Esophagogastric Junction Outflow Obstruction Is Likely to Be a Local Manifestation of Other Primary Diseases: Analysis of Single-Center 4-Year Follow-Up Data
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. High-Resolution Manometry
2.3. Statistical Analysis
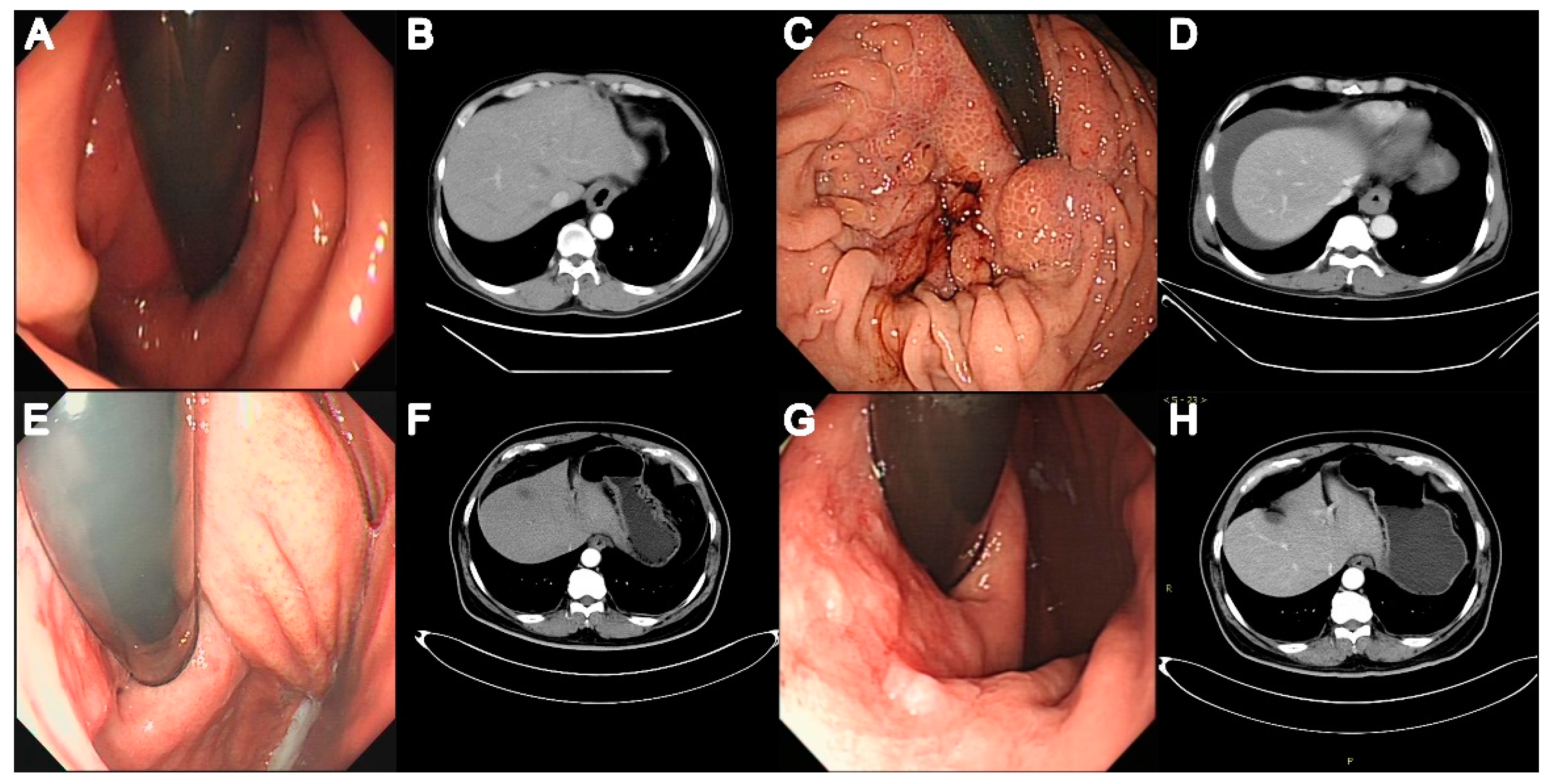
3. Results
3.1. Baseline Characteristics of Patients with AC vs. EGJOO
3.2. Baseline HRM Parameters in Patients with AC vs. EGJOO
3.3. Treatments and Outcomes in EGJOO vs. AC
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kahrilas, P.J.; Bredenoord, A.J.; Fox, M.; Gyawali, C.P.; Roman, S.; Smout, A.J.P.M.; Pandolfino, J.E. The Chicago Classification of esophageal motility disorders, v3.0. Neurogastroenterol. Motil. 2015, 2, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Laique, S.; Singh, T.; Dornblaser, D.; Gadre, A.; Rangan, V.; Fass, R.; Kirby, D.; Chatterjee, S.; Gabbard, S. Clinical Characteristics and Associated Systemic Diseases in Patients with Esophageal “Absent Contractility”—A Clinical Algorithm. J. Clin. Gastroenterol. 2019, 53, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Daum, C.; Sweis, R.; Kaufman, E.; Fuellemann, A.; Anggiansah, A.; Fried, M.; Fox, M. Failure to respond to physiologic challenge characterizes esophageal motility in erosive gastro-esophageal reflux disease. Neurogastroenterol. Motil. 2011, 23, 517-e200. [Google Scholar] [CrossRef] [PubMed]
- Riva, C.G.; Siboni, S.; Sozzi, M.; Lazzari, V.; Asti, E.; Bonavina, L. High-resolution manometry findings after Linx procedure for gastro-esophageal reflux disease. Neurogastroenterol. Motil. 2020, 32, e13750. [Google Scholar] [CrossRef] [PubMed]
- Vaezi, M.F.; Pandolfino, J.E.; Yadlapati, R.H.; Greer, K.B.; Kavitt, R.T. ACG Clinical Guidelines: Diagnosis and Management of Achalasia. Am. J. Gastroenterol. 2020, 115, 1393–1411. [Google Scholar] [CrossRef]
- Achem, S.R.; Vazquez-Elizondo, G.; Fass, R. Jackhammer Esophagus: Current Concepts and Dilemmas. J. Clin. Gastroenterol. 2020; online ahead of print. [Google Scholar] [CrossRef]
- Khalaf, M.; Chowdhary, S.; Elias, P.S.; Castell, D. Distal Esophageal Spasm: A Review. Am. J. Med. 2018, 131, 1034–1040. [Google Scholar] [CrossRef]
