The Role of Transabdominal Ultrasound Elastography in Gastrointestinal Non-Liver Diseases: Current Application and Future Prospectives
Abstract
:1. Introduction
2. Upper Gastrointestinal Tract
3. Lower Gastrointestinal Tract
3.1. Inflammatory Bowel Disease
3.1.1. US-E, Disease Activity and Fibro-Inflammatory Characterization in Crohn’s Disease
3.1.2. US-E in Ulcerative Colitis
3.1.3. US-E and IBD in Paediatric Population
3.1.4. US-E and IBD Treatment
3.2. Appendicitis
3.3. Neoplastic Bowel Lesions
4. Pancreas
| First Author (Year) | Pancreatic Disease | Aim | Study Design (N) | US Device | Elastography Technique | Main Results |
|---|---|---|---|---|---|---|
| Sezgin (2022) [57] | PS | Compare PS to non-PS Correlation with metabolic parameters | Prospective (total 125: PS 68, non-PS 57) | Aplio 500, Toshiba | 2D-SWE | 2D-SWE was higher in PS than in controls (9.08 ± 2.29 vs. 7.13 ± 1.85, p = 0.000). In PS group, 2D-SWE values were significantly correlated with waist circumference (r = 0.335; p = 0.023) and insulin resistance (r = 0.338, p = 0.004). |
| Iino (2021) [58] | PC | Differential diagnosis of lesions | Prospective (total 85: PDAC 36, non-PDAC 16, controls 33) | Aplio 500, Canon | 2D-SWE | 2D-SWE values were higher in PDAC than in non-PDAC (9.8 vs. 7.5 kPa, p = 0.0045). |
| Sezgin (2021) [59] | AP | Compare AP to healthy controls Correlation with AP severity and evolution | Prospective (total 155: AP 81, controls 74) | Aplio 500, Toshiba | 2D-SWE | 2D-SWE values were higher in AP than in controls (10.97 ± 2.26 vs. 7.72 ± 2.50 kPa, p = 0.000) and were not different between mild and severe AP. 2D-SWE values decreased after clinical improvement but remained higher than those in controls after 1 month. |
| Suzuki (2021) [60] | AIP | Compare AIP to healthy controls Relation with response to therapy | Prospective (total 57: controls 34, AIP 23) | Aplio i900, Canon | 2D-SWE | 2D-SWE values were higher in AIP than in controls (30.9 vs. 6.6 kPa, p < 0.001). Decrease in 2D-SWE values was greater than change in the size of the pancreas (p = 0.026). |
| Sanjeevi (2020) [61] | RAP | Compare RAP to healthy controls | Prospective (total 66: controls 35, RAP 31) | Acuson 2000, Siemens | p-SWE | p-SWE values were higher in RAP than in controls (1.27 ± 0.50 vs. 1.00 ± 0.17 m/s, p = 0.001) and correlated with the number of pain episodes. |
| Durmaz (2018) [62] | AP | Compare AP to CP and healthy controls Diagnose AP | Prospective (total 120: controls 70, AP 50) | Aplio 500, Toshiba | 2D-SWE | 2D-SWE values were higher in AP than in controls (3.48 ± 0.52 vs. 2.60 ± 1.63 m/s or 45.71 ± 10.72 vs. 23.77 ± 6.72 kPa, p < 0.001). The sensitivity and specificity were both 98%, using elastic modulus, and 96% and 98.3%, respectively, using SW velocity. |
| Kaya (2018) [63] | AP | Compare AP to healthy controls Diagnose AP Correlation with clinical-laboratory outcome | Prospective (total 187: controls 79, AP 108) | Acuson 2000, Siemens | p-SWE | p-SWE values were higher in AP than in controls (2.43 ± 0.08 vs. 1.27 ± 0.025 m/s, p < 0.001). The sensitivity was 100%, and the specificity to diagnose AP was 98%. No correlation with hospitalization, amylase and blood leukocyte. |
| Kuwahara (2018) [64] | CP | Correlation with endoscopic ultrasound findings | Retrospective (85) | iU22, Philips | p-SWE | Hyperechoic foci with shadowing and lobularity with honeycombing were independently related to p-SWE (B = 2.92 (95% CI 2.12–5.71, p < 0.001) and B = 3.91 (95% CI 1.22–4.62, p = 0.001), respectively). |
| He (2017) [65] | PS | Compare DM to healthy controls Compare complicated DM to uncomplicated DM | Prospective (total 230: controls 115, complicated DM 68, uncomplicated DM 47) | Acuson S2000, Siemens | p-SWE | p-SWE values were higher in DM than in controls (p < 0.001). p-SWE values for pancreatic body were higher in complicated DM than in uncomplicated DM (p < 0.01). |
| Pozzi (2017) [66] | CP | Compare CP to healthy controls Correlation with CP severity | Prospective (total 94: control 42, CP 52) | iU22, Philips | p-SWE | p-SWE values were higher in CP than in controls (4.3 ± 2.4 vs. 2.8 ± 1.1 kPa, p = 0.001). Disease duration, analgesics administration and low body weight were independently associated with p-SWE values. |
| Kuwahara (2016) [67] | - | Correlation with histological pancreatic fibrosis | Retrospective (53) | iU22, Philips | p-SWE | p-SWE values correlated with histological pancreatic fibrosis stage. The AUC for ≥mild, ≥moderate and severe fibrosis was 0.85, 0.84 and 0.87. |
