Neurophysiology of Brain Networks Underlies Symptoms of Parkinson’s Disease: A Basis for Diagnosis and Management
Abstract
:1. Introduction
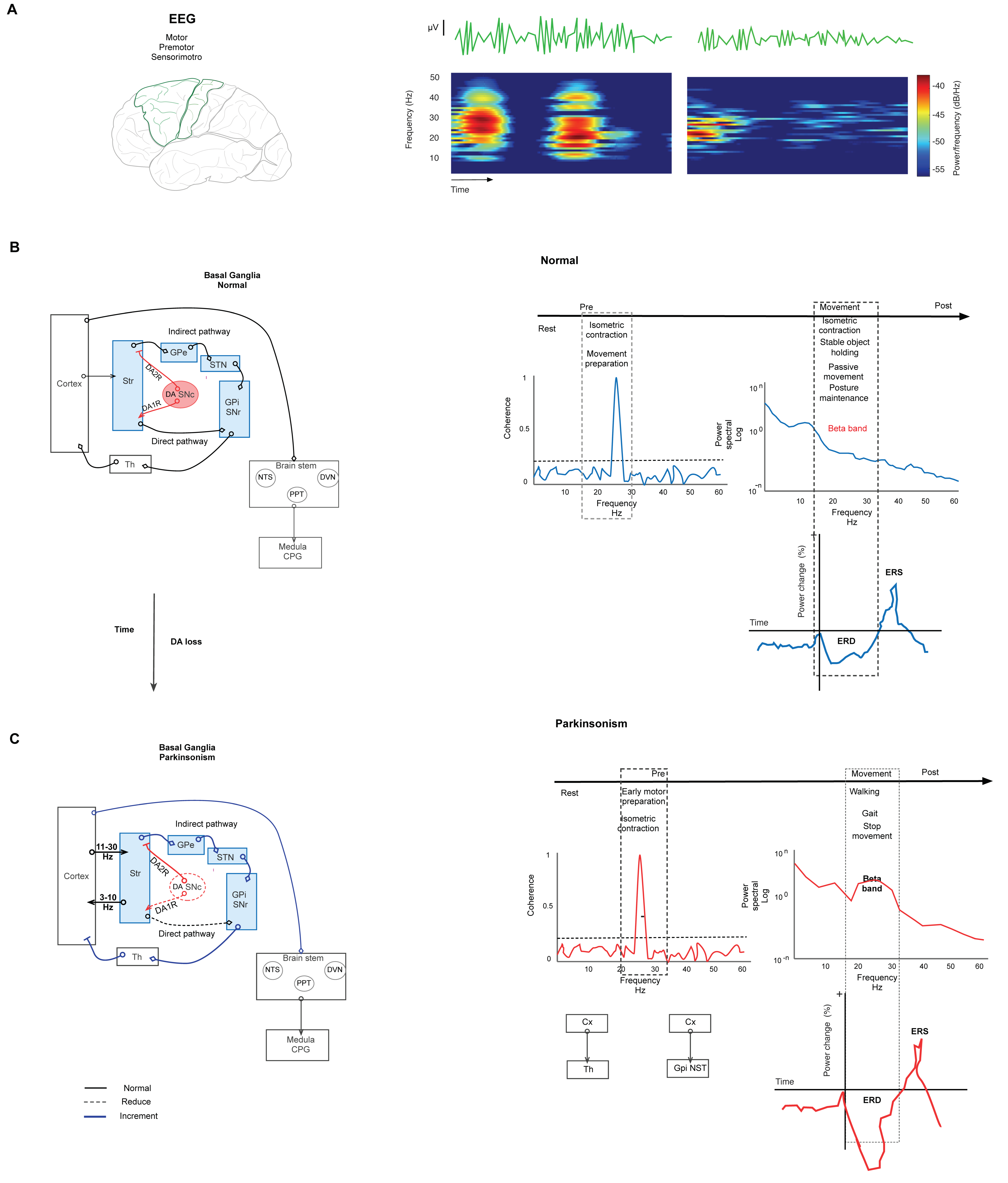
2. Network Involvement in Motor Symptoms
Firing Pattern Implication in the Neurophysiology of the BG Network
3. Network Oscillation
Network Oscillation in Parkinsonism
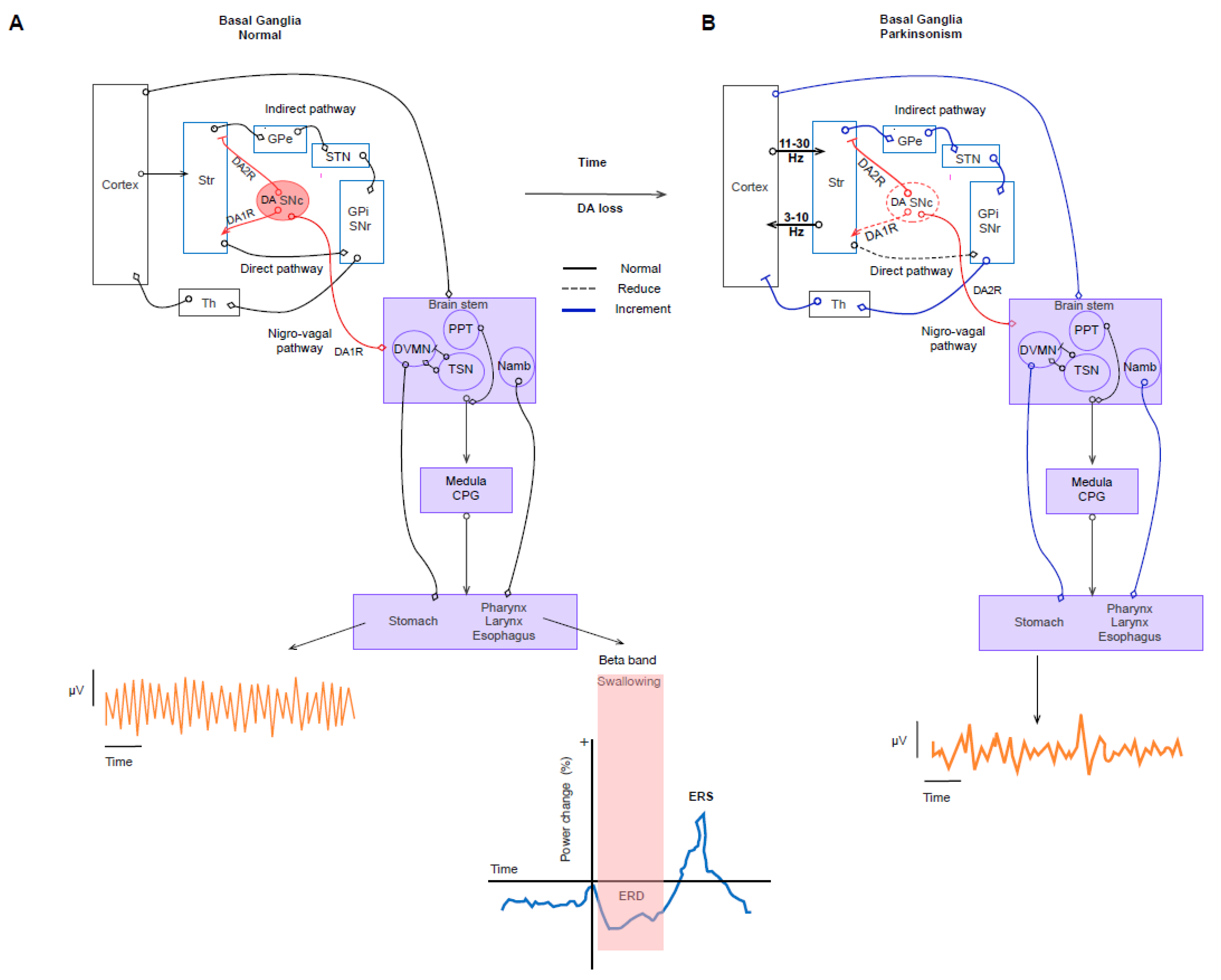
4. Network Involvement in Gastrointestinal Symptoms
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2016 Neurology Collaborators. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, M.J.; Okun, M.S. Diagnosis and Treatment of Parkinson Disease: A Review. JAMA 2020, 323, 548–560. [Google Scholar] [CrossRef]
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385–397. [Google Scholar] [CrossRef]
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450. [Google Scholar] [CrossRef]
- Camacho, M.; Greenland, J.C.; Williams-Gray, C.H. The Gastrointestinal Dysfunction Scale for Parkinson’s Disease. Mov. Disord. 2021, 36, 2358–2366. [Google Scholar] [CrossRef]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef]
- Galvan, A.; Devergnas, A.; Wichmann, T. Alterations in neuronal activity in basal ganglia-thalamocortical circuits in the parkinsonian state. Front. Neuroanat. 2015, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- McGregor, M.M.; Nelson, A.B. Circuit Mechanisms of Parkinson’s Disease. Neuron 2019, 101, 1042–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bove, C.; Travagli, R.A. Neurophysiology of the brain stem in Parkinson’s disease. J. Neurophysiol. 2019, 121, 1856–1864. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Movement disorders. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Homayoun, H. Parkinson Disease. Ann. Intern. Med. 2018, 169, ITC33–ITC48. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Tachibana, Y.; Chiken, S. Cause of parkinsonian symptoms: Firing rate, firing pattern or dynamic activity changes? Basal Ganglia 2015, 5, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef]
- DeLong, M.R. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990, 13, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Parent, A.; Hazrati, L.N. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain research. Brain Res. Rev. 1995, 20, 91–127. [Google Scholar] [CrossRef]
- Kim, H.F.; Hikosaka, O. Parallel basal ganglia circuits for voluntary and automatic behaviour to reach rewards. Brain A J. Neurol. 2015, 138, 1776–1800. [Google Scholar] [CrossRef] [Green Version]
- Meoni, S.; Cury, R.G.; Moro, E. New players in basal ganglia dysfunction in Parkinson’s disease. Prog. Brain Res. 2020, 252, 307–327. [Google Scholar] [CrossRef]
- Alexander, G.E.; Crutcher, M.D.; DeLong, M.R. Basal ganglia-thalamocortical circuits: Parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990, 85, 119–146. [Google Scholar] [CrossRef]
