Intravenous Immunoglobulin in Kawasaki Disease—Evolution and Pathogenic Mechanisms
Abstract
1. Background
2. History of the Evolution of IVIg as a Treatment for KD
3. Mechanisms of Action of IVIg
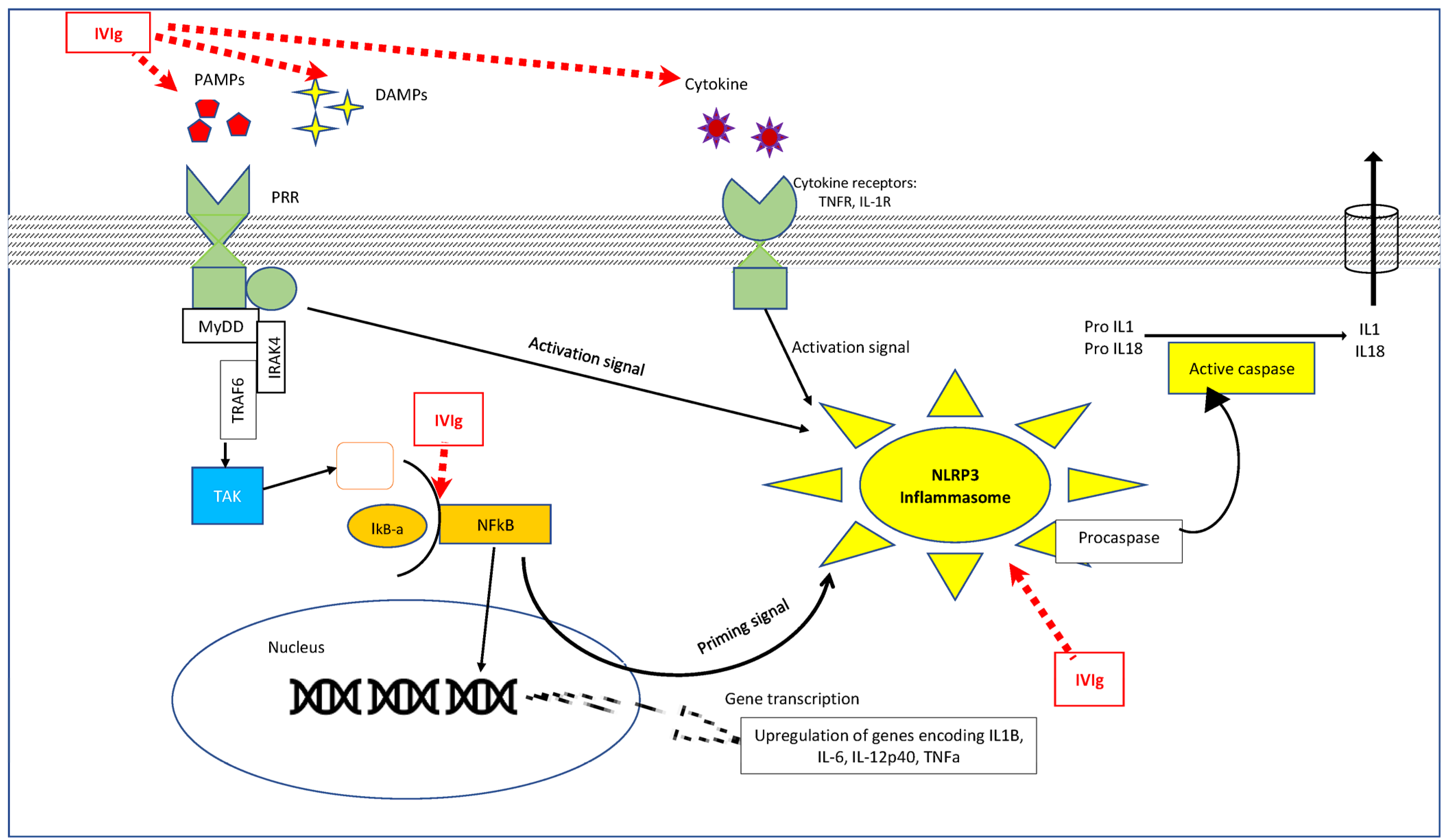
3.1. Effect of IVIg on the Innate Immune System (Figure 1)

3.1.1. Interaction with Neutrophils
3.1.2. Interaction with Macrophages (Figure 1)
3.1.3. Interaction with Vascular Endothelial Cells (Figure 2)
3.1.4. Interaction with NK Cells
3.1.5. Interaction with Dendritic Cells (DCs)
3.1.6. Interactions with Molecules in the Innate Immune System: (Figure 2)
3.2. Adaptive Immunity
3.2.1. Interaction with T Cells
3.2.2. Interaction with Antibodies
4. IVIg Refractory KD
5. Time to IVIg Administration since Onset of Fever—Early versus Late IVIg Treatment
6. Data on the Duration of IVIg Infusion
7. Effect of the Strength of IVIg Concentration—5% versus 10%
8. Recent Data on the Efficacy of Different Doses of IVIg in the Treatment of KD—1 g/kg versus 2 g/kg
9. Side Effect Profile
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Kawasaki, T. Kawasaki Disease in India, Lessons Learnt Over the Last 20 Years. Indian. Pediatr. 2016, 53, 119–124. [Google Scholar] [CrossRef]
- Kawasaki, T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi Allergy 1967, 16, 178–222. [Google Scholar]
- Kato, H.; Koike, S.; Yokoyama, T. Kawasaki disease: Effect of treatment on coronary artery involvement. Pediatrics 1979, 63, 175–179. [Google Scholar] [CrossRef]
- Furusho, K.; Kamiya, T.; Nakano, H.; Kiyosawa, N.; Shinomiya, K.; Hayashidera, T.; Tamura, T.; Hirose, O.; Manabe, Y.; Yokoyama, T.; et al. Intravenous gamma-globulin for Kawasaki disease. Acta Paediatr. Jpn. 1991, 33, 799–804. [Google Scholar] [CrossRef]
- Furusho, K.; Kamiya, T.; Nakano, H.; Kiyosawa, N.; Shinomiya, K.; Hayashidera, T.; Tamura, T.; Hirose, O.; Manabe, Y.; Yokoyama, T.; et al. High-dose intravenous gammaglobulin for Kawasaki disease. Lancet 1984, 2, 1055–1058. [Google Scholar] [CrossRef] [PubMed]
- Engle, M.A.; Fatica, N.S.; Bussel, J.B.; O’Loughlin, J.E.; Snyder, M.S.; Lesser, M.L. Clinical trial of single-dose intravenous gamma globulin in acute Kawasaki disease—Preliminary report. Am. J. Dis. Child. 1989, 143, 1300–1304. [Google Scholar] [CrossRef] [PubMed]