| Llamoza-Torres (2016) [68] | CP | Diagnose CP | Retrospective (total 33: normal 16, CP 17) | Acuson S2000, Siemens | p-SWE | p-SWE values for pancreatic body were higher in CP compared to normal group (1.57 vs. 1.27 m/s, p = 0.037); AUC 0.71 (95% CI 0.532–0.895) to diagnose CP |
| Xie (2015) [55] | AP | Compare AP to healthy controls | Prospective (total 254: controls 210, AP 44) | Acuson S2000, Siemens | p-SWE | p-SWE showed no differences between AP and healthy controls (1.21 ± 0.20 vs. 1.18 ± 0.20 m/s (pancreatic head) and 1.25 ± 0.19 m/s (pancreatic body)). |
| Goya (2014) [69] | AP | Compare B-mode, CT and elastography to diagnose AP | Prospective (88) | Acuson S2000, Siemens | p-SWE | p-SWE showed higher accuracy than B-mode and CT to diagnose AP with 100% sensitivity and 98% specificity |
| Park (2013) [70] | PC | Differential diagnosis benign and malignant lesions | Retrospective (total 27: benign 8, malignant 19) | Acuson S2000, Siemens | p-SWE | Relative p-SWE values were higher for malignancies than for benign lesions (1.5 ± 0.8 vs. 0.4 ± 0.3 m/s, p = 0.011) |
| Mateen (2012) [71] | AP | Compare AP to CP and healthy controls Diagnose AP | Prospective (total 166: controls 52, CP 46, AP 68) | Acuson S2000, Siemens | p-SWE | p-SWE values were higher in AP compared to CP and healthy controls (3.28 ± 0.85 vs. 1.28 ± 0.29 vs. 1.25 ± 0.23 m/s, p < 0.001). Sensitivity, specificity, PPV and NPV of p-SWE to differentiate AP vs. CP and healthy controls were 97.1%, 92.9%, 90.4% and 97.8%, respectively. |
| Yashima (2012) [72] | CP | Compare CP to healthy controls Diagnose CP | Prospective (total 98: controls 52, CP 46) | Acuson S2000, Siemens | p-SWE | p-SWE values for pancreatic head, body and tail were higher in CP than in controls (1.23 ± 0.34, 1.30 ± 0.34 and 1.24 ± 0.50 vs. 1.65 ± 0.71, 2.09 ± 1.03 and 1.68 ± 0.84 m/s, respectively, p < 0.001). Considering pancreatic body, AUC was 0.78 with sensitivity, specificity, PPV and NPV of 75%, 72%, 69% and 78%, respectively. |
4.1. Acute Pancreatitis
4.2. Chronic Pancreatitis
4.3. Metabolic Associated Pancreatic Disease
4.4. Pancreatic Cancer
5. Gallbladder
5.1. Inflammatory Diseases
5.2. Benign and Malignant Lesions
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atkinson, N.S.S.; Bryant, R.V.; Dong, Y.; Maaser, C.; Kucharzik, T.; Maconi, G.; Asthana, A.K.; Blaivas, M.; Goudie, A.; Gilja, O.H.; et al. WFUMB Position Paper. Learning Gastrointestinal Ultrasound: Theory and Practice. Ultrasound Med. Biol. 2016, 42, 2732–2742. [Google Scholar] [CrossRef] [Green Version]
- Grgurevic, I.; Salkic, N.; Bozin, T.; Mustapic, S.; Matic, V.; Dumic-Cule, I.; Tjesic Drinkovic, I.; Bokun, T. Magnitude Dependent Discordance in Liver Stiffness Measurements Using Elastography Point Quantification with Transient Elastography as the Reference Test. Eur. Radiol. 2019, 29, 2448–2456. [Google Scholar] [CrossRef]
- Sidhu, P. Multiparametric Ultrasound (MPUS) Imaging: Terminology Describing the Many Aspects of Ultrasonography. Ultraschall Med. 2015, 36, 315–317. [Google Scholar] [CrossRef]
- Shiina, T.; Nightingale, K.R.; Palmeri, M.L.; Hall, T.J.; Bamber, J.C.; Barr, R.G.; Castera, L.; Choi, B.I.; Chou, Y.-H.; Cosgrove, D.; et al. WFUMB Guidelines and Recommendations for Clinical Use of Ultrasound Elastography: Part 1: Basic Principles and Terminology. Ultrasound Med. Biol. 2015, 41, 1126–1147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, C.F.; Barr, R.G.; Farrokh, A.; Dighe, M.; Hocke, M.; Jenssen, C.; Dong, Y.; Saftoiu, A.; Havre, R.F. Strain Elastography—How To Do It? Ultrasound Int. Open 2017, 3, E137–E149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gennisson, J.-L.; Deffieux, T.; Fink, M.; Tanter, M. Ultrasound Elastography: Principles and Techniques. Diagn. Interv. Imaging 2013, 94, 487–495. [Google Scholar] [CrossRef]
- Trout, A.T.; Qiu, L.; Dillman, J.R. Practical Considerations for Pancreas Ultrasound Elastography: Reply to Rojas-Rojas et al. Pediatr. Radiol. 2021, 51, 1770–1771. [Google Scholar] [CrossRef]
- Ozturk, A.; Grajo, J.R.; Dhyani, M.; Anthony, B.W.; Samir, A.E. Principles of ultrasound elastography. Abdom. Radiol. 2018, 43, 773–785. [Google Scholar] [CrossRef]
- Cui, X.-W.; Li, K.-N.; Yi, A.-J.; Wang, B.; Wei, Q.; Wu, G.-G.; Dietrich, C.F. Ultrasound Elastography. Endosc. Ultrasound 2022, 11, 252–274. [Google Scholar] [CrossRef]
- Ophir, J.; Céspedes, I.; Ponnekanti, H.; Yazdi, Y.; Li, X. Elastography: A Quantitative Method for Imaging the Elasticity of Biological Tissues. Ultrason. Imaging 1991, 13, 111–134. [Google Scholar] [CrossRef] [PubMed]