- Gerfen, C.R.; Engber, T.M.; Mahan, L.C.; Susel, Z.; Chase, T.N.; Monsma, F.J., Jr.; Sibley, D.R. D1 and D2 dopamine receptor-regulated gene expression of striatonigral and striatopallidal neurons. Science 1990, 250, 1429–1432. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Graves, S.M.; Shen, W. Dopaminergic modulation of striatal networks in health and Parkinson’s disease. Curr. Opin. Neurobiol. 2014, 29, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Galvan, A.; Wichmann, T. Pathophysiology of parkinsonism. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2008, 119, 1459–1474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bevan, M.D.; Magill, P.J.; Terman, D.; Bolam, J.P.; Wilson, C.J. Move to the rhythm: Oscillations in the subthalamic nucleus-external globus pallidus network. Trends Neurosci. 2002, 25, 525–531. [Google Scholar] [CrossRef] [PubMed]
- Stanley, G.B. Reading and writing the neural code. Nat. Neurosci. 2013, 16, 259–263. [Google Scholar] [CrossRef]
- Gerstein, G.L. Analysis of Firing Pafferns in Single Neurons. Science 1960, 131, 1811–1812. [Google Scholar] [CrossRef]
- Wichmann, T. Changing views of the pathophysiology of Parkinsonism. Mov. Disord. Off. J. Mov. Disord. Soc. 2019, 34, 1130–1143. [Google Scholar] [CrossRef]
- Schneider, J.S.; Rothblat, D.S. Alterations in intralaminar and motor thalamic physiology following nigrostriatal dopamine depletion. Brain Res. 1996, 742, 25–33. [Google Scholar] [CrossRef]
- Nishibayashi, H.; Ogura, M.; Kakishita, K.; Tanaka, S.; Tachibana, Y.; Nambu, A.; Kita, H.; Itakura, T. Cortically evoked responses of human pallidal neurons recorded during stereotactic neurosurgery. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, J.A.; Schneider, J.S. GABA-opioid interactions in the globus pallidus: [D-Ala2]-Met-enkephalinamide attenuates potassium-evoked GABA release after nigrostriatal lesion. J. Neurochem. 2002, 82, 666–673. [Google Scholar] [CrossRef] [Green Version]
- Galvan, A.; Hu, X.; Smith, Y.; Wichmann, T. Localization and function of GABA transporters in the globus pallidus of parkinsonian monkeys. Exp. Neurol. 2010, 223, 505–515. [Google Scholar] [CrossRef] [Green Version]
- Pasquereau, B.; Turner, R.S. Primary motor cortex of the parkinsonian monkey: Differential effects on the spontaneous activity of pyramidal tract-type neurons. Cereb. Cortex 2011, 21, 1362–1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindenbach, D.; Bishop, C. Critical involvement of the motor cortex in the pathophysiology and treatment of Parkinson’s disease. Neurosci. Biobehav. Rev. 2013, 37, 2737–2750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Llinás, R.R. Intrinsic electrical properties of mammalian neurons and CNS function: A historical perspective. Front. Cell. Neurosci. 2014, 8, 320. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.S.; Glajch, K.E.; Gertler, T.S.; Guzman, J.N.; Mercer, J.N.; Lewis, A.S.; Goldberg, A.B.; Tkatch, T.; Shigemoto, R.; Fleming, S.M.; et al. HCN channelopathy in external globus pallidus neurons in models of Parkinson’s disease. Nat. Neurosci. 2011, 14, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.J.; Ma, T.C.; Ding, Y.; Cheung, T.; Joshi, N.; Sulzer, D.; Mosharov, E.V.; Kang, U.J. Alterations in the intrinsic properties of striatal cholinergic interneurons after dopamine lesion and chronic L-DOPA. eLife 2020, 9, e56920. [Google Scholar] [CrossRef]
