The Impact of Liquid Biopsies Positive for EGFR Mutations on Overall Survival in Non-Small Cell Lung Cancer Patients
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Tissue Biopsy Procedure
2.3. Liquid Biopsy Procedure
2.4. Statistical Analysis
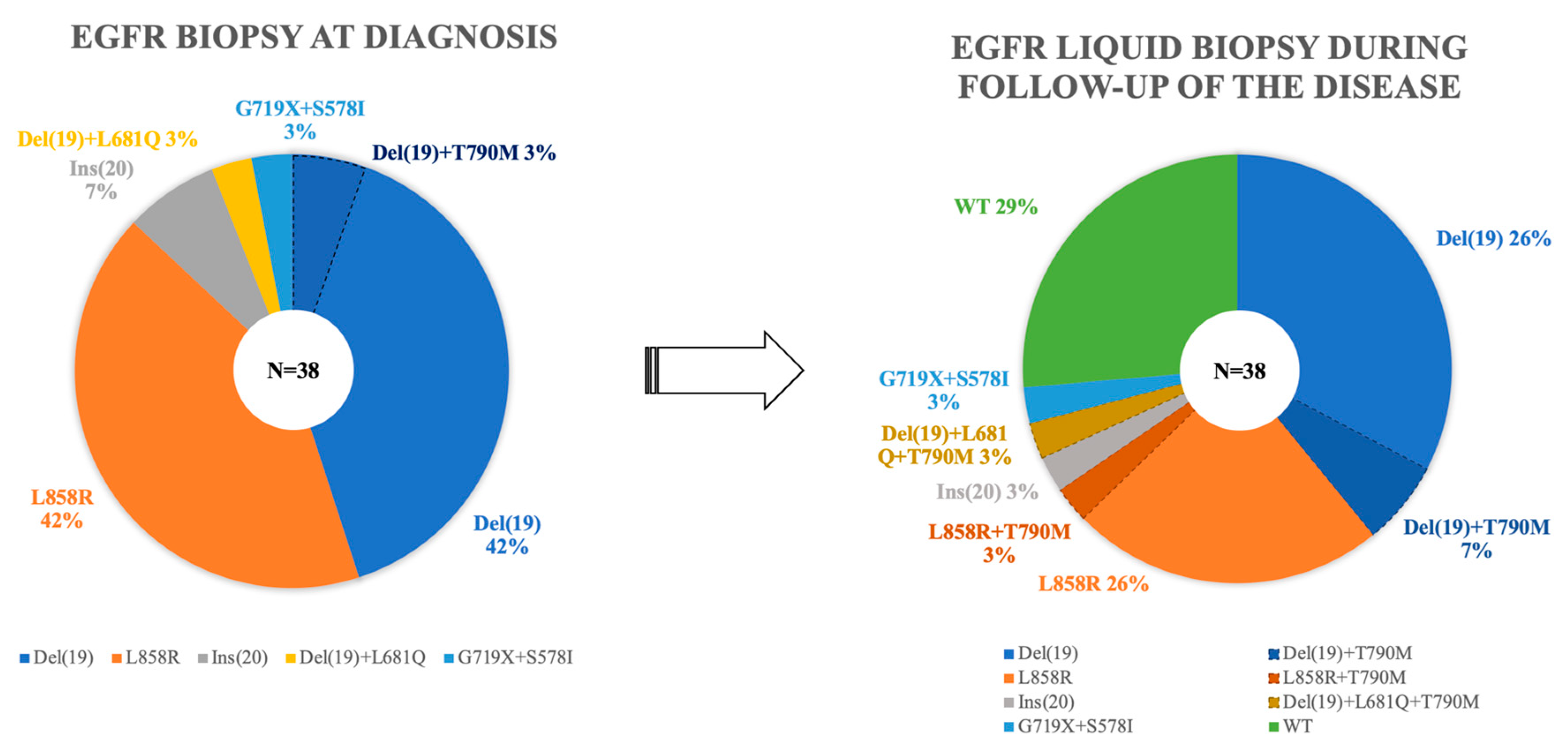
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global Epidemiology of Lung Cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; DVM, A.J. Cancer Statistics, 2022—Siegel—2022—CA: A Cancer Journal for Clinicians—Wiley Online Library. Available online: https://acsjournals.onlinelibrary.wiley.com/doi/full/10.3322/caac.21708?utm_source=newsletter&utm_medium=email&utm_campaign=work-in-progress&utm_content=20230117 (accessed on 21 March 2023).