- Cochlin, D.L.; Ganatra, R.H.; Griffiths, D.F.R. Elastography in the Detection of Prostatic Cancer. Clin. Radiol. 2002, 57, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Garra, B.S.; Cespedes, E.I.; Ophir, J.; Spratt, S.R.; Zuurbier, R.A.; Magnant, C.M.; Pennanen, M.F. Elastography of Breast Lesions: Initial Clinical Results. Radiology 1997, 202, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, D.; Piscaglia, F.; Bamber, J.; Bojunga, J.; Correas, J.-M.; Gilja, O.; Klauser, A.; Sporea, I.; Calliada, F.; Cantisani, V.; et al. EFSUMB Guidelines and Recommendations on the Clinical Use of Ultrasound Elastography. Part 2: Clinical Applications. Ultraschall Med. 2013, 34, 238–253. [Google Scholar] [CrossRef]
- Săftoiu, A.; Gilja, O.H.; Sidhu, P.S.; Dietrich, C.F.; Cantisani, V.; Amy, D.; Bachmann-Nielsen, M.; Bob, F.; Bojunga, J.; Brock, M.; et al. The EFSUMB Guidelines and Recommendations for the Clinical Practice of Elastography in Non-Hepatic Applications: Update 2018. Ultraschall Med. 2019, 40, 425–453. [Google Scholar] [CrossRef] [Green Version]
- Suhara, H.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Nakamura, M.; Miyahara, R.; Ishigami, M.; Hashimoto, S.; Goto, H. Transabdominal Ultrasound Elastography of the Esophagogastric Junction Predicts Reflux Esophagitis. J. Med. Ultrason. 2019, 46, 99–104. [Google Scholar] [CrossRef]
- Ma, L.; Zhu, Q.; Zhang, Y.; Li, J.; Jiang, Y.; Xu, D.; Zeng, X.; Hou, Y.; Liu, H. Esophagus Involvement in Systemic Sclerosis: Ultrasound Parameters and Association with Clinical Manifestations. Arthritis Res. Ther. 2021, 23, 122. [Google Scholar] [CrossRef]
- Gilja, O.H.; Heimdal, A.; Hausken, T.; Gregersen, H.; Matre, K.; Berstad, A.; Ødegaard, S. Strain during Gastric Contractions Can Be Measured Using Doppler Ultrasonography. Ultrasound Med. Biol. 2002, 28, 1457–1465. [Google Scholar] [CrossRef]
- Ahmed, A.B.; Matre, K.; Hausken, T.; Gregersen, H.; Gilja, O.H. Rome III Subgroups of Functional Dyspepsia Exhibit Different Characteristics of Antral Contractions Measured by Strain Rate Imaging—A Pilot Study. Ultraschall Med. 2012, 33, E233–E240. [Google Scholar] [CrossRef]
- Cantisani, V.; Rubini, A.; Miniagio, G. CEUS and Strain Elastography in Gastric Carcinoma. J. Ultrasound 2013, 16, 123–125. [Google Scholar] [CrossRef] [Green Version]
- Lien, W.-C.; Lee, P.-C.; Lin, M.-T.; Chang, C.-H.; Wang, H.-P. Adding Trans-Abdominal Elastography to the Diagnostic Tool for an Ileal Gastrointestinal Stromal Tumor: A Case Report. BMC Med. Imaging 2019, 19, 88. [Google Scholar] [CrossRef]
- Giannetti, A.; Biscontri, M.; Matergi, M. Feasibility of Real-Time Strain Elastography in Colonic Diseases. J. Ultrasound 2014, 17, 321–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu-Ata, N.; Dillman, J.R.; Rubin, J.M.; Collins, M.H.; Johnson, L.A.; Imbus, R.S.; Bonkowski, E.L.; Denson, L.A.; Higgins, P.D.R. Ultrasound Shear Wave Elastography in Pediatric Stricturing Small Bowel Crohn Disease: Correlation with Histology and Second Harmonic Imaging Microscopy. Pediatr. Radiol. 2023, 53, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-J.; Chen, B.-L.; Liang, M.-J.; Chen, S.-L.; Li, X.-H.; Qiu, Y.; Pang, L.-L.; Xia, Q.-Q.; He, Y.; Zeng, Z.-R.; et al. Longitudinal Bowel Behavior Assessed by Bowel Ultrasound to Predict Early Response to Anti-TNF Therapy in Patients with Crohn’s Disease: A Pilot Study. Inflamm. Bowel Dis. 2022, 28, S67–S75. [Google Scholar] [CrossRef] [PubMed]
- Yamada, K.; Ishikawa, T.; Kawashima, H.; Ohno, E.; Iida, T.; Ishikawa, E.; Mizutani, Y.; Sawada, T.; Maeda, K.; Yamamura, T.; et al. Evaluation of Ulcerative Colitis Activity Using Transabdominal Ultrasound Shear Wave Elastography. Quant. Imaging Med. Surg. 2022, 12, 618–626. [Google Scholar] [CrossRef]
- Ma, C.; Huang, P.-L.; Kang, N.; Zhang, J.; Xiao, M.; Zhang, J.-Y.; Cao, X.-C.; Dai, X.-C. The Clinical Value of Multimodal Ultrasound for the Evaluation of Disease Activity and Complications in Inflammatory Bowel Disease. Ann. Palliat. Med. 2020, 9, 4146–4155. [Google Scholar] [CrossRef]
- Ding, S.-S.; Fang, Y.; Wan, J.; Zhao, C.-K.; Xiang, L.-H.; Liu, H.; Pu, H.; Xu, G.; Zhang, K.; Xu, X.-R.; et al. Usefulness of Strain Elastography, ARFI Imaging, and Point Shear Wave Elastography for the Assessment of Crohn Disease Strictures. J. Ultrasound Med. 2019, 38, 2861–2870. [Google Scholar] [CrossRef]