- Patcharatrakul, T.; Alkaddour, A.; Pitisuttithum, P.; Jangsirikul, S.; Vega, K.J.; Clarke, J.O.; Gonlachanvit, S. How to approach esophagogastric junction outflow obstruction? Ann. N. Y. Acad. Sci. 2020, 1481, 210–223. [Google Scholar] [CrossRef]
- Furuzawa-Carballeda, J.; Coss-Adame, E.; Romero-Hernandez, F.; Zuniga, J.; Uribe-Uribe, N.; Aguilar-Leon, D.; Valdovinos, M.A.; Nunez-Alvarez, C.A.; Hernandez-Ramirez, D.F.; Olivares-Martinez, E.; et al. Esophagogastric junction outflow obstruction: Characterization of a new entity? Clinical, manometric, and neuroimmunological description. Neurogastroenterol. Motil. 2020, 32, e13867. [Google Scholar] [CrossRef]
- Clayton, S.B.; Shin, C.M.; Ewing, A.; Blonski, W.; Richter, J. Pneumatic dilation improves esophageal emptying and symptoms in patients with idiopathic esophago-gastric junction outflow obstruction. Neurogastroenterol. Motil. 2019, 31, e13522. [Google Scholar] [CrossRef]
- Filicori, F.; Dunst, C.M.; Sharata, A.; Abdelmoaty, W.F.; Zihni, A.M.; Reavis, K.M.; Demeester, S.R.; Swanstrom, L.L. Long-term outcomes following POEM for non-achalasia motility disorders of the esophagus. Surg. Endosc. 2019, 33, 1632–1639. [Google Scholar] [CrossRef]
- Eckardt, V.F.; Aignherr, C.; Bernhard, G. Predictors of outcome in patients with achalasia treated by pneumatic dilation. Gastroenterology 1992, 103, 1732–1738. [Google Scholar] [CrossRef]
- Jiang, L.Q.; Ye, B.X.; Wang, M.F.; Lin, L. Acid exposure in patients with gastroesophageal reflux disease is associated with esophageal dysmotility. J. Dig. Dis. 2019, 20, 73–77. [Google Scholar] [CrossRef]
- Lynch, K.L.; Yang, Y.X.; Metz, D.C.; Falk, G.W. Clinical presentation and disease course of patients with esophagogastric junction outflow obstruction. Dis. Esophagus 2017, 30, 1–6. [Google Scholar] [CrossRef]
- Ong, A.M.L.; Namasivayam, V.; Wang, Y.T. Evaluation of symptomatic esophagogastric junction outflow obstruction. J. Gastroenterol. Hepatol. 2018, 33, 1745–1750. [Google Scholar] [CrossRef]
- Latrache, S.; Melchior, C.; Desprez, C.; Sidali, S.; Recton, J.; Touchais, O.; van der Eecken, E.; Wuestenberghs, F.; Charpentier, C.; Leroi, A.M.; et al. Is it necessary to perform a morphological assessment for an esophageal motility disorder? A retrospective descriptive study. Clin. Res. Hepatol. Gastroenterol. 2021, 45, 101633. [Google Scholar] [CrossRef]