- Day, M.; Wokosin, D.; Plotkin, J.L.; Tian, X.; Surmeier, D.J. Differential excitability and modulation of striatal medium spiny neuron dendrites. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 11603–11614. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ünal, B.; Shah, F.; Kothari, J.; Tepper, J.M. Anatomical and electrophysiological changes in striatal TH interneurons after loss of the nigrostriatal dopaminergic pathway. Brain Struct. Funct. 2015, 220, 331–349. [Google Scholar] [CrossRef] [Green Version]
- Wichmann, T.; Soares, J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J. Neurophysiol. 2006, 95, 2120–2133. [Google Scholar] [CrossRef] [Green Version]
- Hutchison, W.D.; Lozano, A.M.; Davis, K.D.; Saint-Cyr, J.A.; Lang, A.E.; Dostrovsky, J.O. Differential neuronal activity in segments of globus pallidus in Parkinson’s disease patients. Neuroreport 1994, 5, 1533–1537. [Google Scholar] [CrossRef]
- Magnin, M.; Morel, A.; Jeanmonod, D. Single-unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience 2000, 96, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Sharott, A.; Gulberti, A.; Zittel, S.; Tudor Jones, A.A.; Fickel, U.; Münchau, A.; Köppen, J.A.; Gerloff, C.; Westphal, M.; Buhmann, C.; et al. Activity parameters of subthalamic nucleus neurons selectively predict motor symptom severity in Parkinson’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 6273–6285. [Google Scholar] [CrossRef] [Green Version]
- Plenz, D.; Kital, S.T. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature 1999, 400, 677–682. [Google Scholar] [CrossRef]
- Tachibana, Y.; Iwamuro, H.; Kita, H.; Takada, M.; Nambu, A. Subthalamo-pallidal interactions underlying parkinsonian neuronal oscillations in the primate basal ganglia. Eur. J. Neurosci. 2011, 34, 1470–1484. [Google Scholar] [CrossRef]
- Roy, D.S.; Zhang, Y.; Halassa, M.M.; Feng, G. Thalamic subnetworks as units of function. Nat. Neurosci. 2022, 25, 140–153. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Bouju, C.; Smither, R.A.; Hyland, B.I.; Parr-Brownlie, L.C. Reduced reach-related modulation of motor thalamus neural activity in a rat model of Parkinsn’s disease. J. Neurosci. 2014, 34, 15836–15850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bostan, A.C.; Dum, R.P.; Strick, P.L. Functional Anatomy of Basal Ganglia Circuits with the Cerebral Cortex and the Cerebellum. Prog. Neurol. Surg. 2018, 33, 50–61. [Google Scholar] [CrossRef]
- Milosevic, L.; Kalia, S.K.; Hodaie, M.; Lozano, A.M.; Popovic, M.R.; Hutchison, W.D. Physiological mechanisms of thalamic ventral intermediate nucleus stimulation for tremor suppression. Brain A J. Neurol. 2018, 141, 2142–2155. [Google Scholar] [CrossRef] [PubMed]
- Izhikevich, E.M.; Desai, N.S.; Walcott, E.C.; Hoppensteadt, F.C. Bursts as a unit of neural information: Selective communication via resonance. Trends Neurosci. 2003, 26, 161–167. [Google Scholar] [CrossRef]
- Schnitzler, A.; Gross, J. Normal and pathological oscillatory communication in the brain. Nature reviews. Neuroscience 2005, 6, 285–296. [Google Scholar] [CrossRef]
- Kristeva, R.; Patino, L.; Omlor, W. Beta-range cortical motor spectral power and corticomuscular coherence as a mechanism for effective corticospinal interaction during steady-state motor output. NeuroImage 2007, 36, 785–792. [Google Scholar] [CrossRef] [PubMed]
