Pearls and Pitfalls of Adaptive Optics Ophthalmoscopy in Inherited Retinal Diseases
Abstract
1. Introduction
2. Basic Concepts
2.1. Principles of Adaptive Optics
2.2. Technologies and Devices
2.2.1. AO Flood Illumination Ophthalmoscopy (AOFIO)
2.2.2. AO Scanning Laser Ophthalmoscopy (AOSLO)
2.2.3. AO Optical Coherence Tomography (AO-OCT)
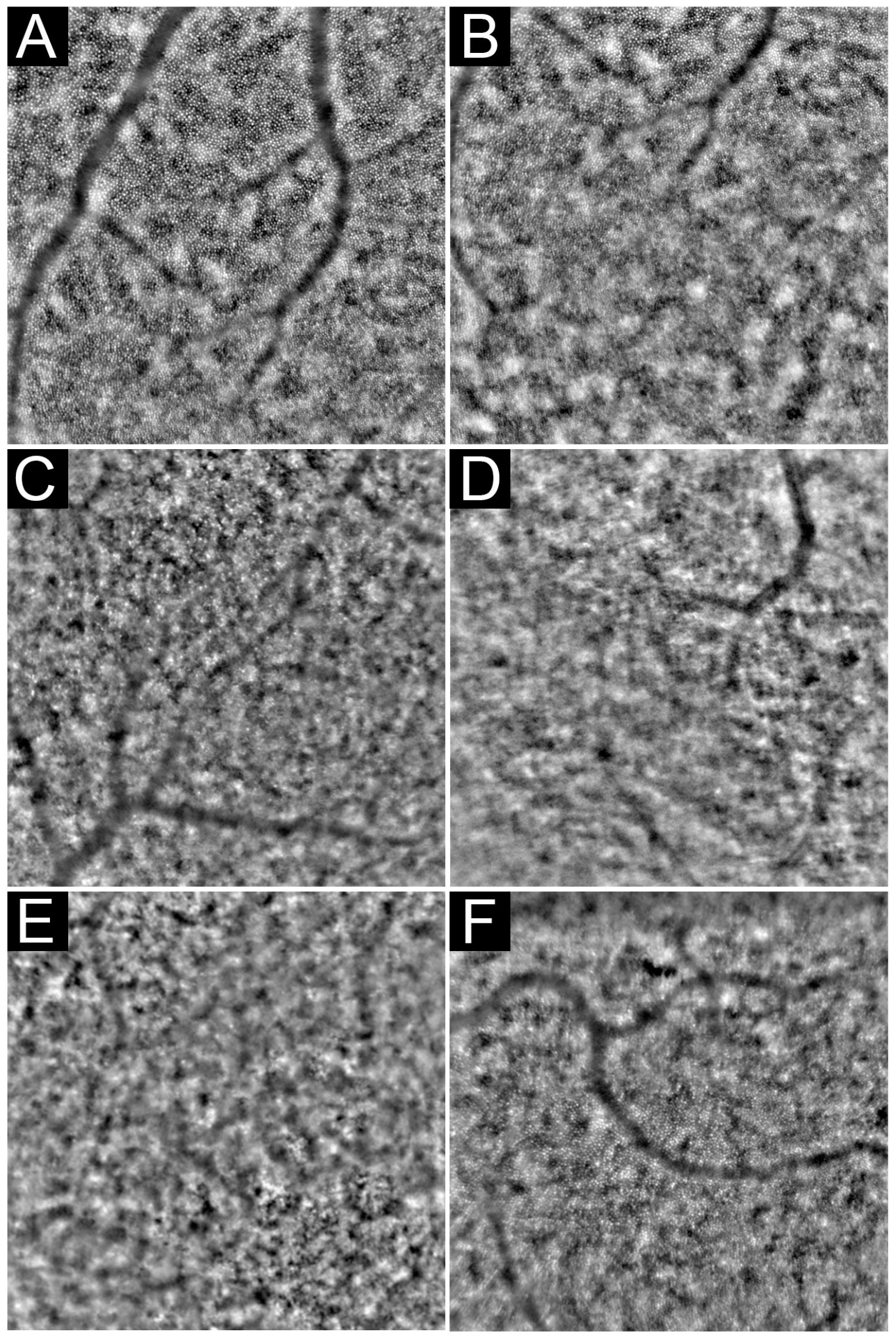
3. Acquisition, Analysis, and Interpretation
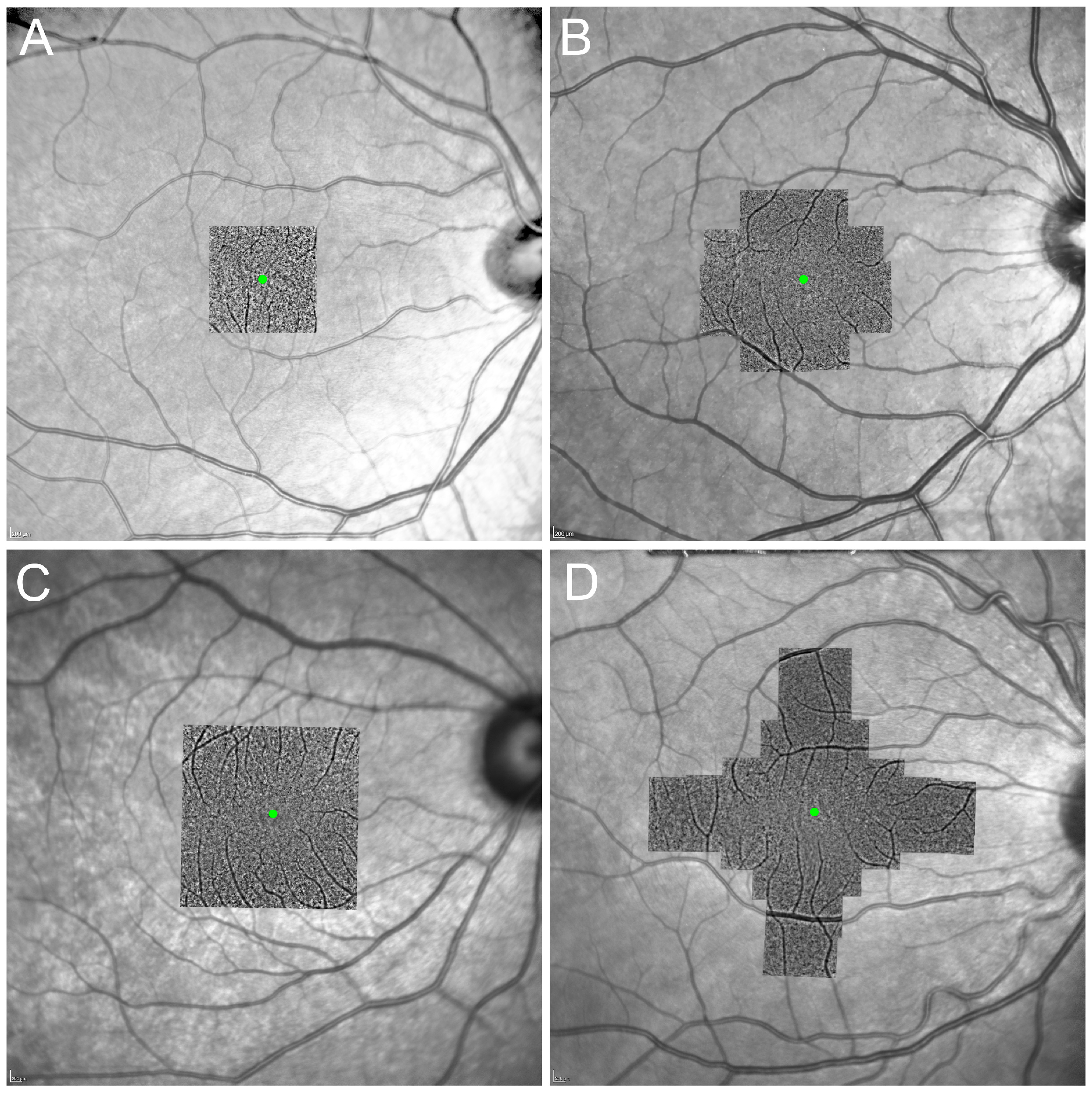
3.1. Image Acquisition Protocols
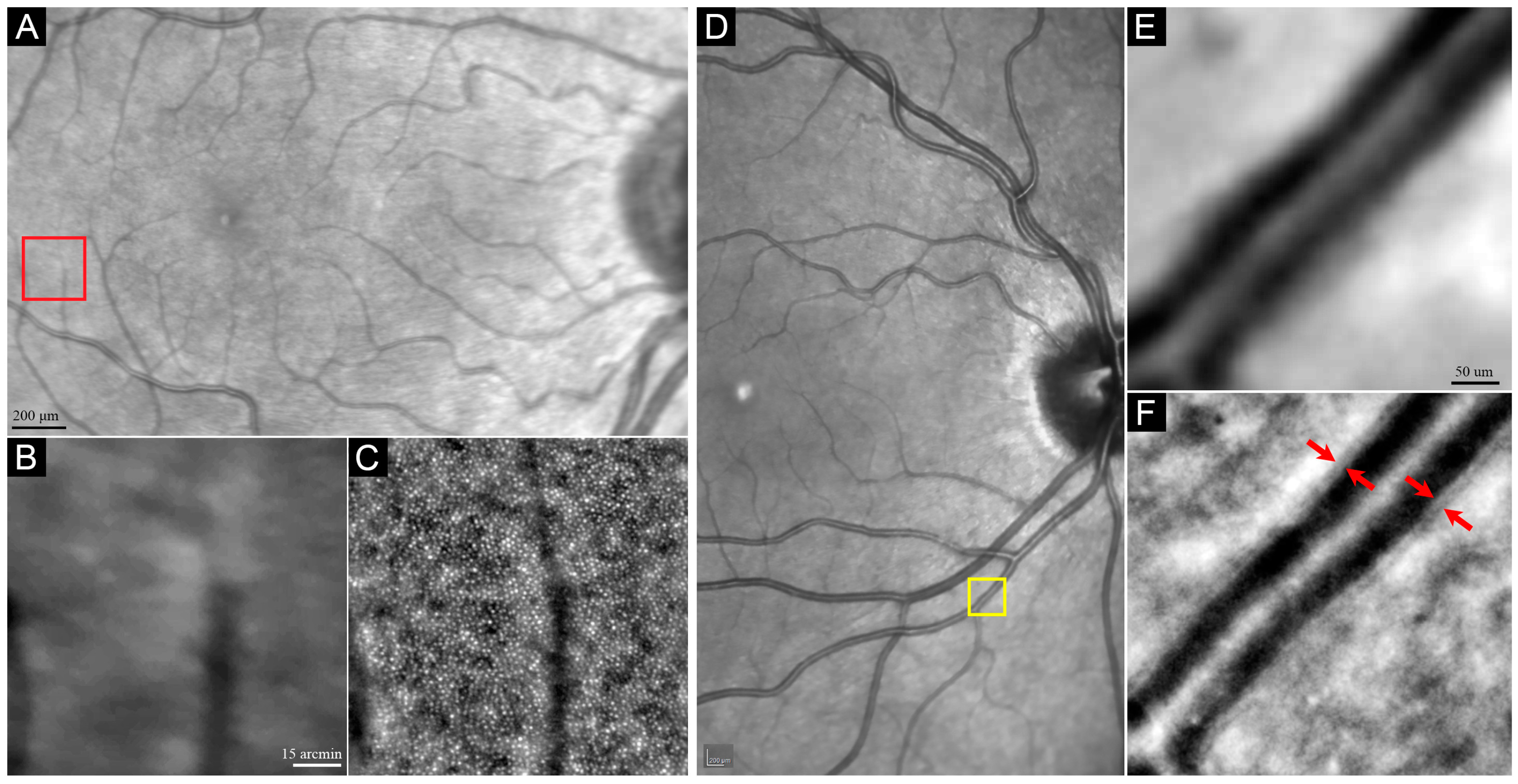
3.1.1. Photoreceptor
3.1.2. Blood Vessel
3.2. Image Analysis
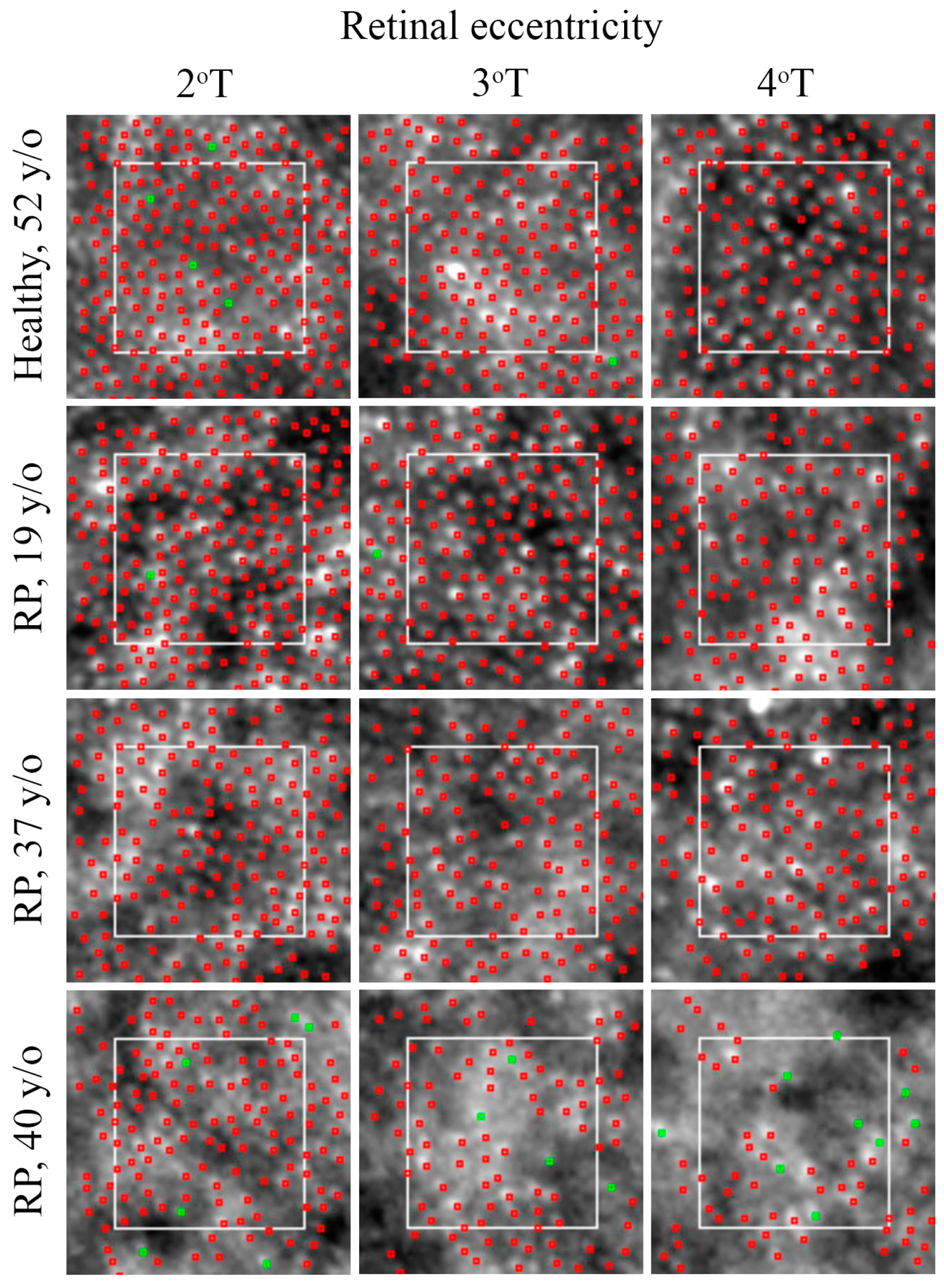
3.2.1. Photoreceptor
3.2.2. Blood Vessel
3.3. Outcome Measures
3.3.1. Photoreceptor
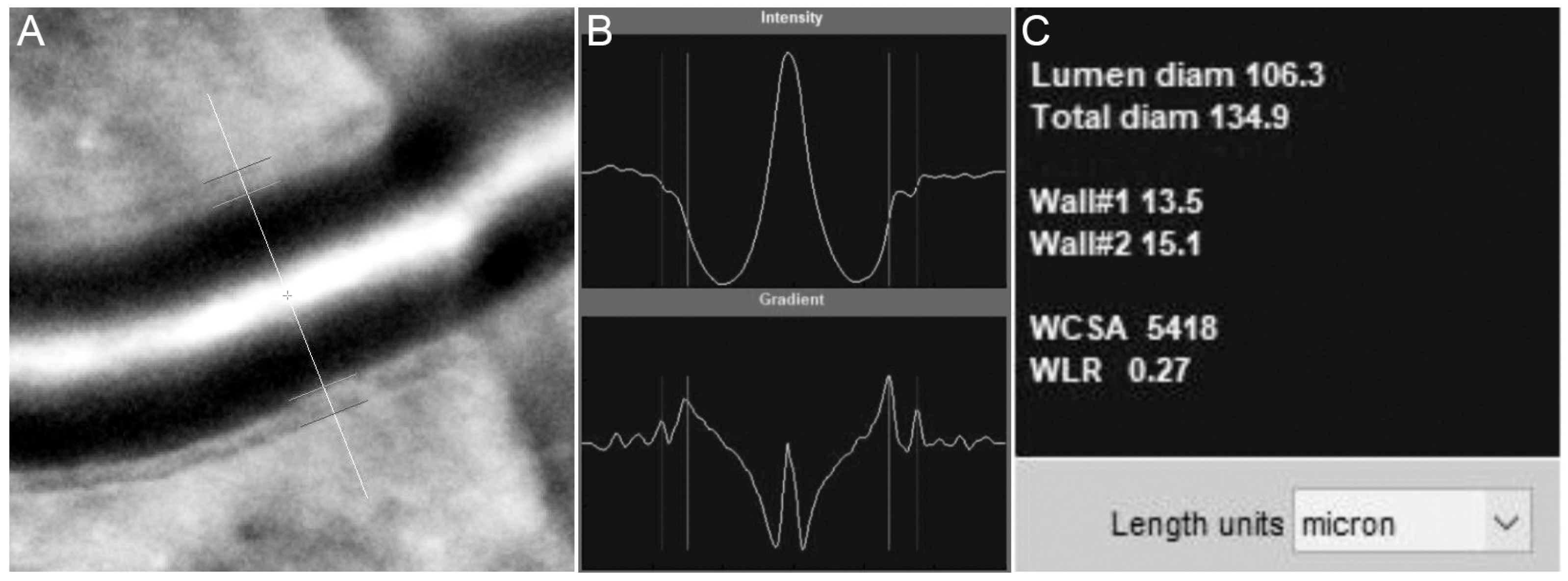
3.3.2. Blood Vessel
4. AO Imaging in Diagnosing Early-Stage IRDs
4.1. Rod-Dominated Dystrophies
4.2. Cone-Dominated Diseases
4.3. Macular Dystrophies
4.3.1. Stargardt Disease
4.3.2. Vitelliform Macular Dystrophies (VMDs)
4.3.3. X-Linked Retinoschisis (XLRS)
4.4. Choroideremia
5. AO imaging in Tracking the Progression of IRDs
6. Limitations
6.1. Resolution
6.2. Image Quality
6.3. Localization
6.4. Structure–Function Correlation
6.5. Cone Matching and Follow-Up Imaging
6.6. Special Considerations in Patients with RP
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Roorda, A.; Duncan, J.L. Advances in imaging of Stargardt disease. Adv. Exp. Med. Biol. 2010, 664, 333–340. [Google Scholar] [CrossRef]
- Talcott, K.E.; Ratnam, K.; Sundquist, S.M.; Lucero, A.S.; Lujan, B.J.; Tao, W.; Porco, T.C.; Roorda, A.; Duncan, J.L. Longitudinal study of cone photoreceptors during retinal degeneration and in response to ciliary neurotrophic factor treatment. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Hillard, J.G.; Gast, T.J.; Chui, T.Y.; Sapir, D.; Burns, S.A. Retinal arterioles in hypo-, normo-, and hypertensive subjects measured using adaptive optics. Transl. Vis. Sci. Technol. 2016, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Luo, T.; Gast, T.J.; Vermeer, T.J.; Burns, S.A. Retinal vascular branching in healthy and diabetic subjects. Investig. Ophthalmol. Vis. Sci. 2017, 58, 2685–2694. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.; Dhamdhere, K.P.; Tiruveedhula, P.; Manzanera, S.; Barez, S.; Bearse, M.A.; Adams, A.J.; Roorda, A. Disruption of the retinal parafoveal capillary network in type 2 diabetes before the onset of diabetic retinopathy. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9257–9266. [Google Scholar] [CrossRef]
- Kurokawa, K.; Liu, Z.; Miller, D.T. Adaptive optics optical coherence tomography angiography for morphometric analysis of choriocapillaris. Biomed. Opt. Express 2017, 8, 1803–1822. [Google Scholar] [CrossRef]
- Heath Jeffery, R.C.; Mukhtar, S.A.; McAllister, I.L.; Morgan, W.H.; Mackey, D.A.; Chen, F.K. Inherited retinal diseases are the most common cause of blindness in the working-age population in Australia. Ophthalmic Genet. 2021, 42, 431–439. [Google Scholar] [CrossRef]
- Newton, F.; Megaw, R. Mechanisms of Photoreceptor Death in Retinitis Pigmentosa. Genes 2020, 11, 1120. [Google Scholar] [CrossRef]
- Ciulla, T.A.; Hussain, R.M.; Berrocal, A.M.; Nagiel, A. Voretigene neparvovec-rzyl for treatment of RPE65-mediated inherited retinal diseases: A model for ocular gene therapy development. Expert Opin. Biol. Ther. 2020, 20, 565–578. [Google Scholar] [CrossRef]
- Cehajic-Kapetanovic, J.; Xue, K.; Martinez-Fernandez de la Camara, C.; Nanda, A.; Davies, A.; Wood, L.J.; Salvetti, A.P.; Fischer, M.D.; Aylward, J.W.; Barnard, A.R.; et al. Initial results from a first-in-human gene therapy trial on X-linked retinitis pigmentosa caused by mutations in RPGR. Nat. Med. 2020, 26, 354–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lillywhite, J.; Zhu, W.; Huang, Z.; Clark, A.M.; Gosstola, N.; Maguire, C.T.; Dykxhoorn, D.; Chen, Z.Y.; Yang, J. Generation and Genetic Correction of USH2A c.2299delG Mutation in Patient-Derived Induced Pluripotent Stem Cells. Genes 2021, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, D.; Heath Jeffery, R.C.; Charng, J.; Sampson, D.M.; McLenachan, S.; Mackey, D.A.; Chen, F.K. Short-Term Parafoveal Cone Loss Despite Preserved Ellipsoid Zone in Rod Cone Dystrophy. Transl. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef] [PubMed]
- Babcock, H.W. The Possibility of Compensating Astronomical Seeing. Publ. Astron. Soc. Pac. 1953, 65, 8. [Google Scholar] [CrossRef]
- Liu, L.; Wu, Z.; Qi, M.; Li, Y.; Zhang, M.; Liao, D.; Gao, P. Application of Adaptive Optics in Ophthalmology. Photonics 2022, 9, 288. [Google Scholar] [CrossRef]
- Hofer, H.; Sredar, N.; Queener, H.; Li, C.; Porter, J. Wavefront sensorless adaptive optics ophthalmoscopy in the human eye. Opt. Express 2011, 19, 14160–14171. [Google Scholar] [CrossRef]
- Tumahai, P.; Moureaux, C.; Meillat, M.; Debellemaniere, G.; Flores, M.; Delbosc, B.; Saleh, M. High-resolution imaging of photoreceptors in healthy human eyes using an adaptive optics retinal camera. Eye 2018, 32, 1723–1730. [Google Scholar] [CrossRef]