- Rieder, E.; Fernandez-Becker, N.Q.; Sarosiek, J.; Guillaume, A.; Azagury, D.E.; Clarke, J.O. Achalasia: Physiology and diagnosis. Ann. N. Y. Acad. Sci. 2020, 1482, 85–94. [Google Scholar] [CrossRef]
- Csucska, M.; Masuda, T.; Bremner, R.M.; Mittal, S.K. Clinical Symptom Presentation of Hypercontractile Peristalsis in the Era of High-Resolution Manometry: A Single-Center Experience. Dig. Dis. 2020, 38, 355–363. [Google Scholar] [CrossRef]
- Blais, P.; Patel, A.; Sayuk, G.S.; Gyawali, C.P. Upper esophageal sphincter (UES) metrics on high-resolution manometry (HRM) differentiate achalasia subtypes. Neurogastroenterol. Motil. 2017, 29, e13136. [Google Scholar] [CrossRef]
- Blais, P.; Bennett, M.C.; Gyawali, C.P. Upper esophageal sphincter metrics on high-resolution manometry differentiate etiologies of esophagogastric junction outflow obstruction. Neurogastroenterol. Motil. 2019, 31, e13558. [Google Scholar] [CrossRef]
- Chavez, Y.H.; Ciarleglio, M.M.; Clarke, J.O.; Nandwani, M.; Stein, E.; Roland, B.C. Upper esophageal sphincter abnormalities: Frequent finding on high-resolution esophageal manometry and associated with poorer treatment response in achalasia. J. Clin. Gastroenterol. 2015, 49, 17–23. [Google Scholar] [CrossRef]
- Krause, A.J.; Su, H.; Triggs, J.R.; Beveridge, C.; Baumann, A.J.; Donnan, E.; Pandolfino, J.E.; Carlson, D.A. Multiple rapid swallows and rapid drink challenge in patients with esophagogastric junction outflow obstruction on high-resolution manometry. Neurogastroenterol. Motil. 2021, 33, e14000. [Google Scholar] [CrossRef]
- Triggs, J.R.; Carlson, D.A.; Beveridge, C.; Kou, W.; Kahrilas, P.J.; Pandolfino, J.E. Functional Luminal Imaging Probe Panometry Identifies Achalasia-Type Esophagogastric Junction Outflow Obstruction. Clin. Gastroenterol. Hepatol. 2020, 18, 2209–2217. [Google Scholar] [CrossRef]
- van Hoeij, F.B.; Smout, A.J.; Bredenoord, A.J. Characterization of idiopathic esophagogastric junction outflow obstruction. Neurogastroenterol. Motil. 2015, 27, 1310–1316. [Google Scholar] [CrossRef]