- Barron, K.S.; Murphy, D.J., Jr.; Silverman, E.D.; Ruttenberg, H.D.; Wright, G.B.; Franklin, W.; Goldberg, S.J.; Higashino, S.M.; Cox, D.G.; Lee, M. Treatment of Kawasaki syndrome: A comparison of two dosage regimens of intravenously administered immune globulin. J. Pediatr. 1990, 117, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Newburger, J.W.; Takahashi, M.; Burns, J.C.; Beiser, A.S.; Chung, K.J.; Duffy, C.E.; Glode, M.P.; Mason, W.H.; Reddy, V.; Sanders, S.P.; et al. The treatment of Kawasaki syndrome with intravenous gamma globulin. N. Engl. J. Med. 1986, 315, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Furusho, K.; Kamiya, T.; Nakano, H.; Kiyosawa, N.; Shinomiya, K.; Hayashidera, T.; Tamura, T.; Hirose, O.; Manabe, Y.; Yokoyama, T.; et al. Japanese gamma globulin trials for Kawasaki disease. Prog. Clin. Biol. Res. 1987, 250, 425–432. [Google Scholar]
- Ogawa, M.; Ogino, H.; Harima, Y.; Kono, S.; Ohkuni, H.; Nishida, M.; Kobayashi, Y.; Yabuuchi, H. The study of efficacy of a high-dose intravenous gammaglobulin therapy for Kawasaki disease. In Proceedings of the Third International Kawasaki Disease Symposium, Tokyo, Japan, 29 November–2 December 1988. [Google Scholar]
- Katoh, T.; Iwasa, K.; Sugiyama, K.; Ando, T.; Nomura, H.; Wada, Y. Prediction of high risk patients and effect of gammaglobulin treatment in Kawasaki disease. In Proceedings of the Third International Kawasaki Disease Symposium, Tokyo, Japan, 29 November–2 December 1988. [Google Scholar]
- Newburger, J.W.; Takahashi, M.; Beiser, A.S.; Burns, J.C.; Bastian, J.; Chung, K.J.; Colan, S.D.; Duffy, C.E.; Fulton, D.R.; Glode, M.P.; et al. A single intravenous infusion of gamma globulin as compared with four infusions in the treatment of acute Kawasaki syndrome. N. Engl. J. Med. 1991, 324, 1633–1639. [Google Scholar] [CrossRef]
- Finberg, R.W.; Newburger, J.W.; Mikati, M.A.; Heller, A.H.; Burns, J.C. Effect of high doses of intravenously administered immune globulin on natural killer cell activity in peripheral blood. J. Pediatr. 1992, 120, 376–380. [Google Scholar] [CrossRef]
- Mouthon, L.; Kaveri, S.V.; Spalter, S.H.; Lacroix-Desmazes, S.; Lefranc, C.; Desai, R.; Kazatchkine, M.D. Mechanisms of action of intravenous immune globulin in immune-mediated diseases. Clin. Exp. Immunol. 1996, 104 (Suppl. S1), 3–9. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. Off. J. Can. Soc. Allergy Clin. Immunol. 2018, 14 (Suppl. S2), 49. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240–273. [Google Scholar] [CrossRef]
- Hara, T.; Nakashima, Y.; Sakai, Y.; Nishio, H.; Motomura, Y.; Yamasaki, S. Kawasaki disease: A matter of innate immunity. Clin. Exp. Immunol. 2016, 186, 134–143. [Google Scholar] [CrossRef]