- Salmon, A.E.; Cooper, R.F.; Langlo, C.S.; Baghaie, A.; Dubra, A.; Carroll, J. An automated reference frame selection (ARFS) algorithm for cone imaging with adaptive optics scanning light ophthalmoscopy. Transl. Vis. Sci. Technol. 2017, 6, 9. [Google Scholar] [CrossRef]
- Bidaut Garnier, M.; Flores, M.; Debellemanière, G.; Puyraveau, M.; Tumahai, P.; Meillat, M.; Schwartz, C.; Montard, M.; Delbosc, B.; Saleh, M. Reliability of cone counts using an adaptive optics retinal camera. Clin. Exp. Ophthalmol. 2014, 42, 833–840. [Google Scholar] [CrossRef]
- Lombardo, M.; Lombardo, G.; Ducoli, P.; Serrao, S. Adaptive optics photoreceptor imaging. Ophthalmology 2012, 119, 1498–1498.e2. [Google Scholar] [CrossRef]
- Muthiah, M.N.; Gias, C.; Chen, F.K.; Zhong, J.; McClelland, Z.; Sallo, F.B.; Peto, T.; Coffey, P.J.; da Cruz, L. Cone photoreceptor definition on adaptive optics retinal imaging. Br. J. Ophthalmol. 2014, 98, 1073–1079. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Li, N.; Kang, J.; He, Y.; Chen, X.-M. Adaptive optics scanning laser ophthalmoscopy in fundus imaging, a review and update. Int. J. Ophthalmol. 2017, 10, 1751. [Google Scholar] [PubMed]
- Scoles, D.; Sulai, Y.N.; Langlo, C.S.; Fishman, G.A.; Curcio, C.A.; Carroll, J.; Dubra, A. In vivo imaging of human cone photoreceptor inner segments. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, E.; Drexler, W. Influence of ocular chromatic aberration and pupil size on transverse resolution in ophthalmic adaptive optics optical coherence tomography. Opt. Express 2005, 13, 8184–8197. [Google Scholar] [CrossRef] [PubMed]
- Reumueller, A.; Wassermann, L.; Salas, M.; Schranz, M.; Hacker, V.; Mylonas, G.; Sacu, S.; Drexler, W.; Pircher, M.; Schmidt-Erfurth, U.; et al. Three-dimensional composition of the photoreceptor cone layers in healthy eyes using adaptive-optics optical coherence tomography (AO-OCT). PLoS ONE 2021, 16, e0245293. [Google Scholar] [CrossRef]
- Zhang, Y.; Rha, J.; Jonnal, R.; Miller, D. Adaptive optics parallel spectral domain optical coherence tomography for imaging the living retina. Opt. Express 2005, 13, 4792–4811. [Google Scholar] [CrossRef]
- Cense, B.; Gao, W.; Brown, J.M.; Jones, S.M.; Jonnal, R.S.; Mujat, M.; Park, B.H.; de Boer, J.F.; Miller, D.T. Retinal imaging with polarization-sensitive optical coherence tomography and adaptive optics. Opt. Express 2009, 17, 21634–21651. [Google Scholar] [CrossRef]
- Torti, C.; Povazay, B.; Hofer, B.; Unterhuber, A.; Carroll, J.; Ahnelt, P.K.; Drexler, W. Adaptive optics optical coherence tomography at 120,000 depth scans/s for non-invasive cellular phenotyping of the living human retina. Opt. Express 2009, 17, 19382–19400. [Google Scholar] [CrossRef]
- Wong, K.S.; Jian, Y.; Cua, M.; Bonora, S.; Zawadzki, R.J.; Sarunic, M.V. In vivo imaging of human photoreceptor mosaic with wavefront sensorless adaptive optics optical coherence tomography. Biomed. Opt. Express 2015, 6, 580–590. [Google Scholar] [CrossRef]
- Davidson, B.; Kalitzeos, A.; Carroll, J.; Dubra, A.; Ourselin, S.; Michaelides, M.; Bergeles, C. Fast adaptive optics scanning light ophthalmoscope retinal montaging. Biomed. Opt. Express 2018, 9, 4317–4328. [Google Scholar] [CrossRef]
- Lowe, D.G. Distinctive image features from scale-invariant keypoints. Int. J. Comput. Vis. 2004, 60, 91–110. [Google Scholar] [CrossRef]
- Martin, J.A.; Roorda, A. Direct and noninvasive assessment of parafoveal capillary leukocyte velocity. Ophthalmology 2005, 112, 2219–2224. [Google Scholar] [CrossRef]
- Tam, J.; Martin, J.A.; Roorda, A. Noninvasive visualization and analysis of parafoveal capillaries in humans. Investig. Ophthalmol. Vis. Sci. 2010, 51, 1691–1698. [Google Scholar] [CrossRef] [PubMed]
- Chui, T.Y.P.; Mo, S.; Krawitz, B.; Menon, N.R.; Choudhury, N.; Gan, A.; Razeen, M.; Shah, N.; Pinhas, A.; Rosen, R.B. Human retinal microvascular imaging using adaptive optics scanning light ophthalmoscopy. Int. J. Retin. Vitr. 2016, 2, 11. [Google Scholar] [CrossRef] [PubMed]
- Carroll, J.; Neitz, M.; Hofer, H.; Neitz, J.; Williams, D.R. Functional photoreceptor loss revealed with adaptive optics: An alternate cause of color blindness. Proc. Natl. Acad. Sci. USA 2004, 101, 8461–8466. [Google Scholar] [CrossRef] [PubMed]
- Wolfing, J.I.; Chung, M.; Carroll, J.; Roorda, A.; Williams, D.R. High-resolution retinal imaging of cone-rod dystrophy. Ophthalmology 2006, 113, 1019.e1. [Google Scholar] [CrossRef]
- Li, K.Y.; Roorda, A. Automated identification of cone photoreceptors in adaptive optics retinal images. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2007, 24, 1358–1363. [Google Scholar] [CrossRef]
- Bukowska, D.M.; Chew, A.L.; Huynh, E.; Kashani, I.; Wan, S.L.; Wan, P.M.; Chen, F.K. Semi-automated identification of cones in the human retina using circle Hough transform. Biomed. Opt. Express 2015, 6, 4676–4693. [Google Scholar] [CrossRef]