- Pérez-Fernández, M.T.; Santander, C.; Marinero, A.; Burgos-Santamaría, D.; Chavarría-Herbozo, C. Characterization and follow-up of esophagogastric junction outflow obstruction detected by high resolution manometry. Neurogastroenterol. Motil. 2016, 28, 116–126. [Google Scholar] [CrossRef]
- Ponds, F.A.; Fockens, P.; Lei, A.; Neuhaus, H.; Beyna, T.; Kandler, J.; Frieling, T.; Chiu, P.W.Y.; Wu, J.C.Y.; Wong, V.W.Y.; et al. Effect of Peroral Endoscopic Myotomy vs Pneumatic Dilation on Symptom Severity and Treatment Outcomes Among Treatment-Naive Patients with Achalasia: A Randomized Clinical Trial. JAMA 2019, 322, 134–144. [Google Scholar] [CrossRef]
- Wang, D.; Wang, X.; Yu, Y.; Xu, X.; Wang, J.; Jia, Y.; Xu, H. Assessment of Esophageal Motor Disorders Using High-resolution Manometry in Esophageal Dysphagia With Normal Endoscopy. J. Neurogastroenterol. Motil. 2019, 25, 61–67. [Google Scholar] [CrossRef]



| EGJOO (n = 70) | AC (n = 79) | p | |
|---|---|---|---|
| Adenocarcinoma of the esophagogastric junction | 5 (2.86%) | 0 (0%) | 0.021 * |
| Autoimmune disease | 7 (10%) | 1 (1.27%) | 0.026 * |
| Esophageal organic disease † | 5 (2.86%) | 5 (6.33%) | 0.999 |
| Herpes zoster | 6 (8.57%) | 5 (6.33%) | 0.601 |
| Family history ‡ | 13 (18.57%) | 2 (2.53%) | 0.002 * |
| Anatomical EGJOO (n = 23) | Functional EGJOO (n = 47) | EGJOO at Baseline (n = 70) | AC with Organic Diseases (n = 11) | True AC (n = 68) | AC at Baseline (n = 79) | |
|---|---|---|---|---|---|---|
| Age (year) | 56.57 ± 15.88 | 52.49 ± 13.81 | 53.83 ± 14.53 | 46.27 ± 15.12 | 44.47 ± 15.85 | 44.72 ± 15.67 c |
| Male | 12 (52.17%) | 19 (40.43%) | 31 (44.29%) | 5 (45.45%) | 35 (51.47%) | 40 (50.63%) |
| BMI (kg/m2) | 22.84 ± 4.58 | 22.32 ± 3.30 | 22.49 ± 3.76 | 20.81 ± 3.55 | 22.04 ± 5.24 | 21.86 ± 5.04 |
| Duration (months) | 24.0 (12.0–54.0) | 24.0 (12.0–36.0) | 24.0 (12.0–36.0) | 96.0 (60.0–336.0) | 48.0 (18.0–120.0) b | 60.0 (24.0–120.0) c |
| Eckardt score | ||||||
| Dysphagia | 1.50 ± 1.26 | 0.67 ± 0.90 a | 0.95 ± 1.10 | 2.55 ± 0.69 | 2.41 ± 0.80 | 2.43 ± 0.78 c |
| Regurgitation | 0.68 ± 0.72 | 0.61 ± 0.77 | 0.63 ± 0.75 | 0.91 ± 0.94 | 0.97 ± 0.90 | 0.96 ± 0.90 c |
| Chest pain | 0.45 ± 0.67 | 0.55 ± 0.77 | 0.52 ± 0.73 | 0.91 ± 1.04 | 0.63 ± 0.91 | 0.67 ± 0.93 |
| Weight loss | 0.23 ± 0.53 | 0.24 ± 0.43 | 0.23 ± 0.46 | 0.64 ± 0.81 | 0.57 ± 0.87 | 0.58 ± 0.86 c |