- Takeshita, S.; Sekine, I.; Fujisawa, T.; Yoshioka, S. Studies of peripheral blood toxic neutrophils as a predictor of coronary risk in Kawasaki disease—The pathogenetic role of hematopoietic colony-stimulating factors (GM-CSF, G-CSF). Acta Paediatr. Jpn. Overseas Ed. 1990, 32, 508–514. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Oharaseki, T.; Yokouchi, Y.; Hiruta, N.; Naoe, S. Kawasaki Disease as a Systemic Vasculitis in Childhood. Ann. Vasc. Dis. 2010, 3, 173–181. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Takeshita, S.; Nakatani, K.; Kawamura, Y.; Tokutomi, T.; Sekine, I. Delayed apoptosis of circulating neutrophils in Kawasaki disease. Clin. Exp. Immunol. 2001, 126, 355–364. [Google Scholar] [CrossRef]
- Takeshita, S.; Kawamura, Y.; Kanai, T.; Yoshida, Y.; Tsujita, Y.; Nonoyama, S. The Role of Neutrophil Activation in the Pathogenesis of Kawasaki Disease. Pediatr. Infect. Dis. 2017, 30, 3. [Google Scholar] [CrossRef]
- Takeshita, S.; Tsujimoto, H.; Nakatani, K. Intravenous immunoglobulin preparations promote apoptosis in lipopolysaccharide-stimulated neutrophils via an oxygen-dependent pathway in vitro. Acta Pathol. Microbiol. Immunol. Scand. 2005, 113, 269–277. [Google Scholar] [CrossRef]
- Tsujimoto, H.; Takeshita, S.; Nakatani, K.; Kawamura, Y.; Tokutomi, T.; Sekine, I. Intravenous immunoglobulin therapy induces neutrophil apoptosis in Kawasaki disease. Clin. Immunol. Orlando Fla. 2002, 103, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Huang, F.C.; Huang, Y.H.; Kuo, H.C.; Li, S.C. Identifying Downregulation of Autophagy Markers in Kawasaki Disease. Children 2020, 7, 166. [Google Scholar] [CrossRef]
- Yamashita, K.; Takaori-Kondo, A.; Mizugishi, K. Exaggerated neutrophil extracellular trap formation in Kawasaki disease: A key phenomenon behind the outbreak in western countries? Ann. Rheum. Dis. 2020, 21, e178. [Google Scholar] [CrossRef] [PubMed]
- Masso-Silva, J.A.; Sakoulas, G.; Olay, J.; Groysberg, V.; Geriak, M.; Nizet, V.; Crotty Alexander, L.E.; Meier, A. Abrogation of neutrophil inflammatory pathways and potential reduction of neutrophil-related factors in COVID-19 by intravenous immunoglobulin. Front. Immunol. 2022, 13, 993720. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Qian, W.; Ling, J.; Xu, T.; Wang, T.; Shi, Y.; Ju, L. Neutrophil Extracellular Traps Formation and Citrullinated Histones 3 Level In Patients with Kawasaki Disease. 2022. Available online: https://www.researchsquare.com/article/rs-2303527/v1 (accessed on 6 July 2023).