- Goertz, R.S.; Lueke, C.; Schellhaas, B.; Pfeifer, L.; Wildner, D.; Neurath, M.F.; Strobel, D. Acoustic Radiation Force Impulse (ARFI) Shear Wave Elastography of the Bowel Wall in Healthy Volunteers and in Ulcerative Colitis. Acta Radiol. Open 2019, 8, 205846011984096. [Google Scholar] [CrossRef]
- Chen, Y.; Mao, R.; Md, X.L.; Cao, Q.; Chen, Z.; Liu, B.; Chen, S.; Chen, B.; He, Y.; Zeng, Z.; et al. Real-Time Shear Wave Ultrasound Elastography Differentiates Fibrotic from Inflammatory Strictures in Patients with Crohn’s Disease. Inflamm. Bowel Dis. 2018, 24, 2183–2190. [Google Scholar] [CrossRef]
- Goertz, R.S.; Lueke, C.; Wildner, D.; Vitali, F.; Neurath, M.F.; Strobel, D. Acoustic Radiation Force Impulse (ARFI) Elastography of the Bowel Wall as a Possible Marker of Inflammatory Activity in Patients with Crohn’s Disease. Clin. Radiol. 2018, 73, 678.e1–678.e5. [Google Scholar] [CrossRef]
- Orlando, S.; Fraquelli, M.; Coletta, M.; Branchi, F.; Magarotto, A.; Conti, C.B.; Mazza, S.; Conte, D.; Basilisco, G.; Caprioli, F. Ultrasound Elasticity Imaging Predicts Therapeutic Outcomes of Patients with Crohn’s Disease Treated with Anti-Tumour Necrosis Factor Antibodies. J. Crohn’s Colitis 2018, 12, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Quaia, E.; Gennari, A.G.; Cova, M.A.; Van Beek, E.J.R. Differentiation of Inflammatory From Fibrotic Ileal Strictures among Patients with Crohn’s Disease Based on Visual Analysis: Feasibility Study Combining Conventional B-Mode Ultrasound, Contrast-Enhanced Ultrasound and Strain Elastography. Ultrasound Med. Biol. 2018, 44, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gui, X.; Chen, W.; Fung, T.; Novak, K.; Wilson, S.R. Ultrasound Shear Wave Elastography and Contrast Enhancement: Effective Biomarkers in Crohn’s Disease Strictures. Inflamm. Bowel Dis. 2017, 23, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Rizzello, F.; Pratico’, C.; Felicani, C.; Fiorini, E.; Brugnera, R.; Mazzotta, E.; Giunchi, F.; Fiorentino, M.; D’Errico, A.; et al. Real-Time Elastography for the Detection of Fibrotic and Inflammatory Tissue in Patients with Stricturing Crohn’s Disease. J. Ultrasound 2017, 20, 273–284. [Google Scholar] [CrossRef]
- Sconfienza, L.M.; Cavallaro, F.; Colombi, V.; Pastorelli, L.; Tontini, G.; Pescatori, L.; Esseridou, A.; Savarino, E.; Messina, C.; Casale, R.; et al. In-Vivo Axial-Strain Sonoelastography Helps Distinguish Acutely-Inflamed from Fibrotic Terminal Ileum Strictures in Patients with Crohn’s Disease: Preliminary Results. Ultrasound Med. Biol. 2016, 42, 855–863. [Google Scholar] [CrossRef]
- Fraquelli, M.; Branchi, F.; Cribiù, F.M.; Orlando, S.; Casazza, G.; Magarotto, A.; Massironi, S.; Botti, F.; Contessini-Avesani, E.; Conte, D.; et al. The Role of Ultrasound Elasticity Imaging in Predicting Ileal Fibrosis in Crohn’s Disease Patients. Inflamm. Bowel Dis. 2015, 21, 2605–2612. [Google Scholar] [CrossRef] [Green Version]
- Fufezan, O.; Asavoaie, C.; Tamas, A.; Farcau, D.; Serban, D. Bowel Elastography—A Pilot Study for Developing an Elastographic Scoring System to Evaluate Disease Activity in Pediatric Crohn’s Disease. Med. Ultrason. 2015, 17, 422–430. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, D.; Ando, T.; Watanabe, O.; Ishiguro, K.; Maeda, O.; Miyake, N.; Nakamura, M.; Miyahara, R.; Ohmiya, N.; Hirooka, Y.; et al. Images of Colonic Real-Time Tissue Sonoelastography Correlate with Those of Colonoscopy and May Predict Response to Therapy in Patients with Ulcerative Colitis. BMC Gastroenterol. 2011, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Adolph, T.E.; Meyer, M.; Schwärzler, J.; Mayr, L.; Grabherr, F.; Tilg, H. The Metabolic Nature of Inflammatory Bowel Diseases. Nat. Rev. Gastroenterol. Hepatol. 2022, 19, 753–767. [Google Scholar] [CrossRef]
- Magro, F.; Langner, C.; Driessen, A.; Ensari, A.; Geboes, K.; Mantzaris, G.J.; Villanacci, V.; Becheanu, G.; Borralho Nunes, P.; Cathomas, G.; et al. European Consensus on the Histopathology of Inflammatory Bowel Disease. J. Crohn’s Colitis 2013, 7, 827–851. [Google Scholar] [CrossRef] [Green Version]
- Magro, F.; Gionchetti, P.; Eliakim, R.; Ardizzone, S.; Armuzzi, A.; Barreiro-de Acosta, M.; Burisch, J.; Gecse, K.B.; Hart, A.L.; Hindryckx, P.; et al. Third European Evidence-Based Consensus on Diagnosis and Management of Ulcerative Colitis. Part 1: Definitions, Diagnosis, Extra-Intestinal Manifestations, Pregnancy, Cancer Surveillance, Surgery, and Ileo-Anal Pouch Disorders. J. Crohn’s Colitis 2017, 11, 649–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.-Y.; Gao, X.; Zhuang, H.; Wu, Y.-T.; Luo, Y.; Jing, J.-G.; Zhang, Y. Using Shear Wave Elasticity in Normal Terminal Ileum of a Healthy Southwest Chinese Population: A Pilot Study of Reference Elasticity Ranges. Quant. Imaging Med. Surg. 2021, 11, 2677–2687. [Google Scholar] [CrossRef] [PubMed]