- WHO Classification of Tumours Editorial Board. Thoracic Tumours; WHO: Geneva, Switzerland, 2021; ISBN 978-92-832-4506-3. [Google Scholar]
- Rábade Castedo, C.; de Granda-Orive, J.I.; González-Barcala, F.J. Incremento de la prevalencia del tabaquismo: ¿causas y actuación? Arch. Bronconeumol. 2019, 55, 557–558. [Google Scholar] [CrossRef]
- Lidón-Moyano, C.; Fu, M.; Ballbè, M.; Martín-Sánchez, J.C.; Matilla-Santander, N.; Martínez, C.; Fernández, E.; Martínez-Sánchez, J.M. Impact of the Spanish smoking laws on tobacco consumption and secondhand smoke exposure: A longitudinal population study. Addict. Behav. 2017, 75, 30–35. [Google Scholar] [CrossRef]
- MacRosty, C.R.; Rivera, M.P. Lung Cancer in Women. Clin. Chest Med. 2020, 41, 53–65. [Google Scholar] [CrossRef]
- Stapelfeld, C.; Dammann, C.; Maser, E. Sex-specificity in lung cancer risk. Int. J. Cancer 2020, 146, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Yang, X.; Huang, Y.; Zhao, M.; Li, M.; Ma, K.; Yin, J.; Zhan, C.; Wang, Q. Trends in the incidence, treatment, and survival of patients with lung cancer in the last four decades. Cancer Manag. Res. 2019, 11, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Rosell, R.; Moran, T.; Queralt, C.; Porta, R.; Cardenal, F.; Camps, C.; Majem, M.; Lopez-Vivanco, G.; Isla, D.; Provencio, M.; et al. Screening for Epidermal Growth Factor Receptor Mutations in Lung Cancer. N. Engl. J. Med. 2009, 361, 958–967. [Google Scholar] [CrossRef]
- Tan, A.C.; Tan, D.S.W. Targeted Therapies for Lung Cancer Patients with Oncogenic Driver Molecular Alterations. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2022, 40, 611–625. [Google Scholar] [CrossRef]
- Gendarme, S.; Bylicki, O.; Chouaid, C.; Guisier, F. ROS-1 Fusions in Non-Small-Cell Lung Cancer: Evidence to Date. Curr. Oncol. Tor. Ont 2022, 29, 641–658. [Google Scholar] [CrossRef]
- Cascetta, P.; Sforza, V.; Manzo, A.; Carillio, G.; Palumbo, G.; Esposito, G.; Montanino, A.; Costanzo, R.; Sandomenico, C.; De Cecio, R.; et al. RET Inhibitors in Non-Small-Cell Lung Cancer. Cancers 2021, 13, 4415. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.; Choi, Y.-L.; Lee, H.; Hwang, S.; Lee, B.; Yang, H.; Chelakkot, C.; Han, J. Selection Strategies and Practical Application of BRAF V600E-Mutated Non-Small Cell Lung Carcinoma. Cancer Res. Treat. 2022, 54, 782–792. [Google Scholar] [CrossRef] [PubMed]
- Imyanitov, E.N.; Iyevleva, A.G.; Levchenko, E.V. Molecular testing and targeted therapy for non-small cell lung cancer: Current status and perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103194. [Google Scholar] [CrossRef] [PubMed]
- Alexander, M.; Kim, S.Y.; Cheng, H. Update 2020: Management of Non-Small Cell Lung Cancer. Lung 2020, 198, 897–907. [Google Scholar] [CrossRef]
- Reck, M.; Carbone, D.P.; Garassino, M.; Barlesi, F. Targeting KRAS in non-small-cell lung cancer: Recent progress and new approaches. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021, 32, 1101–1110. [Google Scholar] [CrossRef]