- Pogosyan, A.; Gaynor, L.D.; Eusebio, A.; Brown, P. Boosting cortical activity at Beta-band frequencies slows movement in humans. Curr. Biol. 2009, 19, 1637–1641. [Google Scholar] [CrossRef] [Green Version]
- Khanna, P.; Carmena, J.M. Neural oscillations: Beta band activity across motor networks. Curr. Opin. Neurobiol. 2015, 32, 60–67. [Google Scholar] [CrossRef]
- Hutchison, W.D.; Dostrovsky, J.O.; Walters, J.R.; Courtemanche, R.; Boraud, T.; Goldberg, J.; Brown, P. Neuronal oscillations in the basal ganglia and movement disorders: Evidence from whole animal and human recordings. J. Neurosci. Off. J. Soc. Neurosci. 2004, 24, 9240–9243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, A. Oscillatory activity in the cortico-basal ganglia-thalamic neural circuits in Parkinson’s disease. Eur. J. Neurosci. 2018, 48, 2869–2878. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Lopes da Silva, F.H. Event-related EEG/MEG synchronization and desynchronization: Basic principles. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 1999, 110, 1842–1857. [Google Scholar] [CrossRef] [PubMed]
- Courtemanche, R.; Fujii, N.; Graybiel, A.M. Synchronous, focally modulated beta-band oscillations characterize local field potential activity in the striatum of awake behaving monkeys. J. Neurosci. Off. J. Soc. Neurosci. 2003, 23, 11741–11752. [Google Scholar] [CrossRef]
- Damborská, A.; Lamoš, M.; Brunet, D.; Vulliemoz, S.; Bočková, M.; Deutschová, B.; Baláž, M.; Rektor, I. Resting-State Phase-Amplitude Coupling Between the Human Subthalamic Nucleus and Cortical Activity: A Simultaneous Intracranial and Scalp EEG Study. Brain Topogr. 2021, 34, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Han, F.; Wang, Q. Exploring phase-amplitude coupling from primary motor cortex-basal ganglia-thalamus network model. Neural Netw. Off. J. Int. Neural Netw. Soc. 2022, 153, 130–141. [Google Scholar] [CrossRef]
- Oswal, A.; Cao, C.; Yeh, C.H.; Neumann, W.J.; Gratwicke, J.; Akram, H.; Horn, A.; Li, D.; Zhan, S.; Zhang, C.; et al. Neural signatures of hyperdirect pathway activity in Parkinson’s disease. Nat. Commun. 2021, 12, 5185. [Google Scholar] [CrossRef]
- Ruskin, D.N.; Bergstrom, D.A.; Kaneoke, Y.; Patel, B.N.; Twery, M.J.; Walters, J.R. Multisecond oscillations in firing rate in the basal ganglia: Robust modulation by dopamine receptor activation and anesthesia. J. Neurophysiol. 1999, 81, 2046–2055. [Google Scholar] [CrossRef]
- Baker, S.N.; Olivier, E.; Lemon, R.N. Coherent oscillations in monkey motor cortex and hand muscle EMG show task-dependent modulation. J. Physiol. 1997, 501, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Conway, B.A.; Halliday, D.M.; Farmer, S.F.; Shahani, U.; Maas, P.; Weir, A.I.; Rosenberg, J.R. Synchronization between motor cortex and spinal motoneuronal pool during the performance of a maintained motor task in man. J. Physiol. 1995, 489, 917–924. [Google Scholar] [CrossRef] [PubMed]