- Dillman, J.R.; Stidham, R.W.; Higgins, P.D.R.; Moons, D.S.; Johnson, L.A.; Keshavarzi, N.R.; Rubin, J.M. Ultrasound Shear Wave Elastography Helps Discriminate Low-Grade from High-Grade Bowel Wall Fibrosis in Ex Vivo Human Intestinal Specimens. J. Ultrasound Med. 2014, 33, 2115–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baumgart, D.C.; Müller, H.P.; Grittner, U.; Metzke, D.; Fischer, A.; Guckelberger, O.; Pascher, A.; Sack, I.; Vieth, M.; Rudolph, B. US-Based Real-Time Elastography for the Detection of Fibrotic Gut Tissue in Patients with Stricturing Crohn Disease. Radiology 2015, 275, 889–899. [Google Scholar] [CrossRef]
- Raine, T.; Bonovas, S.; Burisch, J.; Kucharzik, T.; Adamina, M.; Annese, V.; Bachmann, O.; Bettenworth, D.; Chaparro, M.; Czuber-Dochan, W.; et al. ECCO Guidelines on Therapeutics in Ulcerative Colitis: Medical Treatment. J. Crohn’s Colitis 2022, 16, 2–17. [Google Scholar] [CrossRef] [PubMed]
- Torres, J.; Bonovas, S.; Doherty, G.; Kucharzik, T.; Gisbert, J.P.; Raine, T.; Adamina, M.; Armuzzi, A.; Bachmann, O.; Bager, P.; et al. ECCO Guidelines on Therapeutics in Crohn’s Disease: Medical Treatment. J. Crohn’s Colitis 2020, 14, 4–22. [Google Scholar] [CrossRef] [Green Version]
- Puca, P.; Del Vecchio, L.E.; Ainora, M.E.; Gasbarrini, A.; Scaldaferri, F.; Zocco, M.A. Role of Multiparametric Intestinal Ultrasound in the Evaluation of Response to Biologic Therapy in Adults with Crohn’s Disease. Diagnostics 2022, 12, 1991. [Google Scholar] [CrossRef]
- Cha, S.-W.; Kim, I.Y.; Kim, Y.W. Quantitative Measurement of Elasticity of the Appendix Using Shear Wave Elastography in Patients with Suspected Acute Appendicitis. PLoS ONE 2014, 9, e101292. [Google Scholar] [CrossRef]
- Keven, A.; Tekin, A.F.; Arslan, F.Z.; Özer, H.; Durmaz, M.S. Two-Dimensional Shear Wave Elastography Can Improve the Diagnostic Accuracy of Ultrasonography in Acute Appendicitis. J. Ultrasound 2022, 26, 471–477. [Google Scholar] [CrossRef]
- Kapoor, A.; Kapoor, A.; Mahajan, G. Real-Time Elastography in Acute Appendicitis. J. Ultrasound Med. 2010, 29, 871–877. [Google Scholar] [CrossRef]
- Goya, C.; Hamidi, C.; Okur, M.H.; Icer, M.; Oguz, A.; Hattapoglu, S.; Cetincakmak, M.G.; Teke, M. The Utility of Acoustic Radiation Force Impulse Imaging in Diagnosing Acute Appendicitis and Staging Its Severity. Diagn. Interv. Radiol. 2014, 20, 453–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isik, İ.A.; Ozkan, M.B. Evaluation of the Clinical Effectiveness of Shear Wave Elastography in Pediatric Cases with Acute Appendicitis. Ultrasound Q. 2021, 37, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Arslan, H.; Akdemir, Z.; Yavuz, A.; Gökçal, F.; Parlakgümüş, C.; İslamoglu, N.; Akdeniz, H. Efficacy of Strain Elastography in Diagnosis and Staging of Acute Appendicitis in Pediatric Patients. Med. Sci. Monit. 2018, 24, 855–862. [Google Scholar] [CrossRef] [Green Version]
- Havre, R.; Leh, S.; Gilja, O.; Ødegaard, S.; Waage, J.; Baatrup, G.; Nesje, L. Strain Assessment in Surgically Resected Inflammatory and Neoplastic Bowel Lesions. Ultraschall Med. 2012, 35, 149–158. [Google Scholar] [CrossRef]
- Xie, J.; Zou, L.; Yao, M.; Xu, G.; Zhao, L.; Xu, H.; Wu, R. A Preliminary Investigation of Normal Pancreas and Acute Pancreatitis Elasticity Using Virtual Touch Tissue Quantification (VTQ) Imaging. Med. Sci. Monit. 2015, 21, 1693–1699. [Google Scholar] [CrossRef] [Green Version]
- Stumpf, S.; Jaeger, H.; Graeter, T.; Oeztuerk, S.; Schmidberger, J.; Haenle, M.M.; Kratzer, W. Elasto-Study Group Ulm Influence of Age, Sex, Body Mass Index, Alcohol, and Smoking on Shear Wave Velocity (p-SWE) of the Pancreas. Abdom. Radiol. 2016, 41, 1310–1316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sezgin, O.; Yaraş, S.; Özdoğan, O. Pancreatic Steatosis Is Associated with Both Metabolic Syndrome and Pancreatic Stiffness Detected by Ultrasound Elastography. Dig. Dis. Sci. 2022, 67, 293–304. [Google Scholar] [CrossRef]
- Iino, Y.; Maruyama, H.; Mikata, R.; Yasui, S.; Koroki, K.; Nagashima, H.; Awatsu, M.; Shingyoji, A.; Kusakabe, Y.; Kobayashi, K.; et al. Percutaneous Two-Dimensional Shear Wave Elastography for Diagnosis of Pancreatic Tumor. Diagnostics 2021, 11, 498. [Google Scholar] [CrossRef]
- Sezgin, O.; Yaraş, S.; Özdoğan, O. The Course and Prognostic Value of Increased Pancreas Stiffness Detected by Ultrasound Elastography during Acute Pancreatitis. Pancreatology 2021, 21, 1285–1290. [Google Scholar] [CrossRef]