- Abe, J.; Jibiki, T.; Noma, S.; Nakajima, T.; Saito, H.; Terai, M. Gene expression profiling of the effect of high-dose intravenous Ig in patients with Kawasaki disease. J. Immunol. Baltim. Md. 1950, 174, 5837–5845. [Google Scholar] [CrossRef]
- Nagelkerke, S.Q.; Dekkers, G.; Kustiawan, I.; van de Bovenkamp, F.S.; Geissler, J.; Plomp, R.; Wuhrer, M.; Vidarsson, G.; Rispens, T.; van den Berg, T.K.; et al. Inhibition of FcγR-mediated phagocytosis by IVIg is independent of IgG-Fc sialylation and FcγRIIb in human macrophages. Blood 2014, 124, 3709–3718. [Google Scholar] [CrossRef]
- Nakashima, Y.; Sakai, Y.; Mizuno, Y.; Furuno, K.; Hirono, K.; Takatsuki, S.; Suzuki, H.; Onouchi, Y.; Kobayashi, T.; Tanabe, K.; et al. Lipidomics links oxidized phosphatidylcholines and coronary arteritis in Kawasaki disease. Cardiovasc. Res. 2021, 117, 96–108. [Google Scholar] [CrossRef]
- Di Gioia, M.; Spreafico, R.; Springstead, J.R.; Mendelson, M.M.; Joehanes, R.; Levy, D.; Zanoni, I. Endogenous oxidized phospholipids reprogram cellular metabolism and boost hyperinflammation. Nat. Immunol. 2020, 21, 42–53. [Google Scholar] [CrossRef]
- Hamamichi, Y.; Ichida, F.; Yu, X.; Hirono, K.I.; Uese, K.I.; Hashimoto, I.; Tsubata, S.; Yoshida, T.; Futatani, T.; Kanegane, H.; et al. Neutrophils and mononuclear cells express vascular endothelial growth factor in acute Kawasaki disease: Its possible role in progression of coronary artery lesions. Pediatr. Res. 2001, 49, 74–80. [Google Scholar] [CrossRef]
- Hokibara, S.; Kobayashi, N.; Kobayashi, K.; Shigemura, T.; Nagumo, H.; Takizawa, M.; Yamazaki, T.; Agematsu, K. Markedly elevated CD64 expression on neutrophils and monocytes as a biomarker for diagnosis and therapy assessment in Kawasaki disease. Inflamm. Res. 2016, 65, 579–585. [Google Scholar] [CrossRef]
- Kobayashi, T.; Kimura, H.; Okada, Y.; Inoue, Y.; Kobayashi, T.; Shinohara, M.; Morikawa, A. Increased CD11b expression on polymorphonuclear leucocytes and cytokine profiles in patients with Kawasaki disease. Clin. Exp. Immunol. 2007, 148, 112–118. [Google Scholar] [CrossRef] [PubMed]
- McAlpine, S.M.; Roberts, S.E.; Heath, J.J.; Käsermann, F.; Issekutz, A.C.; Issekutz, T.B.; Derfalvi, B. High Dose Intravenous IgG Therapy Modulates Multiple NK Cell and T Cell Functions in Patients With Immune Dysregulation. Front. Immunol. 2021, 12, 660506. [Google Scholar] [CrossRef] [PubMed]
- Bayry, J.; Lacroix-Desmazes, S.; Carbonneil, C.; Misra, N.; Donkova, V.; Pashov, A.; Chevailler, A.; Mouthon, L.; Weill, B.; Bruneval, P.; et al. Inhibition of maturation and function of dendritic cells by intravenous immunoglobulin. Blood 2003, 101, 758–765. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Chen, Z.; Zhang, F.; Zhang, Q.; Sun, L.; Lv, H.; Wang, B.; Shen, J.; Zhou, X.; Chen, F.; et al. Intravenous Immunoglobulin Therapy Restores the Quantity and Phenotype of Circulating Dendritic Cells and CD4+ T Cells in Children With Acute Kawasaki Disease. Front. Immunol. 2022, 13, 802690. [Google Scholar] [CrossRef]
- Burns, J.C.; Song, Y.; Bujold, M.; Shimizu, C.; Kanegaye, J.T.; Tremoulet, A.H.; Franco, A. Immune-monitoring in Kawasaki disease patients treated with infliximab and intravenous immunoglobulin. Clin. Exp. Immunol. 2013, 174, 337–344. [Google Scholar] [CrossRef]
- Tang, D.; Kang, R.; Coyne, C.B.; Zeh, H.J.; Lotze, M.T. PAMPs and DAMPs: Signal 0s that spur autophagy and immunity. Immunol. Rev. 2012, 249, 158–175. [Google Scholar] [CrossRef]