- Spinks, R.L.; Kraskov, A.; Brochier, T.; Umilta, M.A.; Lemon, R.N. Selectivity for grasp in local field potential and single neuron activity recorded simultaneously from M1 and F5 in the awake macaque monkey. J. Neurosci. Off. J. Soc. Neurosci. 2008, 28, 10961–10971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kilavik, B.E.; Zaepffel, M.; Brovelli, A.; MacKay, W.A.; Riehle, A. The ups and downs of β oscillations in sensorimotor cortex. Exp. Neurol. 2013, 245, 15–26. [Google Scholar] [CrossRef] [Green Version]
- Omlor, W.; Patino, L.; Mendez-Balbuena, I.; Schulte-Mönting, J.; Kristeva, R. Corticospinal beta-range coherence is highly dependent on the pre-stationary motor state. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 8037–8045. [Google Scholar] [CrossRef]
- Paradiso, G.; Cunic, D.; Saint-Cyr, J.A.; Hoque, T.; Lozano, A.M.; Lang, A.E.; Chen, R. Involvement of human thalamus in the preparation of self-paced movement. Brain A J. Neurol. 2004, 127, 2717–2731. [Google Scholar] [CrossRef] [Green Version]
- Parkes, L.M.; Bastiaansen, M.C.; Norris, D.G. Combining EEG and fMRI to investigate the post-movement beta rebound. NeuroImage 2006, 29, 685–696. [Google Scholar] [CrossRef] [Green Version]
- Brown, P. Oscillatory nature of human basal ganglia activity: Relationship to the pathophysiology of Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2003, 18, 357–363. [Google Scholar] [CrossRef]
- Levy, R.; Hutchison, W.D.; Lozano, A.M.; Dostrovsky, J.O. Synchronized neuronal discharge in the basal ganglia of parkinsonian patients is limited to oscillatory activity. J. Neurosci. Off. J. Soc. Neurosci. 2002, 22, 2855–2861. [Google Scholar] [CrossRef] [Green Version]
- Kühn, A.A.; Williams, D.; Kupsch, A.; Limousin, P.; Hariz, M.; Schneider, G.H.; Yarrow, K.; Brown, P. Event-related beta desynchronization in human subthalamic nucleus correlates with motor performance. Brain A J. Neurol. 2004, 127, 735–746. [Google Scholar] [CrossRef]
- Marsden, J.F.; Limousin-Dowsey, P.; Ashby, P.; Pollak, P.; Brown, P. Subthalamic nucleus, sensorimotor cortex and muscle interrelationships in Parkinson’s disease. Brain A J. Neurol. 2001, 124, 378–388. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.; Oliviero, A.; Mazzone, P.; Insola, A.; Tonali, P.; Di Lazzaro, V. Dopamine dependency of oscillations between subthalamic nucleus and pallidum in Parkinson’s disease. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 1033–1038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Volkmann, J.; Joliot, M.; Mogilner, A.; Ioannides, A.A.; Lado, F.; Fazzini, E.; Ribary, U.; Llinás, R. Central motor loop oscillations in parkinsonian resting tremor revealed by magnetoencephalography. Neurology 1996, 46, 1359–1370. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, J.S.; Chung, C.K. Differential beta-band event-related desynchronization during categorical action sequence planning. PLoS ONE 2013, 8, e59544. [Google Scholar] [CrossRef]
- Muthuraman, M.; Palotai, M.; Jávor-Duray, B.; Kelemen, A.; Koirala, N.; Halász, L.; Erőss, L.; Fekete, G.; Bognár, L.; Deuschl, G.; et al. Frequency-specific network activity predicts bradykinesia severity in Parkinson’s disease. NeuroImage Clin. 2021, 32, 102857. [Google Scholar] [CrossRef]
