pyrazines as mTOR Kinase Inhibitors. U.S. Patent 10,167,290 B2, 1 January 2019. [Google Scholar]
- Brentjens, R.J.; Jackson, H.J. Compositions and Methods for Immunotherapy. U.S. Patent 10,124,023 B2, 13 November 2018. [Google Scholar]
- Gurney, A.L.; Lewicki, J.; Satyal, S.H. Compositions and Methods for Diagnosing and Treating Cancer. U.S. Patent 2018/0222997 A1, 9 August 2018. [Google Scholar]
- Eltoukhy, H.; Talasaz, A.A. Methods and Systems for Detecting Genetic Variants. U.S. Patent 9,920,366 B2, 20 March 2018. [Google Scholar]
| NCT Number | Antigens/Biological | Clinical Phase |
|---|---|---|
| NCT00854789 | E75 and GM-CSF | I |
| NCT00892567 | Her-2/neu; CEA and CTA | I |
| NCT02019524 | E39 and J65 peptides | I |
| NCT04270149 | ESR1 peptide vaccine | I |
| NCT04521764 | Helicobacter pylori neutrophil-activating protein | I |
| NCT00343109 | HER-2/neu | II |
| NCT02348320 | Personalised polyepitope DNA vaccine | I |
| NCT02018458 | LA TNBC; ER+/HER-BC | I/II |
| NCT04348747 | Anti-HER2/HER3 DC vaccine; Pembrolizumab | II |
| NCT02061423 | HER-2 pulsed DC vaccine | I |
| NCT01730118 | AdHER-2/neu DC vaccine | I |
| NCT00524277 | HER2-derived peptide GP2; GM-CSF | II |
| NCT01479244 | HER2-derived peptide E75; GM-CSF | I/II |
| NCT01570036 | HER2-derived peptide E75; GM-CSF; Trastuzumab | II |
| NCT00140738 | HER; AS 15 | I/II |
| NCT02061332 | HER; DC vaccine | II |
| NCT00399529 | HER2; GM-CSF; Cyclophosphamide; Trastuzumab | II |
| NCT01479244 | HER2-derived peptide E75; GM-CSF | III |
| S. No. | Patent Number | Date of Publication | Invention Disclosed |
|---|---|---|---|
| 1. | US 2022/0195008 A1 | 23 June 2022 | This invention disclosed the antigenic specificity of the T-cell receptor (TCR) for the melanoma antigen family. The polypeptide in the functional portion of the TCR was found to carry amino acid sequences of length 16–21 [203]. |
| 2. | US 2022/0193199 A1 | 23 June 2022 | This invention described an immune cytokine that is a conjugate and an immunomodulatory antibody. It comprised an interleukin15-containing polypeptide, which is an IL-15Ra sushi domain-containing polypeptide. The immunomodulatory antibody- or antigen-binding fragment was said to be capable of binding PD-1 and PD-L1/L2 [204]. |
| 3. | US 2022/0193079 A1 | 23 June 2022 | A pharmaceutical combination comprising a CDK inhibitor and an antihormonal agent that regulates the P13K/Akt/m TOR pathway or a pharmaceutically acceptable salt was discussed in the patent [205]. |
| 4. | US 2022/0170012 A1 | 22 June 2022 | This invention disclosed the compositions and methods of generating an RNA chimeric-antigen receptor of transfected T-cells for use in adoptive therapy for cancer. The method modified the 5′ end of the RNA or its 7-methyl guanosine cap by the addition of the 5′-end of the eukaryotic messenger, soon after the initiation of the transcription [206]. |
| 5. | US 2022/0184111 A1 | 16 June 2022 | Methods for reducing the cytotoxicity of chemotherapeutic agents towards non-cancer cells and increasing their cytotoxicity towards cancer cells were described in this patent. The patent gave details regarding the administration of an effective amount of the agent to achieve the inhibition of CD47 signalling for an effective chemotherapeutic agent [207]. |
| 6. | US 2022/0185892 A1 | 16 June 2022 | This patent disclosed a method for the treatment of a solid tumour through the administration of an effective quantity of some anti-LAG-3 and anti-PD1 antibodies that carry the CD-R1, -R2, and -R3 domains of the chain [208]. |
| 7. | US 2022/0186323 A1 | Jun16, 2022 | This invention discussed a method for detecting a tumour marker, which, in turn, indicated the presence of a cancer. The marker may be a sample of nucleic acid. The sample was then amplified by polymerase chain reaction, thereby enriching the nucleic acids for the detection of genomic regions [209]. |
| 8. | US 11,291,723B2 | 5 April 2022 | A treatment method for enhancing the efficacy of a therapy for cancer in humans and animals was presented in this invention. This treatment was selective in killing or reducing the growth of the target cell by using the enzyme- Cas nuclease. It was also related to cell populations, system, arrays, cells, RNA, and other means affecting the therapy [210]. |
| 9. | US 2022/0040278 A1 | 10 February 2022 | This patent disclosed cancer immunotherapy relating to tumour-associated T-cell peptide epitopes. The presence of peptides on the tested tissue sample biopsies helped to diagnose cancer. This patent also gave methods, such as antibody detection or spectrometry, for analysing peptides [211]. |
| 10. | US 2022/0010385 A1 | 13 January 2022 | This invention discussed a method to detect the inactivation of the DNA homologous recombination pathway, which helped to detect the BC gene, BRCA, or placental alkaline phosphatase, PALP (also called FANCN), inactivation, which was probably due to a somatic mutation or a mutation in the germ cell line [212]. |