- Suzuki, H.; Ishikawa, T.; Ohno, E.; Iida, T.; Uetsuki, K.; Yashika, J.; Yamada, K.; Yoshikawa, M.; Furukawa, K.; Nakamura, M.; et al. An Initial Trial of Quantitative Evaluation of Autoimmune Pancreatitis Using Shear Wave Elastography and Shear Wave Dispersion in Transabdominal Ultrasound. Pancreatology 2021, 21, 682–687. [Google Scholar] [CrossRef]
- Sanjeevi, R.; John, R.A.; Kurien, R.T.; Dutta, A.K.; Simon, E.G.; David, D.; Joseph, A.J.; Chowdhury, S.D. Acoustic Radiation Force Impulse Imaging of Pancreas in Patients with Early Onset Idiopathic Recurrent Acute Pancreatitis. Eur. J. Gastroenterol. Hepatol. 2020, 32, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, M.S.; Arslan, S.; Özbakır, B.; Güngör, G.; Tolu, İ.; Arslan, F.Z.; Sivri, M.; Koplay, M. Effectiveness of Shear Wave Elastography in the Diagnosis of Acute Pancreatitis on Admission. Med. Ultrason. 2018, 20, 278. [Google Scholar] [CrossRef] [Green Version]
- Kaya, M.; Değirmenci, S.; Göya, C.; Tuncel, E.T.; Uçmak, F.; Kaplan, M.A. The Importance of Acoustic Radiation Force Impulse (ARFI) Elastography in the Diagnosis and Clinical Course of Acute Pancreatitis. Turk. J. Gastroenterol. 2018, 29, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Kuwahara, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Ishikawa, T.; Yamamura, T.; Furukawa, K.; Funasaka, K.; Nakamura, M.; Miyahara, R.; et al. Usefulness of Shear Wave Elastography as a Quantitative Diagnosis of Chronic Pancreatitis: CP Diagnosis by SW-Elastography. J. Gastroenterol. Hepatol. 2018, 33, 756–761. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, H.; Li, X.P.; Zheng, J.-J.; Jin, C.-X. Pancreatic Elastography From Acoustic Radiation Force Impulse Imaging for Evaluation of Diabetic Microangiopathy. Am. J. Roentgenol. 2017, 209, 775–780. [Google Scholar] [CrossRef]
- Pozzi, R.; Parzanese, I.; Baccarin, A.; Giunta, M.; Conti, C.B.; Cantù, P.; Casazza, G.; Tenca, A.; Rosa, R.; Gridavilla, D.; et al. Point Shear-Wave Elastography in Chronic Pancreatitis: A Promising Tool for Staging Disease Severity. Pancreatology 2017, 17, 905–910. [Google Scholar] [CrossRef]
- Kuwahara, T.; Hirooka, Y.; Kawashima, H.; Ohno, E.; Sugimoto, H.; Hayashi, D.; Morishima, T.; Kawai, M.; Suhara, H.; Takeyama, T.; et al. Quantitative Evaluation of Pancreatic Tumor Fibrosis Using Shear Wave Elastography. Pancreatology 2016, 16, 1063–1068. [Google Scholar] [CrossRef]
- Llamoza-Torres, C.J.; Fuentes-Pardo, M.; Álvarez-Higueras, F.J.; Alberca-de-las-Parras, F.; Carballo-Álvarez, F. Usefulness of Percutaneous Elastography by Acoustic Radiation Force Impulse for the Non-Invasive Diagnosis of Chronic Pancreatitis. Rev. Esp. Enferm. Dig. 2016, 108, 450–456. [Google Scholar] [CrossRef] [Green Version]
- Goya, C.; Hamidi, C.; Hattapoğlu, S.; Cetincakmak, M.; Teke, M.; Degirmenci, M.; Kaya, M.; Bilici, A. Use of Acoustic Radiation Force Impulse Elastography to Diagnose Acute Pancreatitis at Hospital Admission Comparison with Sonography and Computed Tomography. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2014, 33, 1453–1460. [Google Scholar] [CrossRef]
- Park, M.K.; Jo, J.; Kwon, H.; Cho, J.H.; Oh, J.Y.; Noh, M.H.; Nam, K.J. Usefulness of Acoustic Radiation Force Impulse Elastography in the Differential Diagnosis of Benign and Malignant Solid Pancreatic Lesions. Ultrasonography 2013, 33, 26–33. [Google Scholar] [CrossRef]
- Mateen, M.A.; Muheet, K.A.; Mohan, R.J.; Rao, P.N.; Majaz, H.M.K.; Rao, G.V.; Reddy, D.N. Evaluation of Ultrasound Based Acoustic Radiation Force Impulse (ARFI) and ESie Touch Sonoelastography for Diagnosis of Inflammatory Pancreatic Diseases. J. Pancreas 2012, 13, 36–44. [Google Scholar]
- Yashima, Y.; Sasahira, N.; Isayama, H.; Kogure, H.; Ikeda, H.; Hirano, K.; Mizuno, S.; Yagioka, H.; Kawakubo, K.; Sasaki, T.; et al. Acoustic Radiation Force Impulse Elastography for Noninvasive Assessment of Chronic Pancreatitis. J. Gastroenterol. 2012, 47, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Whitcomb, D.C.; Shimosegawa, T.; Esposito, I.; Lerch, M.M.; Gress, T.; Mayerle, J.; Drewes, A.M.; Rebours, V.; Akisik, F.; et al. Chronic Pancreatitis. Nat. Rev. Dis. Prim. 2017, 3, 17060. [Google Scholar] [CrossRef]
- Ramkissoon, R.; Gardner, T.B. Pancreatic Steatosis: An Emerging Clinical Entity. Off. J. Am. Coll. Gastroenterol. ACG 2019, 114, 1726. [Google Scholar] [CrossRef]