- Patel, S.A.; Weiss, J. Advances in the Treatment of Non-Small Cell Lung Cancer: Immunotherapy. Clin. Chest Med. 2020, 41, 237–247. [Google Scholar] [CrossRef]
- Isla, D.; Lozano, M.D.; Paz-Ares, L.; Salas, C.; de Castro, J.; Conde, E.; Felip, E.; Gómez-Román, J.; Garrido, P.; Enguita, A.B. New update to the guidelines on testing predictive biomarkers in non-small-cell lung cancer: A National Consensus of the Spanish Society of Pathology and the Spanish Society of Medical Oncology. Clin. Transl. Oncol. Off. Publ. Fed. Span. Oncol. Soc. Natl. Cancer Inst. Mex. 2022, 25, 1252–1267. [Google Scholar] [CrossRef]
- Rolfo, C.; Mack, P.C.; Scagliotti, G.V.; Baas, P.; Barlesi, F.; Bivona, T.G.; Herbst, R.S.; Mok, T.S.; Peled, N.; Pirker, R.; et al. Liquid Biopsy for Advanced Non-Small Cell Lung Cancer (NSCLC): A Statement Paper from the IASLC. J. Thorac. Oncol. 2018, 13, 1248–1268. [Google Scholar] [CrossRef]
- Mosele, F.; Remon, J.; Mateo, J.; Westphalen, C.B.; Barlesi, F.; Lolkema, M.P.; Normanno, N.; Scarpa, A.; Robson, M.; Meric-Bernstam, F.; et al. Recommendations for the use of next-generation sequencing (NGS) for patients with metastatic cancers: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2020, 31, 1491–1505. [Google Scholar] [CrossRef]
- Mlika, M.; Hofman, P.; Dziri, C.; Mezni, F. La biopsie liquide dans le cancer du poumon Liquid biopsy in lung cancer. Tunis. Med. 2017, 95, 965–971. [Google Scholar]
- Bernabé, R.; Hickson, N.; Wallace, A.; Blackhall, F.H. What do we need to make circulating tumour DNA (ctDNA) a routine diagnostic test in lung cancer? Eur. J. Cancer 2017, 81, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Bonanno, L.; Dal Maso, A.; Pavan, A.; Zulato, E.; Calvetti, L.; Pasello, G.; Guarneri, V.; Conte, P.; Indraccolo, S. Liquid biopsy and non-small cell lung cancer: Are we looking at the tip of the iceberg? Br. J. Cancer 2022, 127, 383–393. [Google Scholar] [CrossRef]
- Vicidomini, G.; Cascone, R.; Carlucci, A.; Fiorelli, A.; Di Domenico, M.; Santini, M. Diagnostic and prognostic role of liquid biopsy in non-small cell lung cancer: Evaluation of circulating biomarkers. Explor. Target. Anti-Tumor Ther. 2020, 1, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Rijavec, E.; Coco, S.; Genova, C.; Rossi, G.; Longo, L.; Grossi, F. Liquid Biopsy in Non-Small Cell Lung Cancer: Highlights and Challenges. Cancers 2019, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Heitzer, E.; Haque, I.S.; Roberts, C.E.S.; Speicher, M.R. Current and future perspectives of liquid biopsies in genomics-driven oncology. Nat. Rev. Genet. 2019, 20, 71–88. [Google Scholar] [CrossRef]
- Li, W.; Liu, J.-B.; Hou, L.-K.; Yu, F.; Zhang, J.; Wu, W.; Tang, X.-M.; Sun, F.; Lu, H.-M.; Deng, J.; et al. Liquid biopsy in lung cancer: Significance in diagnostics, prediction, and treatment monitoring. Mol. Cancer 2022, 21, 25. [Google Scholar] [CrossRef]
- Qvick, A.; Stenmark, B.; Carlsson, J.; Isaksson, J.; Karlsson, C.; Helenius, G. Liquid biopsy as an option for predictive testing and prognosis in patients with lung cancer. Mol. Med. 2021, 27, 68. [Google Scholar] [CrossRef]