- Chiu, S.J.; Lokhnygina, Y.; Dubis, A.M.; Dubra, A.; Carroll, J.; Izatt, J.A.; Farsiu, S. Automatic cone photoreceptor segmentation using graph theory and dynamic programming. Biomed. Opt. Express 2013, 4, 924–937. [Google Scholar] [CrossRef]
- Liu, J.; Dubra, A.; Tam, J. A fully automatic framework for cell segmentation on non-confocal adaptive optics images. In Proceedings of the Medical Imaging 2016: Computer-Aided Diagnosis, San Diego, CA, USA, 27 February–3 March 2016; pp. 654–660. [Google Scholar]
- Hamwood, J.; Alonso-Caneiro, D.; Sampson, D.M.; Collins, M.J.; Chen, F.K. Automatic Detection of Cone Photoreceptors With Fully Convolutional Networks. Transl. Vis. Sci. Technol. 2019, 8, 10. [Google Scholar] [CrossRef]
- Lombardo, M.; Serrao, S.; Ducoli, P.; Lombardo, G. Eccentricity dependent changes of density, spacing and packing arrangement of parafoveal cones. Ophthalmic Physiol. Opt. J. Br. Coll. Ophthalmic Opt. (Optom.) 2013, 33, 516–526. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Zmijewska, A.; Wawrzyniak, Z.M.; Ulinska, M.; Szaflik, J.; Dabrowska, A.; Szaflik, J.P. Human photoreceptor cone density measured with adaptive optics technology (rtx1 device) in healthy eyes: Standardization of measurements. Medicine 2017, 96, e7300. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.A.; Akkali, M.C.; Jayadev, C.; Anuj, A.; Yadav, N.K. Morphometric analysis of retinal arterioles in control and hypertensive population using adaptive optics imaging. Indian J. Ophthalmol. 2019, 67, 1673–1677. [Google Scholar] [CrossRef]
- Frangi, A.F.; Niessen, W.J.; Vincken, K.L.; Viergever, M.A. Multiscale vessel enhancement filtering. In Medical Image Computing and Computer-Assisted Intervention—MICCAI’98: First International Conference, Cambridge, MA, USA, 11–13 October 1998; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Zhang, Q.; Sampani, K.; Xu, M.; Cai, S.; Deng, Y.; Li, H.; Sun, J.K.; Karniadakis, G.E. AOSLO-net: A Deep Learning-Based Method for Automatic Segmentation of Retinal Microaneurysms From Adaptive Optics Scanning Laser Ophthalmoscopy Images. Transl. Vis. Sci. Technol. 2022, 11, 7. [Google Scholar] [CrossRef] [PubMed]
- Le, C.T.; Wang, D.; Villanueva, R.; Liu, Z.; Hammer, D.X.; Tao, Y.; Saeedi, O.J. Novel Application of Long Short-Term Memory Network for 3D to 2D Retinal Vessel Segmentation in Adaptive Optics—Optical Coherence Tomography Volumes. Appl. Sci. 2021, 11, 9475. [Google Scholar] [CrossRef]
- Legras, R.; Gaudric, A.; Woog, K. Distribution of cone density, spacing and arrangement in adult healthy retinas with adaptive optics flood illumination. PLoS ONE 2018, 13, e0191141. [Google Scholar] [CrossRef]
- Makiyama, Y.; Ooto, S.; Hangai, M.; Takayama, K.; Uji, A.; Oishi, A.; Ogino, K.; Nakagawa, S.; Yoshimura, N. Macular cone abnormalities in retinitis pigmentosa with preserved central vision using adaptive optics scanning laser ophthalmoscopy. PLoS ONE 2013, 8, e79447. [Google Scholar] [CrossRef]
- Tanna, P.; Kasilian, M.; Strauss, R.; Tee, J.; Kalitzeos, A.; Tarima, S.; Visotcky, A.; Dubra, A.; Carroll, J.; Michaelides, M. Reliability and Repeatability of Cone Density Measurements in Patients With Stargardt Disease and RPGR-Associated Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3608–3615. [Google Scholar] [CrossRef]
- Zayit-Soudry, S.; Sippl-Swezey, N.; Porco, T.C.; Lynch, S.K.; Syed, R.; Ratnam, K.; Menghini, M.; Roorda, A.J.; Duncan, J.L. Repeatability of Cone Spacing Measures in Eyes With Inherited Retinal Degenerations. Investig. Ophthalmol. Vis. Sci. 2015, 56, 6179–6189. [Google Scholar] [CrossRef]
- Mc Glacken-Byrne, A.B.; Prentice, D.; Roshandel, D.; Brown, M.R.; Tuch, P.; Yau, K.S.; Sivadorai, P.; Davis, M.R.; Laing, N.G.; Chen, F.K. High-resolution iris and retinal imaging in multisystemic smooth muscle dysfunction syndrome due to a novel Asn117Lys substitution in ACTA2: A case report. BMC Ophthalmol. 2020, 20, 68. [Google Scholar] [CrossRef]
- Meixner, E.; Michelson, G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: A clinical research. Graefes Arch Clin. Exp. Ophthalmol. 2015, 253, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Koch, E.; Rosenbaum, D.; Brolly, A.; Sahel, J.A.; Chaumet-Riffaud, P.; Girerd, X.; Rossant, F.; Paques, M. Morphometric analysis of small arteries in the human retina using adaptive optics imaging: Relationship with blood pressure and focal vascular changes. J. Hypertens. 2014, 32, 890–898. [Google Scholar] [CrossRef]
- Arichika, S.; Uji, A.; Ooto, S.; Muraoka, Y.; Yoshimura, N. Effects of age and blood pressure on the retinal arterial wall, analyzed using adaptive optics scanning laser ophthalmoscopy. Sci. Rep. 2015, 5, 12283. [Google Scholar] [CrossRef] [PubMed]
- Bakker, E.; Dikland, F.A.; van Bakel, R.; De Jesus, D.A.; Sanchez Brea, L.; Klein, S.; van Walsum, T.; Rossant, F.; Farias, D.C.; Grieve, K.; et al. Adaptive optics ophthalmoscopy: A systematic review of vascular biomarkers. Surv. Ophthalmol. 2022, 67, 369–387. [Google Scholar] [CrossRef] [PubMed]