| 11. | US 11,220,715B2 | 11 January 2022 | An expression of a set of genes as thera-prognostics for the disease-free survival of cancer patients was disclosed in this invention. It gives the use of a paraffin-embedded biopsy material that is compatible with different methods of tumour tissue harvest [213]. |
| 12. | US 2022/0003792 A1 | 6 January 2022 | This invention mentioned the methods to determine the presence or absence of a cancer type in an animal. The procedure determined the concentration of lipid amounts in a sample, which was obtained either from a detected cancer or a treated individual’s body fluid, using the mass spectrometric technique [214]. |
| 13. | US 11,085,084B2 | 10 August 2021 | This patent gave details regarding the identification and analysis of some polynucleotide adaptors, which include cell-free nucleic acids, from sample cancer patients. It also included their detection, diagnosis, and prognosis [215]. |
| 14. | US 2021/0145835 A1 | 20 May 2021 | A pharmaceutical composition that could be utilised for the treatment of patients diagnosed with HER2-positive BC was addressed. The pharmaceutical substance disclosed was (R)-1-(3-(4-amino-3-(4-phenoxyphenyl)-1H pyrazolo [3,4-d] pyrimidin-1-yl) piperidin-1-yl) prop-2-en-1-one [216]. |
| 15. | US 2021/0137936 A1 | 13 May 2021 | This invention described the administration of a therapeutically effective quantity of N-((4,6- dimethyl-2-oxo-1,2-dihydropyridin-3-yl) methyl) -5-(ethyl (tetrahydro-2H-pyran-4-yl) amino)-4- methyl-4′-(morpholino-methyl)-[1,1′-biphenyl]-3-carboxamide hydrobromide. The compound was a polymorph and exhibited an X-ray powder diffraction pattern with characteristic peaks [217]. |
| 16. | US 10,991,448B2 | 27 April 2021 | This patent related to methods for the evaluation of the diagnosis of cancer in a patient. The patient may be subjected to some therapy. The attributes that were measured included the degree of mutation, transcription and translation levels, protein, and interaction. Thus, a probabilistic pathway gave a comparative graph representing the cellular processes [218]. |
| 17. | US 10,987,037 B2 | 27 April 2021 | This invention was related to the collection of live tumour cells using a collecting probe, where the procedure involved placing the probe in a living organism. The probe used was composed of guide wire carrying a binding surface with an optically sensitive dye and an atraumatic tip at the distal end [219]. |
| 18. | US 2021/0093715 A1 | 1 April 2021 | A method for the treatment of patients with metastatic BC that would extend the progression-free survival of the subjects under treatment was disclosed. The therapeutic regime consisted of an effective dose of a chemotherapeutic agent and an anti-VEGF antibody [220]. |
| 19. | US 2021/0047429 A1 | 18 February 2021 | This invention disclosed a method for the treatment of BC with an effective amount of HER2 antibody and a taxane derivative [221]. |
| 20. | US 2021/0040216 A1 | 11 February 2021 | This patent described a method for the treatment of patients diagnosed with HER2-positive BC. The method involved the administration of a therapeutic amount of an antagonist of programmed cell-death (PCD) protein-1 combined with Trastuzumab and Pertuzumab [222]. |
| 21. | US 10,907,214B2 | 2 February 2021 | Certain methods, devices, and kits to be used for the detection of biomarkers in cancer patients were disclosed in this patent. The treatment involved a secretory phospholipase A and a hydrolysable cisplatin-containing liposome. It also discussed the use of a microarray device with an oligonucleotidic probe to assess the responsiveness of the patient [223]. |
| 22. | US 2020/0385484 A1 | 10 December 2020 | The compositions and procedures for treating cancer with chimeric antigen receptor-modified cells were included in the invention. An antigen-binding domain, a transmembrane domain, a co-stimulatory signalling region, and a CD3 zeta signalling domain may all be present in the antigen [224]. |
| 23. | US 10,858,626B2 | 8 December 2020 | This invention provided methods for use in the adoptive immunotherapy of diagnosed cancer patients. It gave the composition, which consisted of specific tumour infiltrating lymphocytes, tyrosine-protein kinase-2 receptor, and a vascular endothelial growth factor recepto inhibitor [225]. |
| 24. | US 2020/0246275 A1 | 6 August 2020 | This invention gave the methods for the treatment of an individual with cancer, which comprised the administration of a therapeutically effective amount of a nanoformulation of some taxanes with albumin and a Bcl-2 inhibitor such as ABT-263 [226]. |