| Total | 2.86 ± 1.64 | 2.05 ± 1.17 a | 2.33 ± 1.39 | 5.00 ± 1.73 | 4.59 ± 1.74 | 4.65 ± 1.73 c |
| Heartburn | 6 (26.09%) | 14 (29.79%) | 20 (28.57%) | 5 (45.45%) | 16 (23.53%) | 21 (24.71%) |
| Nausea | 5 (21.74%) | 5 (10.64%) | 10 (14.28%) | 5 (45.45%) | 26 (38.24%) | 31 (39.24%) c |
| Vomiting | 6 (26.09%) | 4 (8.51%) | 10 (14.28%) | 7 (63.64%) | 32 (47.06%) | 39 (49.37%) c |
| Epigastric pain | 6 (26.09%) | 8 (17.02%) | 14 (20%) | 3 (27.27%) | 12 (17.65%) | 15 (18.99%) |
| Odynophagia | 5 (21.74%) | 12 (25.53%) | 17 (24.29%) | 0 (0%) | 2 (2.94%) | 2 (2.53%) c |
| Cough | 3 (13.04%) | 5 (10.64%) | 8 (11.43%) | 2 (18.18%) | 4 (5.88%) | 6 (7.59%) |
| Aspiration | 0 (0%) | 0 (0%) | 0 (0%) | 2 (18.18%) | 2 (2.94%) | 4 (5.06%) |
| Hoarseness | 1 (4.35%) | 3 (6.38%) | 4 (5.71%) | 0 (0%) | 0 (0%) | 0 (0%) c |
| Belching | 10 (43.48%) | 16 (34.04%) | 26 (37.14%) | 4 (36.36%) | 18 (26.47%) | 22 (27.85%) |
| Globus | 3 (13.04%) | 8 (17.02%) | 11 (15.71%) | 1 (9.09%) | 1 (1.47%) | 2 (2.53%) c |
| Anatomical EGJOO (n = 23) | Functional EGJOO (n = 47) | EGJOO at Baseline (n = 70) | AC with Organic Diseases (n = 11) | True AC (n = 68) | AC at Baseline (n = 79) | |
|---|---|---|---|---|---|---|
| UES mean basal pressure | 47.5 (30.6–58.3) | 58.1 (43.0–76.2) | 50.0 (38.2–70.8) | 50.3 (41.3–67.3) | 65.6 (41.0–87.4) | 62.3 (41.2–86.7) |
| UES residual pressure | 1.5 (−1.9–7.4) | 3.1 (−1.6–8.4) | 2.1 (−1.7–7.7) | 10.8 (3.0–14.8) | 9.4 (5.7–15.4) | 9.4 (5.6–15.1) b |
| UES relaxation time to nadir | 225.0 (129.0–280.0) | 150.0 (101.0–220.0) | 164.0 (110.3–234.5) | 297.0 (201.0–491.0) | 139.5 (101.8–209.0) a | 161.0 (102.0–252.5) |
| UES recovery time | 390.0 (331.0–592.0) | 519.0 (363.0–619.0) | 464.0 (344.5–608.0) | 457.0 (379.0–594.0) | 556.0 (416.0–699.5) | 537.0 (402.5–682.0) b |
| LES basal pressure (proximal) | 42.87 ± 2.83 | 41.98 ± 3.08 | 42.27 ± 3.01 | 44.18 ± 3.28 | 44.82 ± 3.34 | 44.74 ± 3.32 b |
| LES basal pressure (distal) | 46.57 ± 2.74 | 45.93 ± 2.83 | 46.14 ± 2.80 | 48.43 ± 2.68 | 48.05 ± 3.23 | 48.10 ± 3.15 b |
| LES basal pressure (minimum) | 22.37 ± 9.27 | 21.36 ± 8.82 | 21.69 ± 8.91 | 25.41 ± 12.07 | 29.95 ± 14.62 | 29.39 ± 14.34 b |