- Nakamura, A.; Ikeda, K.; Hamaoka, K. Aetiological Significance of Infectious Stimuli in Kawasaki Disease. Front. Pediatr. 2019, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Kusuda, T.; Nakashima, Y.; Murata, K.; Kanno, S.; Nishio, H.; Saito, M.; Tanaka, T.; Yamamura, K.; Sakai, Y.; Takada, H.; et al. Kawasaki disease-specific molecules in the sera are linked to microbe-associated molecular patterns in the biofilms. PLoS ONE 2014, 9, e113054. [Google Scholar] [CrossRef]
- Okuzaki, D.; Ota, K.; Takatsuki, S.I.; Akiyoshi, Y.; Naoi, K.; Yabuta, N.; Saji, T.; Nojima, H. FCN1 (M-ficolin), which directly associates with immunoglobulin G1, is a molecular target of intravenous immunoglobulin therapy for Kawasaki disease. Sci. Rep. 2017, 7, 11334. [Google Scholar] [CrossRef]
- Gupta, A. Role of Inflammasomes in Kawasaki Disease. Indian J. Pediatr. 2023, 90, 5–6. [Google Scholar] [CrossRef]
- Ji, M.L.; Dong, J.Y.; Xu, Y.; Pan, Y.T.; Fan, Z.D.; Yu, H.G. Inositol-Triphosphate 3-Kinase C and DNA Methylation Involvement in NLRP3 Inflammasome Activation in Kawasaki Disease. Indian J. Pediatr. 2023, 90, 22–28. [Google Scholar] [CrossRef]
- Anzai, F.; Watanabe, S.; Kimura, H.; Kamata, R.; Karasawa, T.; Komada, T.; Nakamura, J.; Nagi-Miura, N.; Ohno, N.; Takeishi, Y.; et al. Crucial role of NLRP3 inflammasome in a murine model of Kawasaki disease. J. Mol. Cell Cardiol. 2020, 138, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Fann, D.Y.; Lee, S.Y.; Manzanero, S.; Tang, S.C.; Gelderblom, M.; Chunduri, P.; Bernreuther, C.; Glatzel, M.; Cheng, Y.L.; Thundyil, J.; et al. Intravenous immunoglobulin suppresses NLRP1 and NLRP3 inflammasome-mediated neuronal death in ischemic stroke. Cell Death Dis. 2013, 4, e790. [Google Scholar] [CrossRef] [PubMed]
- Ichiyama, T.; Ueno, Y.; Isumi, H.; Niimi, A.; Matsubara, T.; Furukawa, S. An immunoglobulin agent (IVIG) inhibits NF-kappaB activation in cultured endothelial cells of coronary arteries in vitro. Inflamm. Res. 2004, 53, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Wakiguchi, H.; Hasegawa, S.; Suzuki, Y.; Kudo, K.; Ichiyama, T. Relationship between T-cell HLA-DR expression and intravenous immunoglobulin treatment response in Kawasaki disease. Pediatr. Res. 2015, 77, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.F.; Li, C.R.; Li, Q.; Xia, Y.; Wang, G.B.; Yang, J. Regulatory T cell microRNA expression changes in children with acute Kawasaki disease. Clin. Exp. Immunol. 2014, 178, 384–393. [Google Scholar] [CrossRef] [PubMed]
- Rivino, L.; Gruarin, P.; Häringer, B.; Steinfelder, S.; Lozza, L.; Steckel, B.; Weick, A.; Sugliano, E.; Jarrossay, D.; Kühl, A.A.; et al. CCR6 is expressed on an IL-10-producing, autoreactive memory T cell population with context-dependent regulatory function. J. Exp. Med. 2010, 207, 565–577. [Google Scholar] [CrossRef]
- Burns, J.C.; Touma, R.; Song, Y.; Padilla, R.L.; Tremoulet, A.H.; Sidney, J.; Sette, A.; Franco, A. Fine specificities of natural regulatory T cells after IVIG therapy in patients with Kawasaki disease. Autoimmunity 2015, 48, 181–188. [Google Scholar] [CrossRef]
- Furuno, K.; Yuge, T.; Kusuhara, K.; Takada, H.; Nishio, H.; Khajoee, V.; Ohno, T.; Hara, T. CD25+CD4+ regulatory T cells in patients with Kawasaki disease. J. Pediatr. 2004, 145, 385–390. [Google Scholar] [CrossRef]
- Hicar, M.D. Antibodies and Immunity During Kawasaki Disease. Front. Cardiovasc. Med. 2020, 7, 94. [Google Scholar] [CrossRef]
- Kazatchkine, M.D.; Kaveri, S.V. Immunomodulation of autoimmune and inflammatory diseases with intravenous immune globulin. N. Engl. J. Med. 2001, 345, 747–755. [Google Scholar] [CrossRef] [PubMed]
- McCrindle, B.W.; Rowley, A.H.; Newburger, J.W.; Burns, J.C.; Bolger, A.F.; Gewitz, M.; Baker, A.L.; Jackson, M.A.; Takahashi, M.; Shah, P.B.; et al. Diagnosis, Treatment, and Long-Term Management of Kawasaki Disease: A Scientific Statement for Health Professionals From the American Heart Association. Circulation 2017, 135, e927–e999. [Google Scholar] [CrossRef] [PubMed]