- Meirovitch, Y.; Harris, H.; Dayan, E.; Arieli, A.; Flash, T. Alpha and beta band event-related desynchronization reflects kinematic regularities. J. Neurosci. Off. J. Soc. Neurosci. 2015, 35, 1627–1637. [Google Scholar] [CrossRef] [Green Version]
- Pollok, B.; Krause, V.; Martsch, W.; Wach, C.; Schnitzler, A.; Südmeyer, M. Motor-cortical oscillations in early stages of Parkinson’s disease. J. Physiol. 2012, 590, 3203–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wagner, J.R.; Schaper, M.; Hamel, W.; Westphal, M.; Gerloff, C.; Engel, A.K.; Moll, C.K.E.; Gulberti, A.; Pötter-Nerger, M. Combined Subthalamic and Nigral Stimulation Modulates Temporal Gait Coordination and Cortical Gait-Network Activity in Parkinson’s Disease. Front. Hum. Neurosci. 2022, 16, 812954. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.T.; Amarell, M.; Snijders, A.H.; Florin, E.; Quatuor, E.L.; Schönau, E.; Fink, G.R.; Bloem, B.R.; Timmermann, L. Gait and upper limb variability in Parkinson’s disease patients with and without freezing of gait. J. Neurol. 2014, 261, 330–342. [Google Scholar] [CrossRef]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef]
- Wang, D.D.; Choi, J.T. Brain Network Oscillations During Gait in Parkinson’s Disease. Front. Hum. Neurosci. 2020, 14, 568703. [Google Scholar] [CrossRef] [PubMed]
- Fasano, A.; Visanji, N.P.; Liu, L.W.; Lang, A.E.; Pfeiffer, R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015, 14, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Travagli, R.A.; Browning, K.N.; Camilleri, M. Parkinson disease and the gut: New insights into pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, M.G.; Benarroch, E.E. Neural control of the gastrointestinal tract: Implications for Parkinson disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2008, 23, 1065–1075. [Google Scholar] [CrossRef]
- Suttrup, I.; Warnecke, T. Dysphagia in Parkinson’s Disease. Dysphagia 2016, 31, 24–32. [Google Scholar] [CrossRef]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Travagli, R.A.; Anselmi, L. Vagal neurocircuitry and its influence on gastric motility. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 389–401. [Google Scholar] [CrossRef] [Green Version]
- Cuellar, M.; Harkrider, A.W.; Jenson, D.; Thornton, D.; Bowers, A.; Saltuklaroglu, T. Time-frequency analysis of the EEG mu rhythm as a measure of sensorimotor integration in the later stages of swallowing. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2016, 127, 2625–2635. [Google Scholar] [CrossRef]
- Anselmi, L.; Toti, L.; Bove, C.; Hampton, J.; Travagli, R.A. A Nigro-Vagal Pathway Controls Gastric Motility and Is Affected in a Rat Model of Parkinsonism. Gastroenterology 2017, 153, 1581–1593. [Google Scholar] [CrossRef]
- Warnecke, T.; Schäfer, K.H.; Claus, I.; Del Tredici, K.; Jost, W.H. Gastrointestinal involvement in Parkinson’s disease: Pathophysiology, diagnosis, and management. NPJ Park. Dis. 2022, 8, 31. [Google Scholar] [CrossRef]
- Alfonsi, E.; Versino, M.; Merlo, I.M.; Pacchetti, C.; Martignoni, E.; Bertino, G.; Moglia, A.; Tassorelli, C.; Nappi, G. Electrophysiologic patterns of oral-pharyngeal swallowing in parkinsonian syndromes. Neurology 2007, 68, 583–589. [Google Scholar] [CrossRef]