- Arbour, K.C.; Riely, G.J. Systemic Therapy for Locally Advanced and Metastatic Non–Small Cell Lung Cancer: A Review. JAMA 2019, 322, 764. [Google Scholar] [CrossRef]
- Dubin, S.; Griffin, D. Lung Cancer in Non-Smokers. Mo Med. 2020, 117, 375–379. [Google Scholar]
- Santoro, I.L.; Ramos, R.P.; Franceschini, J.; Jamnik, S.; Fernandes, A.L.G. Non-small cell lung cancer in never smokers: A clinical entity to be identified. Clinics 2011, 66, 1873–1877. [Google Scholar] [CrossRef] [PubMed]
- González-Marrón, A.; Martín-Sánchez, J.C.; Miró, Q.; Matilla-Santander, N.; Cartanyà-Hueso, À.; Mucci, L.; Martínez-Sánchez, J.M. Relation between tobacco control policies and population at high risk of lung cancer in the European Union. Environ. Res. 2019, 179, 108594. [Google Scholar] [CrossRef]
- Sun, P.-L.; Seol, H.; Lee, H.J.; Yoo, S.B.; Kim, H.; Xu, X.; Jheon, S.; Lee, C.-T.; Lee, J.-S.; Chung, J.-H. High Incidence of EGFR Mutations in Korean Men Smokers with No Intratumoral Heterogeneity of Lung Adenocarcinomas: Correlation with Histologic Subtypes, EGFR/TTF-1 Expressions, and Clinical Features. J. Thorac. Oncol. 2012, 7, 323–330. [Google Scholar] [CrossRef]
- Banks, K.C.; Sumner, E.T.; Alabaster, A.; Hsu, D.S.; Quesenberry Jr, C.P.; Sakoda, L.C.; Velotta, J.B. Sociodemographic and clinical characteristics associated with never-smoking status in patients with lung cancer: Findings from a large integrated health system. Transl. Cancer Res. 2022, 11, 3522–3534. [Google Scholar] [CrossRef]
- Mederos, N.; Friedlaender, A.; Peters, S.; Addeo, A. Gender-specific aspects of epidemiology, molecular genetics and outcome: Lung cancer. ESMO Open 2020, 5, e000796. [Google Scholar] [CrossRef]
- Tang, A.; Ahmad, U.; Toth, A.J.; Bourdakos, N.; Raja, S.; Raymond, D.P.; Blackstone, E.H.; Murthy, S.C. Non-small cell lung cancer in never- and ever-smokers: Is it the same disease? J. Thorac. Cardiovasc. Surg. 2021, 161, 1903–1917.e9. [Google Scholar] [CrossRef]
- Isla, D.; Majem, M.; Viñolas, N.; Artal, A.; Blasco, A.; Felip, E.; Garrido, P.; Remón, J.; Baquedano, M.; Borrás, J.M.; et al. A consensus statement on the gender perspective in lung cancer. Clin. Transl. Oncol. 2017, 19, 527–535. [Google Scholar] [CrossRef]
- Alberg, A.J.; Wallace, K.; Silvestri, G.A.; Brock, M.V. Invited Commentary: The Etiology of Lung Cancer in Men Compared With Women. Am. J. Epidemiol. 2013, 177, 613–616. [Google Scholar] [CrossRef] [PubMed]
- Vavalà, T.; Catino, A.; Pizzutilo, P.; Longo, V.; Galetta, D. Gender Differences and Immunotherapy Outcome in Advanced Lung Cancer. Int. J. Mol. Sci. 2021, 22, 11942. [Google Scholar] [CrossRef] [PubMed]
- Uramoto, H.; Tanaka, F. Recurrence after surgery in patients with NSCLC. Transl. Lung Cancer Res. 2014, 3, 242–249. [Google Scholar] [CrossRef]
- Revelo, A.E.; Martin, A.; Velasquez, R.; Kulandaisamy, P.C.; Bustamante, J.; Keshishyan, S.; Otterson, G. Liquid biopsy for lung cancers: An update on recent developments. Ann. Transl. Med. 2019, 7, 349. [Google Scholar] [CrossRef] [PubMed]