| 25. | US 10,695,426B2 | 30 June 2020 | This invention described a combination therapy consisting of antagonists of PCD-1 and lymphoma kinase for use in the treatment of cancer. The treatment consisted of monoclonal antibody with variable regions of seq. ID nos. 13 and 15 with a heavy chain and a light chain [227]. |
| 26. | US 2020/0157177 A1 | 21 May 2020 | An immunotherapeutic strategy for cancer was discussed in this invention. It described the use of tumour-associated T-cell peptide epitopes in combination with certain other associated peptide molecules bound to the major histocompatibility complex [228]. |
| 27. | US 10,633,441B2 | 28 April 2020 | This invention claimed that methods and compositions enhance the immune system response towards cancers. It related antigen receptors that selectively target human mesothelin [229]. |
| 28. | US 2020/0101102 A1 | 2 April 2020 | This patent gave methods for immune response induction in cancer patients using Toll-like receptor-9 agonists being administered intratumourally [230]. |
| 29. | US 10, 596, 112B2 | 24 March 2020 | This invention disclosed the composition and methods of making antibodies and carrier proteins to be used as cancer therapeutic agents. It also discussed a lyophilisation procedure for nanoparticle formulation that contained albumin, antibodies, and some drug such as Paclitaxel [231]. |
| 30. | US 10, 378, 066B2 | 13 August 2019 | A molecular diagnostic test for cancer was presented in this invention. The test was based on the detection of DNA damage and the repair mechanism and was capable of determining cancer in clinically responsive or non-responsive patients [232]. |
| 31. | US 10, 370, 452B2 | 6 August 2019 | This invention related to immunotherapeutics obtained from pluripotent stem cells to generate some phenotypical, functionally expandable T-cells. The cells could be used to target a specific antigen, thereby enhancing the cytotoxic potential, antitumour activity, and, thus, the survival of the patient [233]. |
| 32. | US 10, 344, 096B2 | 9 July 2019 | This patent disclosed some pharmaceutical compositions and their preparation methods. The composition was said to be useful in inhibiting metastasis using receptor tyrosine kinase-like orphan receptor1 antibodies (ROR1-antibodies) [234]. |
| 33. | US 10, 266, 592B2 | 23 April 2019 | This invention described surface-engineered human T-lymphocytes for augmenting the immune response. The designed molecules helped mediate cellular immunotherapy and, thus, were an effective strategy to treat cancer [235]. |
| 34. | US 10, 213, 499B2 | 26 February 2019 | The use of proteins, peptides, and nucleic acids such as immunotherapeutic agents, either alone or in combination, was presented. The peptides could either be peptide epitopes or bound to molecules such as the major histocompatibility complex [236]. |
| 35. | US 10, 167, 290B2 | 1 January 2019 | This invention reported substituted pyrazino [2,3-b] pyrazines as inhibitors of mammalian target of rapamycin (mTOR) kinase. These compounds are used for the treatment of diseases such as cancer, inflammation, or neurogenerative disease [237]. |
| 36. | US 10, 124, 023B2 | 13 November 2018 | This patent explored the use of certain cells such as immune-responsive cells, natural killer cells, T-lymphocytes, and regulatory T-cells, which were capable of expressing an antigen-binding receptor in the activation of immune cells. These were single-chain variable fragments that bound to the antigens with immunosuppressive activity, thereby reducing the suppressive action of the antigen [238]. |
| 37. | US2018/0222997 A1 | 9 August 2018 | Certain novel compositions and methods to diagnose and treat solid tumours by the use of antibodies were described. It was said that the antibody was specifically bound to the extracellular domain of the human Frizzled (FZD) receptors, thereby inhibiting growth of tumour cells [239]. |
| 38. | US 9, 920, 366B2 | 20 March 2018 | This invention described the methods and systems for determining genetic variants and detecting double-stranded DNA [240]. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, G.N.; Fatma, H.; Saraf, S.K. Vaccines in Breast Cancer: Challenges and Breakthroughs. Diagnostics 2023, 13, 2175. https://doi.org/10.3390/diagnostics13132175
Fatima GN, Fatma H, Saraf SK. Vaccines in Breast Cancer: Challenges and Breakthroughs. Diagnostics. 2023; 13(13):2175. https://doi.org/10.3390/diagnostics13132175
Chicago/Turabian StyleFatima, Gul Naz, Hera Fatma, and Shailendra K. Saraf. 2023. "Vaccines in Breast Cancer: Challenges and Breakthroughs" Diagnostics 13, no. 13: 2175. https://doi.org/10.3390/diagnostics13132175
APA StyleFatima, G. N., Fatma, H., & Saraf, S. K. (2023). Vaccines in Breast Cancer: Challenges and Breakthroughs. Diagnostics, 13(13), 2175. https://doi.org/10.3390/diagnostics13132175