- Portman, M.A.; Wiener, H.W.; Silva, M.; Shendre, A.; Shrestha, S. DC-SIGN gene promoter variants and IVIG treatment response in Kawasaki disease. Pediatr. Rheumatol. Online J. 2013, 11, 32. [Google Scholar] [CrossRef]
- Weng, K.P.; Hsieh, K.S.; Ho, T.Y.; Huang, S.H.; Lai, C.R.; Chiu, Y.T.; Huang, S.C.; Lin, C.C.; Hwang, Y.T.; Ger, L.P. IL-1B polymorphism in association with initial intravenous immunoglobulin treatment failure in Taiwanese children with Kawasaki disease. Circ. J. 2010, 74, 544–551. [Google Scholar] [CrossRef]
- Shrestha, S.; Wiener, H.; Shendre, A.; Kaslow, R.A.; Wu, J.; Olson, A.; Bowles, N.E.; Patel, H.; Edberg, J.C.; Portman, M.A. Role of activating FcγR gene polymorphisms in Kawasaki disease susceptibility and intravenous immunoglobulin response. Circ. Cardiovasc. Genet. 2012, 5, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, S.; Wiener, H.W.; Olson, A.K.; Edberg, J.C.; Bowles, N.E.; Patel, H.; Portman, M.A. Functional FCGR2B gene variants influence intravenous immunoglobulin response in patients with Kawasaki disease. J. Allergy Clin. Immunol. 2011, 128, 677–680. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, Y.; Zhou, H.; Wang, Y.; Li, W.; Lu, Z.; Jiang, Z.; Gu, X.; Zheng, H.; Zeng, L.; et al. Association between P2RY12 Gene Polymorphisms and IVIG Resistance in Kawasaki Patients. Cardiovasc. Ther. 2020, 2020, 3568608. [Google Scholar] [CrossRef]
- Ahn, J.G.; Bae, Y.; Shin, D.; Nam, J.; Kim, K.Y.; Kim, D.S. HMGB1 gene polymorphism is associated with coronary artery lesions and intravenous immunoglobulin resistance in Kawasaki disease. Rheumatology 2019, 58, 770–775. [Google Scholar] [CrossRef]
- Kobayashi, T.; Inoue, Y.; Takeuchi, K.; Okada, Y.; Tamura, K.; Tomomasa, T.; Kobayashi, T.; Morikawa, A. Prediction of intravenous immunoglobulin unresponsiveness in patients with Kawasaki disease. Circulation 2006, 113, 2606–2612. [Google Scholar] [CrossRef]
- Sano, T.; Kurotobi, S.; Matsuzaki, K.; Yamamoto, T.; Maki, I.; Miki, K.; Kogaki, S.; Hara, J. Prediction of non-responsiveness to standard high-dose gamma-globulin therapy in patients with acute Kawasaki disease before starting initial treatment. Eur. J. Pediatr. 2007, 166, 131–137. [Google Scholar] [CrossRef]
- Egami, K.; Muta, H.; Ishii, M.; Suda, K.; Sugahara, Y.; Iemura, M.; Matsuishi, T. Prediction of resistance to intravenous immunoglobulin treatment in patients with Kawasaki disease. J. Pediatr. 2006, 149, 237–240. [Google Scholar] [CrossRef]
- Shashaani, N.; Shiari, R.; Karimi, A.; Salehi, S.; Ghanaei, R.; Hassas Yeganeh, M.; Shiari, S.; Rahmani, K.; Javadi Parvaneh, V. Determination of the Relationship Between Kobayashi, Sano, and Egami Criteria and Prevalence of Intravenous Immunoglobulin Resistance and Coronary Artery Aneurysm in Iranian Children with Kawasaki Disease. Open Access Rheumatol. 2020, 12, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, Y.; Tang, Y.; Ding, Y.; Xu, Q.; Sun, L.; Qian, W.; Qian, G.; Qin, L.; Lv, H. Predictors of intravenous immunoglobulin-resistant Kawasaki disease in children: A meta-analysis of 4442 cases. Eur. J. Pediatr. 2018, 177, 1279–1292. [Google Scholar] [CrossRef]
- Terai, M.; Honda, T.; Yasukawa, K.; Higashi, K.; Hamada, H.; Kohno, Y. Prognostic impact of vascular leakage in acute Kawasaki disease. Circulation 2003, 108, 325–330. [Google Scholar] [CrossRef]