- Nesper, P.L.; Scarinci, F.; Fawzi, A.A. Adaptive Optics Reveals Photoreceptor Abnormalities in Diabetic Macular Ischemia. PLoS ONE 2017, 12, e0169926. [Google Scholar] [CrossRef]
- Michaelides, M.; Kaines, A.; Hamilton, R.D.; Fraser-Bell, S.; Rajendram, R.; Quhill, F.; Boos, C.J.; Xing, W.; Egan, C.; Peto, T.; et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: Report 2. Ophthalmology 2010, 117, 1078–1086.e2. [Google Scholar] [CrossRef]
- Menghini, M.; Lujan, B.J.; Zayit-Soudry, S.; Syed, R.; Porco, T.C.; Bayabo, K.; Carroll, J.; Roorda, A.; Duncan, J.L. Correlation of outer nuclear layer thickness with cone density values in patients with retinitis pigmentosa and healthy subjects. Investig. Ophthalmol. Vis. Sci. 2014, 56, 372–381. [Google Scholar] [CrossRef]
- Park, S.P.; Lee, W.; Bae, E.J.; Greenstein, V.; Sin, B.H.; Chang, S.; Tsang, S.H. Early structural anomalies observed by high-resolution imaging in two related cases of autosomal-dominant retinitis pigmentosa. Ophthalmic Surg. Lasers Imaging Retin. 2014, 45, 469–473. [Google Scholar] [CrossRef]
- Tojo, N.; Nakamura, T.; Fuchizawa, C.; Oiwake, T.; Hayashi, A. Adaptive optics fundus images of cone photoreceptors in the macula of patients with retinitis pigmentosa. Clin. Ophthalmol. 2013, 7, 203–210. [Google Scholar] [CrossRef]
- Ochinciuc, R.; Ochinciuc, U.; Balta, G.; Al Barri, L.; Pac, C.; Adrian, T.; Balta, F.; Burcea, M. High-resolution images in macular disorders. Rom. J. Ophthalmol. 2021, 65, 204–211. [Google Scholar] [CrossRef]
- Gale, M.J.; Feng, S.; Titus, H.E.; Smith, T.B.; Pennesi, M.E. Interpretation of Flood-Illuminated Adaptive Optics Images in Subjects with Retinitis Pigmentosa. Adv. Exp. Med. Biol. 2016, 854, 291–297. [Google Scholar] [CrossRef]
- Roshandel, D.; Lamey, T.M.; Charng, J.; Heath Jeffery, R.C.; McLaren, T.L.; Thompson, J.A.; De Roach, J.N.; McLenachan, S.; Mackey, D.A.; Chen, F.K. Microperimetry and Adaptive Optics Imaging Reveal Localized Functional and Structural Changes in Asymptomatic RPGR Mutation Carriers. Investig. Ophthalmol. Vis. Sci. 2023, 64, 3. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, K.; Carroll, J.; Porco, T.C.; Duncan, J.L.; Roorda, A. Relationship between foveal cone structure and clinical measures of visual function in patients with inherited retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5836–5847. [Google Scholar] [CrossRef] [PubMed]
- Roshandel, D.; Thompson, J.A.; Heath Jeffery, R.C.; Sampson, D.M.; Chelva, E.; McLaren, T.L.; Lamey, T.M.; De Roach, J.N.; Durkin, S.R.; Chen, F.K. Multimodal Retinal Imaging and Microperimetry Reveal a Novel Phenotype and Potential Trial End Points in CRB1-Associated Retinopathies. Transl. Vis. Sci. Technol. 2021, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.W.; Johnson, R.D.; Langlo, C.S.; Cooper, R.F.; Razeen, M.M.; Russillo, M.C.; Dubra, A.; Connor, T.B., Jr.; Han, D.P.; Pennesi, M.E.; et al. Assessing Photoreceptor Structure in Retinitis Pigmentosa and Usher Syndrome. Investig. Ophthalmol. Vis. Sci. 2016, 57, 2428–2442. [Google Scholar] [CrossRef]
- Muthiah, M.N.; Kalitzeos, A.; Oprych, K.; Singh, N.; Georgiou, M.; Wright, G.A.; Robson, A.G.; Arno, G.; Khan, K.; Michaelides, M. Novel disease-causing variant in RDH12 presenting with autosomal dominant retinitis pigmentosa. Br. J. Ophthalmol. 2022, 106, 1274–1281. [Google Scholar] [CrossRef]
- Lindberg, C.R.; Fishman, G.A.; Anderson, R.J.; Vasquez, V. Contrast sensitivity in retinitis pigmentosa. Br. J. Ophthalmol. 1981, 65, 855–858. [Google Scholar] [CrossRef]
- Akeo, K.; Hiida, Y.; Saga, M.; Inoue, R.; Oguchi, Y. Correlation between contrast sensitivity and visual acuity in retinitis pigmentosa patients. Ophthalmologica 2002, 216, 185–191. [Google Scholar] [CrossRef]
- Choi, S.S.; Doble, N.; Hardy, J.L.; Jones, S.M.; Keltner, J.L.; Olivier, S.S.; Werner, J.S. In vivo imaging of the photoreceptor mosaic in retinal dystrophies and correlations with visual function. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2080–2092. [Google Scholar] [CrossRef]
- Duncan, J.L.; Zhang, Y.; Gandhi, J.; Nakanishi, C.; Othman, M.; Branham, K.E.; Swaroop, A.; Roorda, A. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3283–3291. [Google Scholar] [CrossRef]
- Song, H.; Rossi, E.A.; Stone, E.; Latchney, L.; Williams, D.; Dubra, A.; Chung, M. Phenotypic diversity in autosomal-dominant cone-rod dystrophy elucidated by adaptive optics retinal imaging. Br. J. Ophthalmol. 2018, 102, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Latchney, L.; Williams, D.; Chung, M. Fluorescence adaptive optics scanning laser ophthalmoscope for detection of reduced cones and hypoautofluorescent spots in fundus albipunctatus. JAMA Ophthalmol. 2014, 132, 1099–1104. [Google Scholar] [CrossRef] [PubMed]
