Intracranial Hemorrhage in Hospitalized Patients Following Percutaneous Coronary Intervention: A Large Cohort Analysis from a Single Center
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Definitions of Diseases
2.3. Clinical Outcomes
2.4. Statistical Analysis
3. Results
3.1. General Characteristics
3.2. Imaging Characteristics
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Capodanno, D.; Bhatt, D.L.; Eikelboom, J.W.; Fox, K.A.A.; Geisler, T.; Michael Gibson, C.; Gonzalez-Juanatey, J.R.; James, S.; Lopes, R.D.; Mehran, R.; et al. Dual-pathway inhibition for secondary and tertiary antithrombotic prevention in cardiovascular disease. Nat. Rev. Cardiol. 2020, 17, 242–257. [Google Scholar] [CrossRef]
- Valgimigli, M.; Costa, F.; Lokhnygina, Y.; Clare, R.M.; Wallentin, L.; Moliterno, D.J.; Armstrong, P.W.; White, H.D.; Held, C.; Aylward, P.E.; et al. Trade-off of myocardial infarction vs. bleeding types on mortality after acute coronary syndrome: Lessons from the Thrombin Receptor Antagonist for Clinical Event Reduction in Acute Coronary Syndrome (TRACER) randomized trial. Eur. Heart J. 2017, 38, 804–810. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Hager, R.; Wojdyla, D.; White, H.D.; Armstrong, P.W.; Alexander, J.H.; Tricoci, P.; Lopes, R.D.; Ohman, E.M.; Roe, M.T.; et al. Meta-analysis of intracranial hemorrhage in acute coronary syndromes: Incidence, predictors, and clinical outcomes. J. Am. Heart Assoc. 2015, 4, e001512. [Google Scholar] [CrossRef]
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation 2020, 141, e139–e596. [Google Scholar] [CrossRef]
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics-2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef]
- Yusuf, S.; Zhao, F.; Mehta, S.R.; Chrolavicius, S.; Tognoni, G.; Fox, K.K. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N. Engl. J. Med. 2001, 345, 494–502. [Google Scholar]
- Wiviott, S.D.; Braunwald, E.; McCabe, C.H.; Montalescot, G.; Ruzyllo, W.; Gottlieb, S.; Neumann, F.-J.; Ardissino, D.; De Servi, S.; Murphy, S.A.; et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2007, 357, 2001–2015. [Google Scholar] [CrossRef]
- Wallentin, L.; Becker, R.C.; Budaj, A.; Cannon, C.P.; Emanuelsson, H.; Held, C.; Horrow, J.; Husted, S.; James, S.; Katus, H.; et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N. Engl. J. Med. 2009, 361, 1045–1057. [Google Scholar] [CrossRef]
- Werner, N.; Bauer, T.; Hochadel, M.; Zahn, R.; Weidinger, F.; Marco, J.; Hamm, C.; Gitt, A.K.; Zeymer, U. Incidence and clinical impact of stroke complicating percutaneous coronary intervention: Results of the Euro heart survey percutaneous coronary interventions registry. Circ. Cardiovasc. Interv. 2013, 6, 362–369. [Google Scholar] [CrossRef]
- Lee, P.H.; Park, S.; Nam, H.; Kang, D.Y.; Kang, S.J.; Lee, S.W.; Kim, Y.H.; Park, S.W.; Lee, C.W. Intracranial Bleeding After Percutaneous Coronary Intervention: Time-Dependent Incidence, Predictors, and Impact on Mortality. J. Am. Heart Assoc. 2021, 10, e019637. [Google Scholar] [CrossRef]
- Van Asch, C.J.; Luitse, M.J.; Rinkel, G.J.; van der Tweel, I.; Algra, A.; Klijn, C.J. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: A systematic review and meta-analysis. Lancet Neurol. 2010, 9, 167–176. [Google Scholar] [CrossRef]
- Cordonnier, C.; Demchuk, A.; Ziai, W.; Anderson, C.S. Intracerebral haemorrhage: Current approaches to acute management. Lancet 2018, 392, 1257–1268. [Google Scholar] [CrossRef] [PubMed]