- Varlotto, J.; Fakiris, A.; Flickinger, J.; Medford-Davis, L.; Liss, A.; Shelkey, J.; Belani, C.; DeLuca, J.; Recht, A.; Maheshwari, N.; et al. Matched-pair and propensity score comparisons of outcomes of patients with clinical stage I non-small cell lung cancer treated with resection or stereotactic radiosurgery: Comparison of SBRT to Surgery in NSCLC. Cancer 2013, 119, 2683–2691. [Google Scholar] [CrossRef]
- Varlotto, J.M.; Recht, A.; Flickinger, J.C.; Medford-Davis, L.N.; Dyer, A.-M.; DeCamp, M.M. Varying recurrence rates and risk factors associated with different definitions of local recurrence in patients with surgically resected, stage I nonsmall cell lung cancer. Cancer 2010, 116, 2390–2400. [Google Scholar] [CrossRef]
- Kadota, K.; Nitadori, J.; Sima, C.S.; Ujiie, H.; Rizk, N.P.; Jones, D.R.; Adusumilli, P.S.; Travis, W.D. Tumor Spread through Air Spaces is an Important Pattern of Invasion and Impacts the Frequency and Location of Recurrences after Limited Resection for Small Stage I Lung Adenocarcinomas. J. Thorac. Oncol. 2015, 10, 806–814. [Google Scholar] [CrossRef]
- Bains, S.; Eguchi, T.; Warth, A.; Yeh, Y.-C.; Nitadori, J.-I.; Woo, K.M.; Chou, T.-Y.; Dienemann, H.; Muley, T.; Nakajima, J.; et al. Procedure-Specific Risk Prediction for Recurrence in Patients Undergoing Lobectomy or Sublobar Resection for Small (≤2 cm) Lung Adenocarcinoma: An International Cohort Analysis. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2019, 14, 72–86. [Google Scholar] [CrossRef]
- Eguchi, T.; Kadota, K.; Park, B.J.; Travis, W.D.; Jones, D.R.; Adusumilli, P.S. The New IASLC/ATS/ERS Lung Adenocarcinoma Classification: What the surgeon should know. Semin. Thorac. Cardiovasc. Surg. 2014, 26, 210–222. [Google Scholar] [CrossRef]
- Keyhanian, K.; Sekhon, H.S. Do fine needle aspirate cytomorphological features correlate with positron emission tomography findings of metastatic non-small cell lung carcinoma in lymph nodes? Cancer Med. 2023, 12, 8218–8227. [Google Scholar] [CrossRef]
- Travis, W.D.; Brambilla, E.; Noguchi, M.; Nicholson, A.G.; Geisinger, K.R.; Yatabe, Y.; Beer, D.G.; Powell, C.A.; Riely, G.J.; Van Schil, P.E.; et al. International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J. Thorac. Oncol. Off. Publ. Int. Assoc. Study Lung Cancer 2011, 6, 244–285. [Google Scholar] [CrossRef]
- Righi, L.; Graziano, P.; Fornari, A.; Rossi, G.; Barbareschi, M.; Cavazza, A.; Pelosi, G.; Scagliotti, G.V.; Papotti, M. Immunohistochemical subtyping of nonsmall cell lung cancer not otherwise specified in fine-needle aspiration cytology. Cancer 2011, 117, 3416–3423. [Google Scholar] [CrossRef] [PubMed]
- de Koning, H.J.; van der Aalst, C.M.; de Jong, P.A.; Scholten, E.T.; Nackaerts, K.; Heuvelmans, M.A.; Lammers, J.-W.J.; Weenink, C.; Yousaf-Khan, U.; Horeweg, N.; et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N. Engl. J. Med. 2020, 382, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Horgan, D.; Čufer, T.; Gatto, F.; Lugowska, I.; Verbanac, D.; Carvalho, Â.; Lal, J.A.; Kozaric, M.; Toomey, S.; Ivanov, H.Y.; et al. Accelerating the Development and Validation of Liquid Biopsy for Early Cancer Screening and Treatment Tailoring. Healthc. Basel Switz. 2022, 10, 1714. [Google Scholar] [CrossRef] [PubMed]