- Kominami, A.; Ueno, S.; Kominami, T.; Nakanishi, A.; Ito, Y.; Fujinami, K.; Tsunoda, K.; Hayashi, T.; Kikuchi, S.; Kameya, S.; et al. Case of cone dystrophy with normal fundus appearance associated with biallelic POC1B variants. Ophthalmic Genet. 2018, 39, 255–262. [Google Scholar] [CrossRef] [PubMed]
- Roorda, A.; Zhang, Y.; Duncan, J.L. High-resolution in vivo imaging of the RPE mosaic in eyes with retinal disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2297–2303. [Google Scholar] [CrossRef] [PubMed]
- Kubota, D.; Gocho, K.; Kikuchi, S.; Akeo, K.; Miura, M.; Yamaki, K.; Takahashi, H.; Kameya, S. CEP250 mutations associated with mild cone-rod dystrophy and sensorineural hearing loss in a Japanese family. Ophthalmic Genet. 2018, 39, 500–507. [Google Scholar] [CrossRef]
- Ammar, M.J.; Scavelli, K.T.; Uyhazi, K.E.; Bedoukian, E.C.; Serrano, L.W.; Edelstein, I.D.; Vergilio, G.; Cooper, R.F.; Morgan, J.I.W.; Kumar, P.; et al. Enhanced S-Cone Syndrome: Visual Function, Cross-Sectional Imaging, and Cellular Structure with Adaptive Optics Ophthalmoscopy. Retin. Cases Brief Rep. 2021, 15, 694–701. [Google Scholar] [CrossRef]
- Park, S.P.; Hong, I.H.; Tsang, S.H.; Lee, W.; Horowitz, J.; Yzer, S.; Allikmets, R.; Chang, S. Disruption of the human cone photoreceptor mosaic from a defect in NR2E3 transcription factor function in young adults. Graefe’s Arch. Clin. Exp. Ophthalmol. 2013, 251, 2299–2309. [Google Scholar] [CrossRef]
- Moore, A.; Strauss, R.; Dubis, A.; Cooper, R.; Ba-Abbad, R.; Moore, A.; Webster, A.; Dubra, A.; Carroll, J.; Michaelides, M. Retinal Architecture in RGS9-and R9AP-Associated Retinal Dysfunction (Bradyopsia). Am. J. Ophthalmol. 2015, 160, 1269–1275. [Google Scholar]
- Song, H.; Rossi, E.A.; Latchney, L.; Bessette, A.; Stone, E.; Hunter, J.J.; Williams, D.R.; Chung, M. Cone and rod loss in Stargardt disease revealed by adaptive optics scanning light ophthalmoscopy. JAMA Ophthalmol. 2015, 133, 1198–1203. [Google Scholar] [CrossRef]
- Chen, Y.; Ratnam, K.; Sundquist, S.M.; Lujan, B.; Ayyagari, R.; Gudiseva, V.H.; Roorda, A.; Duncan, J.L. Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3281–3292. [Google Scholar] [CrossRef]
- Razeen, M.M.; Cooper, R.F.; Langlo, C.S.; Goldberg, M.R.; Wilk, M.A.; Han, D.P.; Connor, T.B.; Fishman, G.A.; Collison, F.T.; Sulai, Y.N. Correlating photoreceptor mosaic structure to clinical findings in Stargardt disease. Transl. Vis. Sci. Technol. 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Scoles, D.; Sulai, Y.N.; Cooper, R.F.; Higgins, B.P.; Johnson, R.D.; Carroll, J.; Dubra, A.; Stepien, K.E. Photoreceptor Inner Segment Morphology in Best Vitelliform Macular Dystrophy. Retina 2017, 37, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Aguilera, N.; Bower, A.J.; Li, J.; Ullah, E.; Dubra, A.; Cukras, C.; Brooks, B.P.; Jeffrey, B.G.; Hufnagel, R.B.; et al. Photoreceptor and Retinal Pigment Epithelium Relationships in Eyes With Vitelliform Macular Dystrophy Revealed by Multimodal Adaptive Optics Imaging. Investig. Ophthalmol. Vis. Sci. 2022, 63, 27. [Google Scholar] [CrossRef] [PubMed]
- Kay, D.B.; Land, M.E.; Cooper, R.F.; Dubis, A.M.; Godara, P.; Dubra, A.; Carroll, J.; Stepien, K.E. Outer retinal structure in best vitelliform macular dystrophy. JAMA Ophthalmol. 2013, 131, 1207–1215. [Google Scholar] [CrossRef]
- Ambrosio, L.; Williams, J.S.; Gutierrez, A.; Swanson, E.A.; Munro, R.J.; Ferguson, R.D.; Fulton, A.B.; Akula, J.D. Carbonic anhydrase inhibition in X-linked retinoschisis: An eye on the photoreceptors. Exp. Eye Res. 2021, 202, 108344. [Google Scholar] [CrossRef]
- Duncan, J.L.; Ratnam, K.; Birch, D.G.; Sundquist, S.M.; Lucero, A.S.; Zhang, Y.; Meltzer, M.; Smaoui, N.; Roorda, A. Abnormal cone structure in foveal schisis cavities in X-linked retinoschisis from mutations in exon 6 of the RS1 gene. Investig. Ophthalmol. Vis. Sci. 2011, 52, 9614–9623. [Google Scholar] [CrossRef]
- Akeo, K.; Kameya, S.; Gocho, K.; Kubota, D.; Yamaki, K.; Takahashi, H. Detailed Morphological Changes of Foveoschisis in Patient with X-Linked Retinoschisis Detected by SD-OCT and Adaptive Optics Fundus Camera. Case Rep. Ophthalmol. Med. 2015, 2015, 432782. [Google Scholar] [CrossRef]
- Morgan, J.I.; Han, G.; Klinman, E.; Maguire, W.M.; Chung, D.C.; Maguire, A.M.; Bennett, J. High-resolution adaptive optics retinal imaging of cellular structure in choroideremia. Investig. Ophthalmol. Vis. Sci. 2014, 55, 6381–6397. [Google Scholar] [CrossRef]
- Sun, L.W.; Johnson, R.D.; Williams, V.; Summerfelt, P.; Dubra, A.; Weinberg, D.V.; Stepien, K.E.; Fishman, G.A.; Carroll, J. Multimodal Imaging of Photoreceptor Structure in Choroideremia. PLoS ONE 2016, 11, e0167526. [Google Scholar] [CrossRef]
- Tuten, W.S.; Vergilio, G.K.; Young, G.J.; Bennett, J.; Maguire, A.M.; Aleman, T.S.; Brainard, D.H.; Morgan, J.I.W. Visual Function at the Atrophic Border in Choroideremia Assessed with Adaptive Optics Microperimetry. Ophthalmol. Retin. 2019, 3, 888–899. [Google Scholar] [CrossRef]