- Friedman, K.G.; Gauvreau, K.; Hamaoka-Okamoto, A.; Tang, A.; Berry, E.; Tremoulet, A.H.; Mahavadi, V.S.; Baker, A.; deFerranti, S.D.; Fulton, D.R.; et al. Coronary Artery Aneurysms in Kawasaki Disease: Risk Factors for Progressive Disease and Adverse Cardiac Events in the US Population. J. Am. Heart Assoc. 2016, 5, e003289. [Google Scholar] [CrossRef]
- McCrindle, B.W.; Cifra, B. The role of echocardiography in Kawasaki disease. Int. J. Rheum. Dis. 2018, 21, 50–55. [Google Scholar] [CrossRef]
- Chbeir, D.; Gaschignard, J.; Bonnefoy, R.; Beyler, C.; Melki, I.; Faye, A.; Meinzer, U. Kawasaki disease: Abnormal initial echocardiogram is associated with resistance to IV Ig and development of coronary artery lesions. Pediatr. Rheumatol. Online J. 2018, 16, 48. [Google Scholar] [CrossRef]
- Bar-Meir, M.; Kalisky, I.; Schwartz, A.; Somekh, E.; Tasher, D.; Israeli Kawasaki Group. Prediction of Resistance to Intravenous Immunoglobulin in Children With Kawasaki Disease. J. Pediatr. Infect. Dis. Soc. 2018, 7, 25–29. [Google Scholar] [CrossRef]
- Son, M.B.F.; Gauvreau, K.; Kim, S.; Tang, A.; Dedeoglu, F.; Fulton, D.R.; Lo, M.S.; Baker, A.L.; Sundel, R.P.; Newburger, J.W. Predicting Coronary Artery Aneurysms in Kawasaki Disease at a North American Center: An Assessment of Baseline z Scores. J. Am. Heart Assoc. 2017, 6, e005378. [Google Scholar] [CrossRef] [PubMed]
- Samadli, S.; Liu, F.F.; Mammadov, G.; Wang, J.J.; Liu, H.H.; Wu, Y.F.; Luo, H.H.; Wu, Y.; Chen, W.X.; Zhang, D.D.; et al. The time option of IVIG treatment is associated with therapeutic responsiveness and coronary artery abnormalities but not with clinical classification in the acute episode of Kawasaki disease. Pediatr. Rheumatol. Online J. 2019, 17, 53. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y.; Inuzuka, R.; Shindo, T.; Mafune, R.; Hayashi, T.; Hirata, Y.; Shimizu, N.; Inatomi, J.; Yokoyama, Y.; Namai, Y.; et al. Effect of I.V. immunoglobulin in the first 4 days of illness in Kawasaki disease. Pediatr. Int. 2018, 60, 334–341. [Google Scholar] [PubMed]
- Cai, J.H.; Tang, M.; Zhang, H.X.; Dan Luo, E.; Zhang, R.; Shuai, S.P.; Liang, H.; Tao, W.J.; Wu, M.J.; Wen, Y.; et al. Therapeutic Window of Intravenous Immunoglobulin (IVIG) and its correlation with IVIG-resistant in Kawasaki Disease: A retrospective study. J. Pediatr. 2023, 99, 161–167. [Google Scholar] [CrossRef]
- Yan, F.; Zhang, H.; Xiong, R.; Cheng, X.; Chen, Y.; Zhang, F. Effect of Early Intravenous Immunoglobulin Therapy in Kawasaki Disease: A Systematic Review and Meta-Analysis. Front. Pediatr. 2020, 8, 593435. [Google Scholar] [CrossRef]
- Guo, Y.; Tian, X.; Wang, X.; Xiao, Z. Adverse Effects of Immunoglobulin Therapy. Front. Immunol. 2018, 9, 1299. [Google Scholar] [CrossRef]
- Fukui, S.; Seki, M.; Minami, T.; Kotani, K.; Oka, K.; Yokomizo, A.; Matsubara, D.; Sato, T.; Nozaki, Y.; Saito, M.; et al. Efficacy and safety associated with the infusion speed of intravenous immunoglobulin for the treatment of Kawasaki disease: A randomized controlled trial. Pediatr. Rheumatol. Online J. 2021, 19, 107. [Google Scholar] [CrossRef]
- Downie, M.L.; Manlhiot, C.; Latino, G.A.; Collins, T.H.; Chahal, N.; Yeung, R.S.; McCrindle, B.W. Variability in Response to Intravenous Immunoglobulin in the Treatment of Kawasaki Disease. J. Pediatr. 2016, 179, 124–130.e1. [Google Scholar] [CrossRef] [PubMed]
- Oda, T.; Nagata, H.; Nakashima, Y.; Nanishi, E.; Takada, Y.; Nishimura, M.; Kubo, E.; Hatae, K.; Ohga, S. Clinical Utility of Highly Purified 10% Liquid Intravenous Immunoglobulin in Kawasaki Disease. J. Pediatr. 2019, 214, 227–230. [Google Scholar] [CrossRef]