- Smits, M.M.; van Geenen, E.J.M. The Clinical Significance of Pancreatic Steatosis. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Uchida, H.; Hirooka, Y.; Itoh, A.; Kawashima, H.; Hara, K.; Nonogaki, K.; Kasugai, T.; Ohno, E.; Ohmiya, N.; Niwa, Y.; et al. Feasibility of Tissue Elastography Using Transcutaneous Ultrasonography for the Diagnosis of Pancreatic Diseases. Pancreas 2009, 38, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Zaro, R.; Dina, L.; Pojoga, C.; Vesa, S.; Badea, R. Evaluation of the Pancreatic Tumors by Transabdominal Shear Wave Elastography: Preliminary Results of a Pilot Study. Med. Ultrason. 2018, 20, 285. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Ishikawa, T.; Ohno, E.; Iida, T.; Furukawa, K.; Nakamura, M.; Honda, T.; Ishigami, M.; Kinoshita, F.; Kawashima, H.; et al. Variability Measurements Provide Additional Value to Shear Wave Elastography in the Diagnosis of Pancreatic Cancer. Sci. Rep. 2021, 11, 7409. [Google Scholar] [CrossRef]
- Kawada, N.; Tanaka, S.; Uehara, H.; Katayama, K.; Hosoki, T.; Takami, M.; Tomita, Y. Alteration of Strain Ratio Evaluated by Transabdominal Ultrasound Elastography May Predict the Efficacy of Preoperative Chemoradiation Performed for Pancreatic Ductal Carcinoma: Preliminary Results. Hepatogastroenterology 2014, 61, 480–483. [Google Scholar]
- Kiewiet, J.J.S.; Leeuwenburgh, M.M.N.; Bipat, S.; Bossuyt, P.M.M.; Stoker, J.; Boermeester, M.A. A Systematic Review and Meta-Analysis of Diagnostic Performance of Imaging in Acute Cholecystitis. Radiology 2012, 264, 708–720. [Google Scholar] [CrossRef] [Green Version]
- Ko, A.; Lee, E.S.; Park, H.J.; Park, S.B.; Kim, H.S.; Choi, B.I. Added Value of 2D Shear Wave Imaging of the Gallbladder Bed of the Liver for Acute Cholecystitis. Ultrasonography 2020, 39, 384–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Choi, D.S.; Bae, K.; Cho, J.M.; Jeong, C.Y.; Kim, H.O. Added Value of Point Shear-Wave Elastography in the Diagnosis of Acute Cholecystitis. Eur. Radiol. 2017, 27, 1517–1526. [Google Scholar] [CrossRef] [PubMed]
- Teber, M.A.; Tan, S.; Dönmez, U.; İpek, A.; Uçar, A.E.; Yıldırım, H.; Aslan, A.; Arslan, H. The Use of Real-Time Elastography in the Assessment of Gallbladder Polyps: Preliminary Observations. Med. Ultrason. 2014, 16, 304–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kapoor, A.; Kapoor, A.; Mahajan, G. Differentiating Malignant from Benign Thickening of the Gallbladder Wall by the Use of Acoustic Radiation Force Impulse Elastography. J. Ultrasound Med. 2011, 30, 1499–1507. [Google Scholar] [CrossRef]
- Soundararajan, R.; Dutta, U.; Bhatia, A.; Gupta, P.; Nahar, U.; Kaman, L.; Bhattacharya, A.; Kumar, A.; Sandhu, M.S. Two-dimensional Shear Wave Elastography: Utility in Differentiating Gallbladder Cancer from Chronic Cholecystitis. J. Ultrasound Med. 2023, 42, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
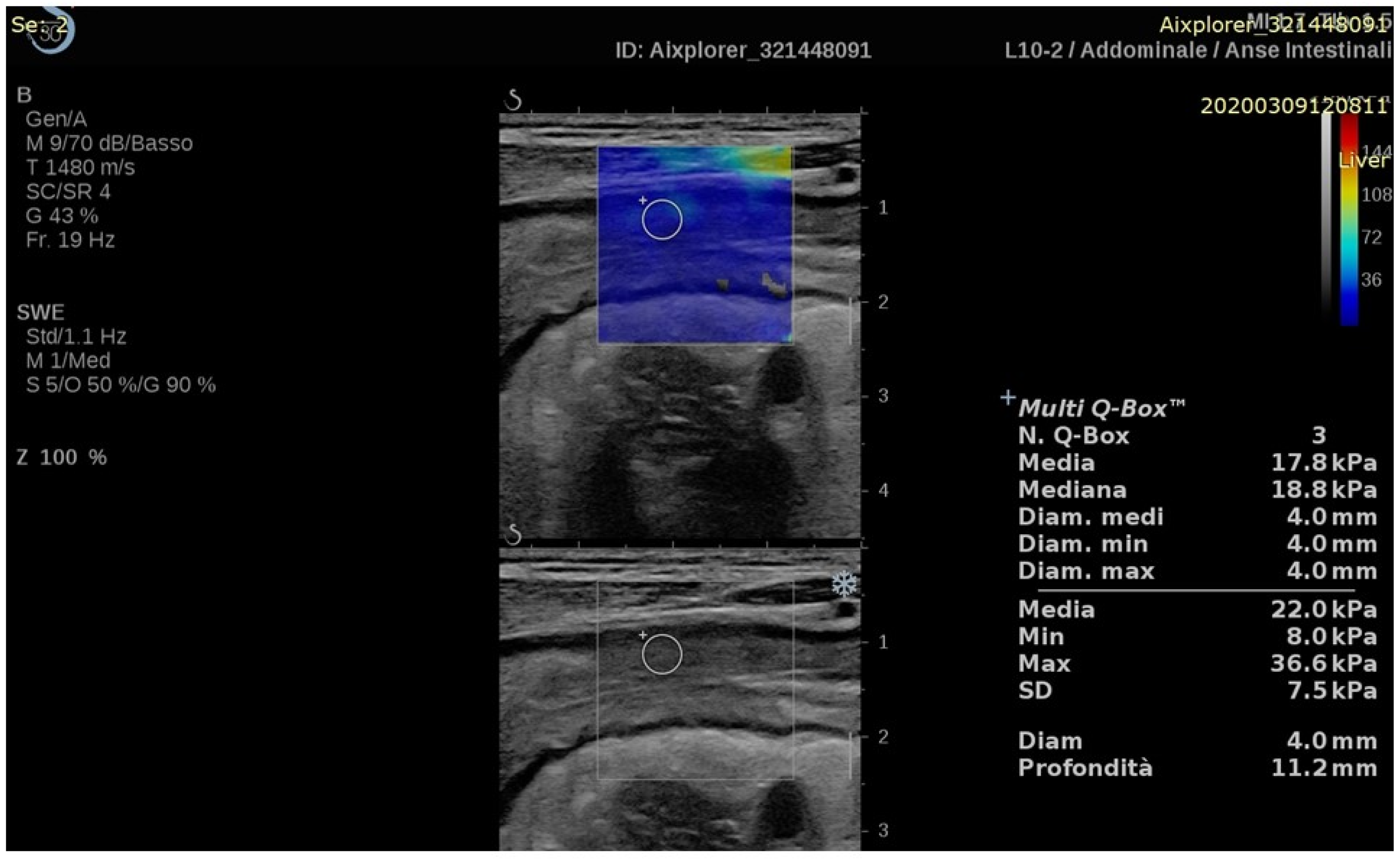

| First Author (Year) | IBD | Aim | Study Design (N) | US Device | Elastography Technique | Main Results | Reference Method |
|---|---|---|---|---|---|---|---|
| Abu Ata (2023) [23] | CD | Stricture characterization | Prospective (18) | Acuson S3000, Siemens | 2D-SWE | 2D-SWE values negatively correlated with mucosal inflammation (r = −0.50, p = 0.04) and positively correlated with muscularis propria inner layer smooth muscle hypertrophy (r = 0.72, p = 0.002). No correlation was found between 2D-SWE values and fibrosis. | Histology |
| Chen (2022) [24] | CD | Response to therapy | Prospective (30) | Aixplorer, SuperSonic Imagine | 2D-SWE | 2D-SWE values decreased in responding patients two weeks after starting therapy (15.3 ± 4.7 vs. 12.6 ± 3.3 kPa, p = 0.003). At baseline, 2D-SWE values were higher in non-responders’ group (15.3 ± 4.7 vs. 21.3 ± 8.7 kPa, p = 0.022). | Clinical data and endoscopy |
| Yamada (2021) [25] | UC | Disease activity | Cross-sectional (26) | Aplio i900, Canon | 2D-SWE | 2D-SWE values were higher in mucosal healing group compared to active disease group (2.40 (IQR, 2.18–3.38) vs. 1.62 (IQR, 1.44–1.95) m/s, p = 0.007). | Endoscopy |
| Ma (2020) [26] | CD/UC | Disease activity | Retrospective (30) | Logiq E8, GE | 2D-SWE | SWE values were higher in fibrosis group compared to inflammation group (3.63 ± 0.86 vs. 2.51 ± 0.66 m/s, p = 0.004). | CEUS and traditional US |
| Ding (2019) [27] | CD | Stricture characterization | Prospective (25) | Acuson S2000, Siemens | SE (colour scale classification) p-SWE | p-SWE values were higher in fibrotic strictures compared to inflammatory stenosis (1.57 ± 0.60 vs. 2.59 ± 0.97 m/s, p < 0.05) and superior to SE in differentiating stenosis properties. | Histology |