- Labeit, B.; Claus, I.; Muhle, P.; Lapa, S.; Suntrup-Krueger, S.; Dziewas, R.; Osada, N.; Warnecke, T. Oropharyngeal freezing and its relation to dysphagia—An analogy to freezing of gait. Park. Relat. Disord. 2020, 75, 1–6. [Google Scholar] [CrossRef]
- Qualman, S.J.; Haupt, H.M.; Yang, P.; Hamilton, S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology 1984, 87, 848–856. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.L.; Shan, D.E.; Chen, C.Y.; Luo, J.C.; Chang, F.Y.; Lee, S.D.; Wu, H.C.; Chen, J.D. Impaired gastric myoelectrical activity in patients with Parkinson’s disease and effect of levodopa treatment. Dig. Dis. Sci. 2004, 49, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Doi, H.; Sakakibara, R.; Sato, M.; Masaka, T.; Kishi, M.; Tateno, A.; Tateno, F.; Tsuyusaki, Y.; Takahashi, O. Plasma levodopa peak delay and impaired gastric emptying in Parkinson’s disease. J. Neurol. Sci. 2012, 319, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Hardoff, R.; Sula, M.; Tamir, A.; Soil, A.; Front, A.; Badarna, S.; Honigman, S.; Giladi, N. Gastric emptying time and gastric motility in patients with Parkinson’s disease. Mov. Disord. Off. J. Mov. Disord. Soc. 2001, 16, 1041–1047. [Google Scholar] [CrossRef]
- Cao, J.; Wang, X.; Chen, J.; Zhang, N.; Liu, Z. The vagus nerve mediates the stomach-brain coherence in rats. NeuroImage 2022, 263, 119628. [Google Scholar] [CrossRef]
- Kaneoke, Y.; Koike, Y.; Sakurai, N.; Washimi, Y.; Hirayama, M.; Hoshiyama, M.; Takahashi, A. Gastrointestinal dysfunction in Parkinson’s disease detected by electrogastroenterography. J. Auton. Nerv. Syst. 1995, 50, 275–281. [Google Scholar] [CrossRef]
- Naftali, T.; Gadoth, N.; Huberman, M.; Novis, B. Electrogastrography in patients with Parkinson’s disease. Can. J. Neurol. Sci. 2005, 32, 82–86. [Google Scholar] [CrossRef] [Green Version]
- Soykan, I.; Lin, Z.; Bennett, J.P.; McCallum, R.W. Gastric myoelectrical activity in patients with Parkinson’s disease: Evidence of a primary gastric abnormality. Dig. Dis. Sci. 1999, 44, 927–931. [Google Scholar] [CrossRef]
- Araki, N.; Yamanaka, Y.; Poudel, A.; Fujinuma, Y.; Katagiri, A.; Kuwabara, S.; Asahina, M. Electrogastrography for diagnosis of early-stage Parkinson’s disease. Park. Relat. Disord. 2021, 86, 61–66. [Google Scholar] [CrossRef]
- Ashraf, W.; Wszolek, Z.K.; Pfeiffer, R.F.; Normand, M.; Maurer, K.; Srb, F.; Edwards, L.L.; Quigley, E.M. Anorectal function in fluctuating (on-off) Parkinson’s disease: Evaluation by combined anorectal manometry and electromyography. Mov. Disord. 1995, 10, 650–657. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Waninger, S.; Berka, C.; Stevanovic Karic, M.; Korszen, S.; Mozley, P.D.; Henchcliffe, C.; Kang, Y.; Hesterman, J.; Mangoubi, T.; Verma, A. Neurophysiological Biomarkers of Parkinson’s Disease. J. Park. Dis. 2020, 10, 471–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Berardelli, A.; Bhattacharya, A.; Bologna, M.; Chen, K.S.; Fasano, A.; Helmich, R.C.; Hutchison, W.D.; Kamble, N.; Kühn, A.A.; et al. Clinical neurophysiology of Parkinson’s disease and parkinsonism. Clin. Neurophysiol. Pract. 2022, 7, 201–227. [Google Scholar] [CrossRef]
- Soliman, H.; Coffin, B.; Gourcerol, G. Gastroparesis in Parkinson Disease: Pathophysiology, and Clinical Management. Brain Sci. 2021, 11, 831. [Google Scholar] [CrossRef] [PubMed]