- Suzuki, T.; Michihata, N.; Yoshikawa, T.; Saito, K.; Matsui, H.; Fushimi, K.; Yasunaga, H. Low- versus high-concentration intravenous immunoglobulin for children with Kawasaki disease in the acute phase. Int. J. Rheum. Dis. 2022, 25, 576–583. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Suh, W.; Rhim, J.W. High-Concentration Intravenous Immunoglobulin May Influence the Course of Fever and Rate of Reported Treatment Resistance in Children With Kawasaki Disease: A Single-Center Retrospective Analysis. Paediatr. Drugs 2022, 24, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Qin, L.J.; Wang, H.W.; Hu, X.F.; Liu, Q.J.; Shi, H.; Wei, Y.X.; Chen, Q.J.; Chen, P.X. Therapeutic effectiveness of intravenous immunoglobulin at 1 g/kg and 2 g/kg on Kawasaki disease: A comparative and follow-up study. Zhonghua Er Ke Za Zhi Chin. J. Pediatr. 2006, 44, 891–895. [Google Scholar]
- Yeo, J.S.; Choi, J.W. Effectiveness of Medium-Dose Intravenous Immunoglobulin (1 g/kg) in the Treatment of Kawasaki Disease. Korean Circ. J. 2010, 40, 81–85. [Google Scholar] [CrossRef]
- He, L.; Liu, F.; Yan, W.; Huang, M.; Huang, M.; Xie, L.; Guo, Y.; Xu, X.; Chu, C.; Wu, L.; et al. Randomized trial of different initial intravenous immunoglobulin regimens in Kawasaki disease. Pediatr. Int. 2021, 63, 757–763. [Google Scholar] [CrossRef]
- Suzuki, T.; Michihata, N.; Yoshikawa, T.; Hata, T.; Matsui, H.; Fushimi, K.; Yasunaga, H. High-dose versus low-dose intravenous immunoglobulin for treatment of children with Kawasaki disease weighing 25 kg or more. Eur. J. Pediatr. 2020, 179, 1901–1907. [Google Scholar] [CrossRef] [PubMed]
- Ichihashi, K.; Shiraishi, H.; Momoi, M. Prediction of responsiveness or non-responsiveness to treatment of acute Kawasaki disease using 1 gram per kilogram of immunoglobulin--an effective and cost-saving schedule of therapy. Cardiol. Young 2009, 19, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, M.; Sugawara, D.; Makita, E.; Hirakubo, Y.; Nonaka, K.; Yamashita, S.; Ichihashi, K. Stratified therapy for Kawasaki disease using a new scoring system to predict the response to a lower dose of intravenous immunoglobulin therapy. Cardiol. Young 2022, 32, 405–409. [Google Scholar] [CrossRef]
- Burns, J.C.; Roberts, S.C.; Tremoulet, A.H.; He, F.; Printz, B.F.; Ashouri, N.; Jain, S.S.; Michalik, D.E.; Sharma, K.; Truong, D.T.; et al. Infliximab versus second intravenous immunoglobulin for treatment of resistant Kawasaki disease in the USA (KIDCARE): A randomised, multicentre comparative effectiveness trial. Lancet Child. Adolesc. Health 2021, 5, 852–861. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nadig, P.L.; Joshi, V.; Pilania, R.K.; Kumrah, R.; Kabeerdoss, J.; Sharma, S.; Suri, D.; Rawat, A.; Singh, S. Intravenous Immunoglobulin in Kawasaki Disease—Evolution and Pathogenic Mechanisms. Diagnostics 2023, 13, 2338. https://doi.org/10.3390/diagnostics13142338
Nadig PL, Joshi V, Pilania RK, Kumrah R, Kabeerdoss J, Sharma S, Suri D, Rawat A, Singh S. Intravenous Immunoglobulin in Kawasaki Disease—Evolution and Pathogenic Mechanisms. Diagnostics. 2023; 13(14):2338. https://doi.org/10.3390/diagnostics13142338
Chicago/Turabian StyleNadig, Pallavi L., Vibhu Joshi, Rakesh Kumar Pilania, Rajni Kumrah, Jayakanthan Kabeerdoss, Saniya Sharma, Deepti Suri, Amit Rawat, and Surjit Singh. 2023. "Intravenous Immunoglobulin in Kawasaki Disease—Evolution and Pathogenic Mechanisms" Diagnostics 13, no. 14: 2338. https://doi.org/10.3390/diagnostics13142338
APA StyleNadig, P. L., Joshi, V., Pilania, R. K., Kumrah, R., Kabeerdoss, J., Sharma, S., Suri, D., Rawat, A., & Singh, S. (2023). Intravenous Immunoglobulin in Kawasaki Disease—Evolution and Pathogenic Mechanisms. Diagnostics, 13(14), 2338. https://doi.org/10.3390/diagnostics13142338