- Aguilera, N.; Liu, T.; Bower, A.J.; Li, J.; Abouassali, S.; Lu, R.; Giannini, J.; Pfau, M.; Bender, C.; Smelkinson, M.G.; et al. Widespread subclinical cellular changes revealed across a neural-epithelial-vascular complex in choroideremia using adaptive optics. Commun. Biol. 2022, 5, 893. [Google Scholar] [CrossRef] [PubMed]
- Foote, K.G.; Rinella, N.; Tang, J.; Bensaid, N.; Zhou, H.; Zhang, Q.; Wang, R.K.; Porco, T.C.; Roorda, A.; Duncan, J.L. Cone Structure Persists Beyond Margins of Short-Wavelength Autofluorescence in Choroideremia. Investig. Ophthalmol. Vis. Sci. 2019, 60, 4931–4942. [Google Scholar] [CrossRef] [PubMed]
- Morgan, J.I.W.; Jiang, Y.Y.; Vergilio, G.K.; Serrano, L.W.; Pearson, D.J.; Bennett, J.; Maguire, A.M.; Aleman, T.S. Short-term Assessment of Subfoveal Injection of Adeno-Associated Virus-Mediated hCHM Gene Augmentation in Choroideremia Using Adaptive Optics Ophthalmoscopy. JAMA Ophthalmol. 2022, 140, 411–420. [Google Scholar] [CrossRef] [PubMed]
- Bower, A.J.; Liu, T.; Aguilera, N.; Li, J.; Liu, J.; Lu, R.; Giannini, J.P.; Huryn, L.A.; Dubra, A.; Liu, Z.; et al. Integrating adaptive optics-SLO and OCT for multimodal visualization of the human retinal pigment epithelial mosaic. Biomed. Opt. Express 2021, 12, 1449–1466. [Google Scholar] [CrossRef]
- Morgan, J.I.W.; Chen, M.; Huang, A.M.; Jiang, Y.Y.; Cooper, R.F. Cone Identification in Choroideremia: Repeatability, Reliability, and Automation Through Use of a Convolutional Neural Network. Transl. Vis. Sci. Technol. 2020, 9, 40. [Google Scholar] [CrossRef]
- Sampson, D.M.; Roshandel, D.; Chew, A.L.; Wang, Y.; Stevenson, P.G.; Cooper, M.N.; Ong, E.; Wong, L.; La, J.; Alonso-Caneiro, D.; et al. Retinal Differential Light Sensitivity Variation Across the Macula in Healthy Subjects: Importance of Cone Separation and Loci Eccentricity. Transl. Vis. Sci. Technol. 2021, 10, 16. [Google Scholar] [CrossRef]
- Ueno, S.; Koyanagi, Y.; Kominami, T.; Ito, Y.; Kawano, K.; Nishiguchi, K.M.; Rivolta, C.; Nakazawa, T.; Sonoda, K.-H.; Terasaki, H. Clinical characteristics and high resolution retinal imaging of retinitis pigmentosa caused by RP1 gene variants. Jpn. J. Ophthalmol. 2020, 64, 485–496. [Google Scholar] [CrossRef]
- Kalitzeos, A.; Kumaran, N.; Georgiou, M.; Singh, N.; Kane, T.; Kasilian, M.; Dubra, A.; Carroll, J.; Michaelides, M. Natural history of foveal cone structure in RPE65-associated Leber congenital amaurosis (LCA). Investig. Ophthalmol. Vis. Sci. 2019, 60, 4584. [Google Scholar]
- Huang, Z.; Zhang, D.; Thompson, J.A.; Jamuar, S.S.; Roshandel, D.; Jennings, L.; Mellough, C.; Charng, J.; Chen, S.-C.; McLaren, T.L. Deep clinical phenotyping and gene expression analysis in a patient with RCBTB1-associated retinopathy. Ophthalmic Genet. 2021, 42, 266–275. [Google Scholar] [CrossRef]
- Nakamura, T.; Ueda-Consolvo, T.; Oiwake, T.; Hayashi, A. Correlation between outer retinal layer thickness and cone density in patients with resolved central serous chorioretinopathy. Graefes Arch Clin. Exp. Ophthalmol. 2016, 254, 2347–2354. [Google Scholar] [CrossRef]
- Kabataş, N.; Doğan, A.Ş.; Kabataş, E.U.; Acar, M.; Biçer, T.; Gürdal, C. The effect of Demodex infestation on blepharitis and the ocular symptoms. Eye Contact Lens Sci. Clin. Pract. 2017, 43, 64–67. [Google Scholar] [CrossRef] [PubMed]
- Chew, A.L.; Sampson, D.M.; Kashani, I.; Chen, F.K. Agreement in Cone Density Derived from Gaze-Directed Single Images Versus Wide-Field Montage Using Adaptive Optics Flood Illumination Ophthalmoscopy. Transl. Vis. Sci. Technol. 2017, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Lazareva, A.; Asad, M.; Slabaugh, G. Learning to deblur adaptive optics retinal images. In Proceedings of the International Conference Image Analysis and Recognition, Montreal, QC, Canada, 5–7 July 2017; pp. 497–506. [Google Scholar]
- Sampson, D.M.; Alonso-Caneiro, D.; Chew, A.L.; La, J.; Roshandel, D.; Wang, Y.; Khan, J.C.; Chelva, E.; Stevenson, P.G.; Chen, F.K. Evaluation of focus and deep learning methods for automated image grading and factors influencing image quality in adaptive optics ophthalmoscopy. Sci. Rep. 2021, 11, 16641. [Google Scholar] [CrossRef] [PubMed]
- Merino, D.; Duncan, J.L.; Tiruveedhula, P.; Roorda, A. Observation of cone and rod photoreceptors in normal subjects and patients using a new generation adaptive optics scanning laser ophthalmoscope. Biomed. Opt. Express 2011, 2, 2189–2201. [Google Scholar] [CrossRef]
- Feng, S.; Gale, M.J.; Fay, J.D.; Faridi, A.; Titus, H.E.; Garg, A.K.; Michaels, K.V.; Erker, L.R.; Peters, D.; Smith, T.B.; et al. Assessment of Different Sampling Methods for Measuring and Representing Macular Cone Density Using Flood-Illuminated Adaptive Optics. Investig. Ophthalmol. Vis. Sci. 2015, 56, 5751–5763. [Google Scholar] [CrossRef] [PubMed]
- Debellemaniere, G.; Flores, M.; Tumahai, P.; Meillat, M.; Bidaut Garnier, M.; Delbosc, B.; Saleh, M. Assessment of parafoveal cone density in patients taking hydroxychloroquine in the absence of clinically documented retinal toxicity. Acta Ophthalmol. 2015, 93, e534–e540. [Google Scholar] [CrossRef]