- Jamal-Hanjani, M.; Wilson, G.A.; McGranahan, N.; Birkbak, N.J.; Watkins, T.B.K.; Veeriah, S.; Shafi, S.; Johnson, D.H.; Mitter, R.; Rosenthal, R.; et al. Tracking the Evolution of Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 376, 2109–2121. [Google Scholar] [CrossRef] [PubMed]
- Reita, D.; Pabst, L.; Pencreach, E.; Guérin, E.; Dano, L.; Rimelen, V.; Voegeli, A.-C.; Vallat, L.; Mascaux, C.; Beau-Faller, M. Molecular Mechanism of EGFR-TKI Resistance in EGFR-Mutated Non-Small Cell Lung Cancer: Application to Biological Diagnostic and Monitoring. Cancers 2021, 13, 4926. [Google Scholar] [CrossRef] [PubMed]
- Pao, W.; Girard, N. New driver mutations in non-small-cell lung cancer. Lancet Oncol. 2011, 12, 175–180. [Google Scholar] [CrossRef] [PubMed]
- Attili, I.; Karachaliou, N.; Conte, P.; Bonanno, L.; Rosell, R. Therapeutic approaches for T790M mutation positive non-small-cell lung cancer. Expert Rev. Anticancer Ther. 2018, 18, 1021–1030. [Google Scholar] [CrossRef]
- Attili, I.; Passaro, A.; Pisapia, P.; Malapelle, U.; de Marinis, F. Uncommon EGFR Compound Mutations in Non-Small Cell Lung Cancer (NSCLC): A Systematic Review of Available Evidence. Curr. Oncol. 2022, 29, 255–266. [Google Scholar] [CrossRef]
- Cheng, G.; Song, Z.; Chen, D. Clinical efficacy of first-generation EGFR-TKIs in patients with advanced non-small-cell lung cancer harboring EGFR exon 20 mutations. OncoTargets Ther. 2016, 9, 4181–4186. [Google Scholar] [CrossRef]
- Pascual, J.; Attard, G.; Bidard, F.-C.; Curigliano, G.; De Mattos-Arruda, L.; Diehn, M.; Italiano, A.; Lindberg, J.; Merker, J.D.; Montagut, C.; et al. ESMO recommendations on the use of circulating tumour DNA assays for patients with cancer: A report from the ESMO Precision Medicine Working Group. Ann. Oncol. 2022, 33, 750–768. [Google Scholar] [CrossRef]
- Hindson, C.M.; Chevillet, J.R.; Briggs, H.A.; Gallichotte, E.N.; Ruf, I.K.; Hindson, B.J.; Vessella, R.L.; Tewari, M. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat. Methods 2013, 10, 1003–1005. [Google Scholar] [CrossRef]
- Diehl, F.; Li, M.; He, Y.; Kinzler, K.W.; Vogelstein, B.; Dressman, D. BEAMing: Single-molecule PCR on microparticles in water-in-oil emulsions. Nat. Methods 2006, 3, 551–559. [Google Scholar] [CrossRef]
- Mardis, E.R. DNA sequencing technologies: 2006-2016. Nat. Protoc. 2017, 12, 213–218. [Google Scholar] [CrossRef]
- Mok, T.; Wu, Y.-L.; Lee, J.S.; Yu, C.-J.; Sriuranpong, V.; Sandoval-Tan, J.; Ladrera, G.; Thongprasert, S.; Srimuninnimit, V.; Liao, M.; et al. Detection and Dynamic Changes of EGFR Mutations from Circulating Tumor DNA as a Predictor of Survival Outcomes in NSCLC Patients Treated with First-line Intercalated Erlotinib and Chemotherapy. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2015, 21, 3196–3203. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Mei, J.; Kang, R.; Deng, S.; Chen, Y.; Yang, Y.; Feng, G.; Deng, Y.; Gan, F.; Lin, Y.; et al. Perioperative ctDNA-Based Molecular Residual Disease Detection for Non-Small Cell Lung Cancer: A Prospective Multicenter Cohort Study (LUNGCA-1). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2022, 28, 3308–3317. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Lee, J.-C.; Choi, C.-M. Clinical Applications of Liquid Biopsy in Non-Small Cell Lung Cancer Patients: Current Status and Recent Advances in Clinical Practice. J. Clin. Med. 2021, 10, 2236. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Frullanti, E. Different Liquid Biopsies for the Management of Non-Small Cell Lung Cancer in the Mutational Oncology Era. Med. Sci. 2023, 11, 8. [Google Scholar] [CrossRef]
- Canale, M.; Pasini, L.; Bronte, G.; Delmonte, A.; Cravero, P.; Crinò, L.; Ulivi, P. Role of liquid biopsy in oncogene-addicted non-small cell lung cancer. Transl. Lung Cancer Res. 2019, 8, S265–S279. [Google Scholar] [CrossRef]
- Kapeleris, J.; Ebrahimi Warkiani, M.; Kulasinghe, A.; Vela, I.; Kenny, L.; Ladwa, R.; O’Byrne, K.; Punyadeera, C. Clinical Applications of Circulating Tumour Cells and Circulating Tumour DNA in Non-Small Cell Lung Cancer—An Update. Front. Oncol. 2022, 12, 859152. [Google Scholar] [CrossRef]
- Soria, J.-C.; Ohe, Y.; Vansteenkiste, J.; Reungwetwattana, T.; Chewaskulyong, B.; Lee, K.H.; Dechaphunkul, A.; Imamura, F.; Nogami, N.; Kurata, T.; et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 113–125. [Google Scholar] [CrossRef]

| Characteristics | Number of Patients [n (%)] EGFR Mutations Detected by Liquid Biopsy | Total of Cases (n = 38) | p | |
|---|---|---|---|---|
| No (n = 11) | Yes (n = 27) | |||
| Age, years * | 68 (52–83) | 73 (43–85) | 68 (43–85) | NS |
| Gender | ||||
| Male | 5 (45) | 12 (44) | 17 (45) | NS |
| Female | 6 (55) | 15 (56) | 21 (55) | |
| Smoking history | ||||
| Smoker or ex-smoker | 5 (45) | 8 (30) | 13 (34) | NS |
| Nonsmoker | 6 (55) | 19 (70) | 25 (66) | |
| ECOG ** | ||||
| 0 | 6 (55) | 18 (67) | 24 (63) | NS |
| 1 | 5 (45) | 9 (33) | 14 (34) | |
| Symptoms | ||||
| Pneumological (cough, dyspnea) | 4 (36) | 18 (67) | 22 (58) | NS |
| Non-pneumological | 3 (27) | 6 (22) | 9 (24) | |
| Asymptomatic | 4 (36) | 3 (11) | 7 (18) | |
| AJCC stage *** | ||||
| I | 0 (0) | 3 (11) | 3 (8) | 0.02 |
| II | 2 (18) | 0 (0) | 2 (5) | |
| III | 3 (27) | 2 (18) | 5 (13) | |
| IV | 6 (55) | 22 (81) | 28 (74) | |
| Surgery | ||||
| Yes | 1 (9) | 4 (15) | 5 (13) | NS |
| No | 10 (91) | 23 (85) | 33 (87) | |
| Oncologic Treatment | ||||
| None | 1 (9) | 0 (0) | 1 (3) | |
| Chemotherapy | 3 (27) | 1 (4) | 4 (10) | 0.01 |
| Radiotherapy | 1 (9) | 0 (0) | 1 (3) | |
| Tyrosine kinase inhibitor | 6 (55) | 26 (96) | 32 (84) | |
| Exitus | ||||
| Yes | 4 (33) | 23 (85) | 27 (29) | 0.002 |
| No | 7 (67) | 4 (15) | 11 (71) | |
| OS (months) | 104 ± 19 | 29 ± 4 | 48 ± 10 | 0.001 |
| Characteristics | Number of Patients [n (%)] EGFR Mutations Detected by Liquid Biopsy | Total of Cases (n = 38) | p | |
|---|---|---|---|---|
| No (n = 11) | Yes (n = 27) | |||
| Histology | ||||
| Adenocarcinoma | 10 (91) | 26 (97) | 36 (95) | NS |