| LES basal pressure (mean) | 31.0 (25.1–37.2) | 28.2 (24.9–37.4) | 29.0 (25.1–37.3) | 32.1 (23.9–44.5) | 36.7 (28.3–48.3) | 36.0 (28.2–48.2) b |
| IRP | 19.2 (16.5–21.8) | 17.8 (16.5–21.6) | 17.9 (16.5–21.7) | 29.9 (21.6–38.5) | 31.4 (22.0–37.6) | 30.4 (22.1–37.6) b |
| LES residual pressure (maximum) | 25.5 (20.6–30.7) | 23.2 (21.1–27.2) | 23.7 (21.1–30.1) | 39.8 (26.4–49.7) | 41.0 (28.1–51.5) | 40.6 (27.8–49.7) b |
| Esophageal length | 25.38 ± 2.17 | 25.14 ± 1.75 | 25.22 ± 1.89 | 26.51 ± 2.37 | 27.34 ± 2.63 | 27.24 ± 2.60 b |
| LES length | 3.69 ± 0.81 | 3.95 ± 1.05 | 3.87 ± 0.98 | 4.26 ± 1.29 | 3.45 ± 0.79 | 3.55 ± 0.90 b |
| LES abdomen length | 2.7 (2.6–4.1) | 3.0 (2.4–3.6) | 3.0 (2.4–3.6) | 3.5 (2.5–4.8) | 2.7 (2.1–3.3) | 2.7 (2.1–3.4) |
| LES relaxation (%) | 44.35 ± 11.50 | 43.45 ± 11.21 | 43.74 ± 11.23 | 7.56 ± 10.93 | 19.67 ± 17.44 a | 18.18 ± 17.19 b |
| Hiatus hernia, n (%) | 1 (4.35%) | 0 (0%) | 1 (1.43%) | 0 (0%) | 2 (2.94%) | 2 (2.53%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Yu, T.; Zhu, F.; Xu, Y.; Bao, Y.; Zhang, L.; Lin, L.; Tang, Y. Esophagogastric Junction Outflow Obstruction Is Likely to Be a Local Manifestation of Other Primary Diseases: Analysis of Single-Center 4-Year Follow-Up Data. Diagnostics 2023, 13, 2329. https://doi.org/10.3390/diagnostics13142329
Wang Y, Yu T, Zhu F, Xu Y, Bao Y, Zhang L, Lin L, Tang Y. Esophagogastric Junction Outflow Obstruction Is Likely to Be a Local Manifestation of Other Primary Diseases: Analysis of Single-Center 4-Year Follow-Up Data. Diagnostics. 2023; 13(14):2329. https://doi.org/10.3390/diagnostics13142329
Chicago/Turabian StyleWang, Yan, Ting Yu, Feng Zhu, Ying Xu, Yun Bao, Ling Zhang, Lin Lin, and Yurong Tang. 2023. "Esophagogastric Junction Outflow Obstruction Is Likely to Be a Local Manifestation of Other Primary Diseases: Analysis of Single-Center 4-Year Follow-Up Data" Diagnostics 13, no. 14: 2329. https://doi.org/10.3390/diagnostics13142329
APA StyleWang, Y., Yu, T., Zhu, F., Xu, Y., Bao, Y., Zhang, L., Lin, L., & Tang, Y. (2023). Esophagogastric Junction Outflow Obstruction Is Likely to Be a Local Manifestation of Other Primary Diseases: Analysis of Single-Center 4-Year Follow-Up Data. Diagnostics, 13(14), 2329. https://doi.org/10.3390/diagnostics13142329