- Roshandel, D.; Sampson, D.M.; Mackey, D.A.; Chen, F.K. Impact of Reference Center Choice on Adaptive Optics Imaging Cone Mosaic Analysis. Investig. Ophthalmol. Vis. Sci. 2022, 63, 12. [Google Scholar] [CrossRef]
- Ferguson, R.D.; Zhong, Z.; Hammer, D.X.; Mujat, M.; Patel, A.H.; Deng, C.; Zou, W.; Burns, S.A. Adaptive optics scanning laser ophthalmoscope with integrated wide-field retinal imaging and tracking. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 2010, 27, A265–A277. [Google Scholar] [CrossRef]
- Yang, Q.; Zhang, J.; Nozato, K.; Saito, K.; Williams, D.R.; Roorda, A.; Rossi, E.A. Closed-loop optical stabilization and digital image registration in adaptive optics scanning light ophthalmoscopy. Biomed. Opt. Express 2014, 5, 3174–3191. [Google Scholar] [CrossRef]
- Sheehy, C.K.; Tiruveedhula, P.; Sabesan, R.; Roorda, A. Active eye-tracking for an adaptive optics scanning laser ophthalmoscope. Biomed. Opt. Express 2015, 6, 2412–2423. [Google Scholar] [CrossRef]
- Yeo, B.; Sabuncu, M.; Vercauteren, T.; Ayache, N.; Fischl, B.; Golland, P. Spherical demons: Fast surface registration. In Proceedings of the International Conference on Medical Image Computing and Computer-Assisted Intervention, New York, NY, USA, 6–10 September 2008; pp. 745–753. [Google Scholar]
- Lee, S.; Lebed, E.; Sarunic, M.V.; Beg, M.F. Exact surface registration of retinal surfaces from 3-D optical coherence tomography images. IEEE Trans. Biomed. Eng. 2014, 62, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Foote, K.G.; De la Huerta, I.; Gustafson, K.; Baldwin, A.; Zayit-Soudry, S.; Rinella, N.; Porco, T.C.; Roorda, A.; Duncan, J.L. Cone spacing correlates with retinal thickness and microperimetry in patients with inherited retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1234–1243. [Google Scholar] [CrossRef]
- Viana, K.Í.; Messias, A.; Siqueira, R.C.; Rodrigues, M.W.; Jorge, R. Structure-functional correlation using adaptive optics, OCT, and microperimetry in a case of occult macular dystrophy. Arq. Bras. De Oftalmol. 2017, 80, 118–121. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, S.G.; Cideciyan, A.V.; Huang, W.C.; Sumaroka, A.; Roman, A.J.; Schwartz, S.B.; Luo, X.; Sheplock, R.; Dauber, J.M.; Swider, M. TULP1 mutations causing early-onset retinal degeneration: Preserved but insensitive macular cones. Investig. Ophthalmol. Vis. Sci. 2014, 55, 5354–5364. [Google Scholar] [CrossRef] [PubMed]
- Makiyama, Y.; Ooto, S.; Hangai, M.; Ogino, K.; Gotoh, N.; Oishi, A.; Yoshimura, N. Cone abnormalities in fundus albipunctatus associated with RDH5 mutations assessed using adaptive optics scanning laser ophthalmoscopy. Am. J. Ophthalmol. 2014, 157, e551–e554. [Google Scholar] [CrossRef] [PubMed]
- Garway-Heath, D.F.; Caprioli, J.; Fitzke, F.W.; Hitchings, R.A. Scaling the hill of vision: The physiological relationship between light sensitivity and ganglion cell numbers. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1774–1782. [Google Scholar]
- Chew, A.L.; Sampson, D.M.; Chelva, E.; Khan, J.C.; Chen, F.K. Perifoveal interdigitation zone loss in hydroxychloroquine toxicity leads to subclinical bull’s eye lesion appearance on near-infrared reflectance imaging. Doc. Ophthalmol. 2018, 136, 57–68. [Google Scholar] [CrossRef]
- Datlinger, F.; Wassermann, L.; Reumueller, A.; Hajdu, D.; Steiner, I.; Salas, M.; Drexler, W.; Pircher, M.; Schmidt-Erfurth, U.; Pollreisz, A. Assessment of Detailed Photoreceptor Structure and Retinal Sensitivity in Diabetic Macular Ischemia Using Adaptive Optics-OCT and Microperimetry. Investig. Ophthalmol. Vis. Sci. 2021, 62, 1. [Google Scholar] [CrossRef]
- Foote, K.G.; Wong, J.J.; Boehm, A.E.; Bensinger, E.; Porco, T.C.; Roorda, A.; Duncan, J.L. Comparing Cone Structure and Function in RHO- and RPGR-Associated Retinitis Pigmentosa. Investig. Ophthalmol. Vis. Sci. 2020, 61, 42. [Google Scholar] [CrossRef]
- Wang, Q.; Tuten, W.S.; Lujan, B.J.; Holland, J.; Bernstein, P.S.; Schwartz, S.D.; Duncan, J.L.; Roorda, A. Adaptive optics microperimetry and OCT images show preserved function and recovery of cone visibility in macular telangiectasia type 2 retinal lesions. Investig. Ophthalmol. Vis. Sci. 2015, 56, 778–786. [Google Scholar] [CrossRef]
- Mariotti, L.; Devaney, N.; Lombardo, G.; Lombardo, M. Understanding the changes of cone reflectance in adaptive optics flood illumination retinal images over three years. Biomed. Opt. Express 2016, 7, 2807–2822. [Google Scholar] [CrossRef] [PubMed]
- Bensinger, E.; Wang, Y.; Roorda, A. Patches of Dysflective Cones in Eyes With No Known Disease. Investig. Ophthalmol. Vis. Sci. 2022, 63, 29. [Google Scholar] [CrossRef] [PubMed]
- Rha, J.; Schroeder, B.; Godara, P.; Carroll, J. Variable optical activation of human cone photoreceptors visualized using a short coherence light source. Opt. Lett. 2009, 34, 3782–3784. [Google Scholar] [CrossRef] [PubMed]