| Undifferentiated carcinoma | 1 (9) | 1 (3) | 2 (5) | |
| TTF1 expression | ||||
| Positive | 10 (91) | 25 (93) | 35 (91) | NS |
| Negative | 1 (9) | 2 (7) | 3 (9) | |
| PD-L1 expression * | ||||
| Negative (0%) | 4 (50) | 12 (48) | 16 (48) | NS |
| Positive | 4 (50) | 13 (52) | 17 (52) | |
| Variable | N | Univariate Analysis | Multivariate Analysis | HR (95% CI) |
|---|---|---|---|---|
| Age | ||||
| <68 years | 15 (39) | NS | ||
| ≥68 years | 23 (61) | |||
| Gender | ||||
| Male | 17 (45) | 0.1 | NS | |
| Female | 21 (55) | |||
| Smoking history | ||||
| Smoker or ex-smoker | 13 (34) | |||
| Nonsmoker | 25 (66) | NS | ||
| ECOG * | ||||
| 0 | 24 (63) | |||
| 1 | 14 (37) | 0.05 | NS | |
| AJCC stage | ||||
| I | 3 (7) | |||
| II | 2 (5) | NS | ||
| III | 5 (13) | |||
| IV | 28 (74) | |||
| Surgery | ||||
| No | 33 (87) | NS | ||
| Yes | 5 (13) | |||
| Oncologic treatment | ||||
| None | 1 (3) | |||
| Chemotherapy | 4 (10) | 0.08 | NS | |
| Radiotherapy | 1 (3) | |||
| Tyrosine kinase inhibitor | 32 (84) | |||
| TTF1expression | ||||
| Positive | 35 (92) | NS | ||
| Negative | 3 (8) | |||
| PD-L1expression | ||||
| Positive (≥1%) | 17 (52) | NS | ||
| Negative | 16 (48) | |||
| EGFR status on liquid biopsy | ||||
| Positive | 27 (71) | 0.004 | 0.008 | 4.7 (1.5–12.9) |
| Negative | 11 (29) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roldan Ruiz, J.; Fuentes Gago, M.G.; Chinchilla Tabora, L.M.; Gonzalez Morais, I.; Sayagués, J.M.; Abad Hernández, M.; Cordovilla Pérez, M.R.; Ludeña de la Cruz, M.D.; del Barco Morillo, E.; Rodriguez Gonzalez, M. The Impact of Liquid Biopsies Positive for EGFR Mutations on Overall Survival in Non-Small Cell Lung Cancer Patients. Diagnostics 2023, 13, 2347. https://doi.org/10.3390/diagnostics13142347
Roldan Ruiz J, Fuentes Gago MG, Chinchilla Tabora LM, Gonzalez Morais I, Sayagués JM, Abad Hernández M, Cordovilla Pérez MR, Ludeña de la Cruz MD, del Barco Morillo E, Rodriguez Gonzalez M. The Impact of Liquid Biopsies Positive for EGFR Mutations on Overall Survival in Non-Small Cell Lung Cancer Patients. Diagnostics. 2023; 13(14):2347. https://doi.org/10.3390/diagnostics13142347
Chicago/Turabian StyleRoldan Ruiz, Jonnathan, Marta Gracia Fuentes Gago, Luis Miguel Chinchilla Tabora, Idalia Gonzalez Morais, José María Sayagués, Mar Abad Hernández, Maria Rosa Cordovilla Pérez, Maria Dolores Ludeña de la Cruz, Edel del Barco Morillo, and Marta Rodriguez Gonzalez. 2023. "The Impact of Liquid Biopsies Positive for EGFR Mutations on Overall Survival in Non-Small Cell Lung Cancer Patients" Diagnostics 13, no. 14: 2347. https://doi.org/10.3390/diagnostics13142347
APA StyleRoldan Ruiz, J., Fuentes Gago, M. G., Chinchilla Tabora, L. M., Gonzalez Morais, I., Sayagués, J. M., Abad Hernández, M., Cordovilla Pérez, M. R., Ludeña de la Cruz, M. D., del Barco Morillo, E., & Rodriguez Gonzalez, M. (2023). The Impact of Liquid Biopsies Positive for EGFR Mutations on Overall Survival in Non-Small Cell Lung Cancer Patients. Diagnostics, 13(14), 2347. https://doi.org/10.3390/diagnostics13142347