- Bowyer, S.M. Coherence a measure of the brain networks: Past and present. Neuropsychiatr. Electrophysiol. 2016, 2, 1. [Google Scholar] [CrossRef]
- Chen, K.S.; Chen, R. Principles of Electrophysiological Assessments for Movement Disorders. J. Mov. Disord. 2020, 13, 27–38. [Google Scholar] [CrossRef]
- Cassim, F.; Szurhaj, W.; Sediri, H.; Devos, D.; Bourriez, J.; Poirot, I.; Derambure, P.; Defebvre, L.; Guieu, J. Brief and sustained movements: Differences in event-related (de)synchronization (ERD/ERS) patterns. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2000, 111, 2032–2039. [Google Scholar] [CrossRef]
- Leocani, L.; Toro, C.; Manganotti, P.; Zhuang, P.; Hallett, M. Event-related coherence and event-related desynchronization/synchronization in the 10 Hz and 20 Hz EEG during self-paced movements. Electroencephalogr. Clin. Neurophysiol. 1997, 104, 199–206. [Google Scholar] [CrossRef]
- Vinding, M.C.; Tsitsi, P.; Waldthaler, J.; Oostenveld, R.; Ingvar, M.; Svenningsson, P.; Lundqvist, D. Reduction of spontaneous cortical beta bursts in Parkinson’s disease is linked to symptom severity. Brain Commun. 2020, 2, fcaa052. [Google Scholar] [CrossRef] [PubMed]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut-Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef] [PubMed]
- Grenham, S.; Clarke, G.; Cryan, J.F.; Dinan, T.G. Brain-gut-microbe communication in health and disease. Front. Physiol. 2011, 2, 94. [Google Scholar] [CrossRef] [Green Version]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.H.; Chong, C.W.; Lim, S.Y.; Yap, I.K.S.; Teh, C.S.J.; Loke, M.F.; Song, S.L.; Tan, J.Y.; Ang, B.H.; Tan, Y.Q.; et al. Gut microbial ecosystem in Parkinson disease: New clinicobiological insights from multi-omics. Ann. Neurol. 2021, 89, 546–559. [Google Scholar] [CrossRef]
- Bullich, C.; Keshavarzian, A.; Garssen, J.; Kraneveld, A.; Perez-Pardo, P. Gut Vibes in Parkinson’s Disease: The Microbiota-Gut-Brain Axis. Mov. Disord. Clin. Pract. 2019, 6, 639–651. [Google Scholar] [CrossRef]
- Forsythe, P.; Bienenstock, J.; Kunze, W.A. Vagal pathways for microbiome-brain-gut axis communication. Adv. Exp. Med. Biol. 2014, 817, 115–133. [Google Scholar] [CrossRef]
- Tan, A.H.; Lim, S.Y.; Lang, A.E. The microbiome-gut-brain axis in Parkinson disease- from basic research to the clinic. Nat. Rev. Neurology 2022, 18, 476–495. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acosta-Mejia, M.T.; Villalobos, N. Neurophysiology of Brain Networks Underlies Symptoms of Parkinson’s Disease: A Basis for Diagnosis and Management. Diagnostics 2023, 13, 2394. https://doi.org/10.3390/diagnostics13142394
Acosta-Mejia MT, Villalobos N. Neurophysiology of Brain Networks Underlies Symptoms of Parkinson’s Disease: A Basis for Diagnosis and Management. Diagnostics. 2023; 13(14):2394. https://doi.org/10.3390/diagnostics13142394
Chicago/Turabian StyleAcosta-Mejia, Martha Teresa, and Nelson Villalobos. 2023. "Neurophysiology of Brain Networks Underlies Symptoms of Parkinson’s Disease: A Basis for Diagnosis and Management" Diagnostics 13, no. 14: 2394. https://doi.org/10.3390/diagnostics13142394