- Jonnal, R.S.; Besecker, J.R.; Derby, J.C.; Kocaoglu, O.P.; Cense, B.; Gao, W.; Wang, Q.; Miller, D.T. Imaging outer segment renewal in living human cone photoreceptors. Opt. Express 2010, 18, 5257–5270. [Google Scholar] [CrossRef] [PubMed]



| FIO | SLO | |
|---|---|---|
| Availability | Commercial | Research custom-made |
| Field | Large (4°) | Small (0.2–1.0°) |
| Illumination | Diffuse | Focused single spot |
| Imaging technique | Single flash | Scanning laser |
| Transverse resolution | 2–3 µm | 1–2 µm |
| Axial resolution | 200–300 µm | 100 µm |
| Motion artefacts | Low | high |
| Visible structures | ||
| Cones | Yes a | Yes |
| Rods | No | Yes |
| RPE | Yes b | Yes |
| RGC | No c | Yes |
| Retinal vessels | Yes | Yes |
| Choriocapillaris | No c | Yes |
| IRD Type | AO Technology | Findings of AO Imaging |
|---|---|---|
| Retinitis pigmentosa | AOSLO, AOFIO | Significant cone loss in the central retina despite intact ONL, EZ, and normal FAF, irregular cone mosaic, lower cone density, decreases the percent of hexagonality, hypo-reflective blurred cones in eccentricities, increased cone spacing, visible RPE, and no detectable cone mosaic in areas of complete INL loss |
| RPGR Female carrier | AOFIO | Irregular cone mosaic and decreased cone density in asymptomatic patients with normal visual acuity |
| CRB1 | AOFIO | Decreased cone density despite normal visual acuity and OCT |
| RDH12 | AOSLO | Reduced cone density along the temporal meridian |
| GUCA1A | AOSLO | Truncated cone in outer segments, reduced cone reflectance (dark cones) |
| POC1B | AOFIO | Disruption of photoreceptor in outer segments with preservation of the inner segments blurred EZ and significant cone mosaic disruption around the fovea |
| NR2E3 (Enhanced S-cone syndrome) | AOSLO | Higher cone density at temporal parafovea with lower total photoreceptor densities, diminished outer segment wave-guide signals, disrupted cone arrangement, larger cone cells with dark patchy-like lesions in the macula |
| RGS9/R9AP (bradyopsia) | AOSLO | Reserved integrity of cone mosaic and cone density within the normal range. A small area of central hypo-reflective lesion of non-waveguiding cones with minimal decrease in peak cone density reported in a patient |
| ABCA4 (Stargardt disease) | AOSLO | Disrupted cone mosaic, decreased cone density from central to peripheral retina, increased cone spacing in regions with normal OCT and FAF, starry-night cone pattern and increased cone spacing in areas of increased FAF, lack of cone mosaic with visible RPE cells in the atrophic zone, significant cone loss and abnormally enlarged rod photoreceptors despite intact EZ, no correlation between the quantity of lipofuscin accumulation in RPE cells and reflectivity shown in AOSLO |
| VMD | AOSLO | Reduced cone and RPE density and enlarged inner segments within the vitelliform lesion, reactive subretinal cells shown as mobile disk-like structures, patchy areas of significant photoreceptor disruption surrounded by contiguous photoreceptor mosaic |
| RS1 (XLRS) | AOSLO, AO-OCT | Spoke-wheel appearance in offset aperture imaging, increased cone diameter, decreased cone density, severe abnormalities at the OPL in AO-OCT of clinically normal regions, no detectable cone mosaic in areas of EZ and IZ loss |
| CHM (Choroideremia) | AOSLO, AOICG, AO-OCT | Intact cone mosaic up to the border of retinal atrophy, ill-defined cone edges, groups of cones with high reflectance relative to the surrounding cone mosaic, bubble-like hyper-reflective spots with dark edges, large remnants of cone inner segments within outer retinal tubulations, widespread large RPEs, choriocapillaris flow voids in areas of increased cone spacing, disruption of RPE blood barrier in AOICG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ashourizadeh, H.; Fakhri, M.; Hassanpour, K.; Masoudi, A.; Jalali, S.; Roshandel, D.; Chen, F.K. Pearls and Pitfalls of Adaptive Optics Ophthalmoscopy in Inherited Retinal Diseases. Diagnostics 2023, 13, 2413. https://doi.org/10.3390/diagnostics13142413
Ashourizadeh H, Fakhri M, Hassanpour K, Masoudi A, Jalali S, Roshandel D, Chen FK. Pearls and Pitfalls of Adaptive Optics Ophthalmoscopy in Inherited Retinal Diseases. Diagnostics. 2023; 13(14):2413. https://doi.org/10.3390/diagnostics13142413
Chicago/Turabian StyleAshourizadeh, Helia, Maryam Fakhri, Kiana Hassanpour, Ali Masoudi, Sattar Jalali, Danial Roshandel, and Fred K. Chen. 2023. "Pearls and Pitfalls of Adaptive Optics Ophthalmoscopy in Inherited Retinal Diseases" Diagnostics 13, no. 14: 2413. https://doi.org/10.3390/diagnostics13142413
APA StyleAshourizadeh, H., Fakhri, M., Hassanpour, K., Masoudi, A., Jalali, S., Roshandel, D., & Chen, F. K. (2023). Pearls and Pitfalls of Adaptive Optics Ophthalmoscopy in Inherited Retinal Diseases. Diagnostics, 13(14), 2413. https://doi.org/10.3390/diagnostics13142413