| Goertz (2019) [28] | UC | Disease activity | Prospective (20) | Acuson S2000, Siemens | p-SWE | p-SWE values were not correlated with MAYO subscore or C-reactive protein level. | Clinical and laboratory data |
| Chen (2018) [29] | CD | Stricture characterization | Prospective (35) | Aixplorer, SuperSonic Imagine | 2D-SWE | 2D-SWE values were higher in severe fibrosis compared to mild or moderate fibrosis (23.0 ± 6.3 vs. 17.4 ± 3.8 and 14.4 ± 2.1 kPa, p = 0.008). | Histology |
| Goertz (2018) [30] | CD | Disease activity | Retrospective (77) Prospective (21) | Acuson S2000, Siemens | p-SWE | No significant correlation was found between ileal or sigma’s p-SWE values and disease activity indicators. | Clinical and laboratory data |
| Orlando (2018) [31] | CD | Response to therapy | Prospective (30) | iU22, Philips | SE (SR) | SR values at baseline were lower in patients who achieved mucosal healing after 14 weeks compared to patients not achieving this endpoint (1.06 vs. 1.58, p = 0.03). | Traditional US (mucosal healing = bowel wall thickness < 3 mm) |
| Quaia (2018) [32] | CD | Stricture characterization | Prospective (20) | iU22, Philips | SE (colour scale classification) | Combination of B-mode, CEUS and SE showed higher diagnostic accuracy than each single technique. | Histology |
| Lu (2017) [33] | CD | Stricture characterization | Prospective (105 CEUS/15 histology) | Acuson S3000, Siemens or Epiq 5, Philips | p-SWE | p-SWE values moderately correlated with muscular hypertrophy (r = 0.59, p = 0.02) and negatively correlated with peak enhancement (r = −0.59, p = 0.02). No correlation was found with fibrosis or inflammation. | Histology |
| Serra (2017) [34] | CD | Stricture characterization | Prospective (32) | iU22, Philips | SE (SR) | No correlation was found between SR and inflammatory and fibrosis scores. | Histology |
| Sconfienza (2016) [35] | CD | Stricture characterization | Prospective (16) | MyLab 70 XvG, Esaote | SE (colour scale score) | SE score was lower in inflammatory strictures compared to fibrotic strictures (16 (IQR 16–18) vs. 20 (IQR, 17.5–22), p = 0.003). | Magnetic resonance elastography |
| Fraquelli (2015) [36] | CD | Stricture characterization | Prospective (23) | iU22, Philips | SE (colour scale classification and SR) | SR values were associated with ileal fibrosis at multivariate analysis (R2 = 0.75, p < 0.0001). No significant association was found between colour scale values and fibrosis. | Histology |
| Fufezan (2015) [37] | CD | Disease activity | Prospective (14) | Xario V 2.0 (Toshiba) | SE (colour scale classification) and SR) | Colour classes and SR results were significantly associated with disease activity markers (CRP-SE p = 0.0104, CRP-SR p = 0.0721, ESR-SE p = 0.0008, ESR-SR p = 0.0123, CAL-SE p =0.0139). | Laboratory data |
| Ishikawa (2011) [38] | UC | Disease activity | Prospective (37) | EUB-8500, Hitachi | SE (colour scale classification) | Elastography and colonoscopy findings were significantly associated (p < 0.001). | Endoscopy |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paratore, M.; Garcovich, M.; Ainora, M.E.; Del Vecchio, L.E.; Cuccia, G.; Riccardi, L.; Pompili, M.; Gasbarrini, A.; Zocco, M.A. The Role of Transabdominal Ultrasound Elastography in Gastrointestinal Non-Liver Diseases: Current Application and Future Prospectives. Diagnostics 2023, 13, 2266. https://doi.org/10.3390/diagnostics13132266
Paratore M, Garcovich M, Ainora ME, Del Vecchio LE, Cuccia G, Riccardi L, Pompili M, Gasbarrini A, Zocco MA. The Role of Transabdominal Ultrasound Elastography in Gastrointestinal Non-Liver Diseases: Current Application and Future Prospectives. Diagnostics. 2023; 13(13):2266. https://doi.org/10.3390/diagnostics13132266
Chicago/Turabian StyleParatore, Mattia, Matteo Garcovich, Maria Elena Ainora, Livio Enrico Del Vecchio, Giuseppe Cuccia, Laura Riccardi, Maurizio Pompili, Antonio Gasbarrini, and Maria Assunta Zocco. 2023. "The Role of Transabdominal Ultrasound Elastography in Gastrointestinal Non-Liver Diseases: Current Application and Future Prospectives" Diagnostics 13, no. 13: 2266. https://doi.org/10.3390/diagnostics13132266
APA StyleParatore, M., Garcovich, M., Ainora, M. E., Del Vecchio, L. E., Cuccia, G., Riccardi, L., Pompili, M., Gasbarrini, A., & Zocco, M. A. (2023). The Role of Transabdominal Ultrasound Elastography in Gastrointestinal Non-Liver Diseases: Current Application and Future Prospectives. Diagnostics, 13(13), 2266. https://doi.org/10.3390/diagnostics13132266