- Hemphill, J.C., 3rd; Bonovich, D.C.; Besmertis, L.; Manley, G.T.; Johnston, S.C. The ICH score: A simple, reliable grading scale for intracerebral hemorrhage. Stroke 2001, 32, 891–897. [Google Scholar] [CrossRef]
- Raposeiras-Roubín, S.; Abu-Assi, E.; Caneiro Queija, B.; Cobas Paz, R.; D’Ascenzo, F.; Henriques, J.P.S.; Saucedo, J.; González-Juanatey, J.; Wilton, S.B.; Kikkert, W.J.; et al. Incidence, predictors and prognostic impact of intracranial bleeding within the first year after an acute coronary syndrome in patients treated with percutaneous coronary intervention. Eur. Heart J. Acute Cardiovasc. Care 2020, 9, 764–770. [Google Scholar] [CrossRef]
- Broderick, J.P.; Brott, T.G.; Duldner, J.E.; Tomsick, T.; Huster, G. Volume of intracerebral hemorrhage. A powerful and easy-to-use predictor of 30-day mortality. Stroke 1993, 24, 987–993. [Google Scholar] [CrossRef]
- Myint, P.K.; Kwok, C.S.; Roffe, C.; Kontopantelis, E.; Zaman, A.; Berry, C.; Ludman, P.F.; de Belder, M.A.; Mamas, M.A.; British Cardiovascular Intervention Society and the National Institute for Cardiovascular Outcomes Research. Determinants and Outcomes of Stroke Following Percutaneous Coronary Intervention by Indication. Stroke 2016, 47, 1500–1507. [Google Scholar] [CrossRef]
- Guptill, J.T.; Mehta, R.H.; Armstrong, P.W.; Horton, J.; Laskowitz, D.; James, S.; Granger, C.B.; Lopes, R.D. Stroke after primary percutaneous coronary intervention in patients with ST-segment elevation myocardial infarction: Timing, characteristics, and clinical outcomes. Circ. Cardiovasc. Interv. 2013, 6, 176–183. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, M.J.; Xavier, D.; Liu, L.; Zhang, H.; Chin, S.L.; Rao-Melacini, P.; Rangarajan, S.; Islam, S.; Pais, P.; McQueen, M.J.; et al. Risk factors for ischaemic and intracerebral haemorrhagic stroke in 22 countries (the INTERSTROKE study): A case-control study. Lancet 2010, 376, 112–123. [Google Scholar] [CrossRef]
- Greenberg, S.M.; Ziai, W.C.; Cordonnier, C.; Dowlatshahi, D.; Francis, B.; Goldstein, J.N.; Hemphill, J.C., 3rd; Johnson, R.; Keigher, K.M.; Mack, W.J.; et al. 2022 Guideline for the Management of Patients with Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke 2022, 53, e282–e361. [Google Scholar] [CrossRef]
- Jolink, W.M.T.; Klijn, C.J.M.; Brouwers, P.J.A.M.; Kappelle, L.J.; Vaartjes, I. Time trends in incidence, case fatality, and mortality of intracerebral hemorrhage. Neurology 2015, 85, 1318–1324. [Google Scholar] [CrossRef]
- Zahuranec, D.B.; Lisabeth, L.D.; Sánchez, B.N.; Smith, M.A.; Brown, D.L.; Garcia, N.M.; Skolarus, L.E.; Meurer, W.J.; Burke, J.F.; Adelman, E.E.; et al. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology 2014, 82, 2180–2186. [Google Scholar] [CrossRef]
- Kwok, C.S.; Kontopantelis, E.; Myint, P.K.; Zaman, A.; Berry, C.; Keavney, B.; Nolan, J.; Ludman, P.F.; de Belder, M.A.; Buchan, I.; et al. Stroke following percutaneous coronary intervention: Type-specific incidence, outcomes and determinants seen by the British Cardiovascular Intervention Society 2007–2012. Eur. Heart J. 2015, 36, 1618–1628. [Google Scholar] [CrossRef]
- Law, Z.K.; Desborough, M.; Roberts, I.; Al-Shahi Salman, R.; England, T.J.; Werring, D.J.; Robinson, T.; Krishnan, K.; Dineen, R.; Laska, A.C.; et al. Outcomes in Antiplatelet-Associated Intracerebral Hemorrhage in the TICH-2 Randomized Controlled Trial. J. Am. Heart Assoc. 2021, 10, e019130. [Google Scholar] [CrossRef]
- Davis, S.M.; Broderick, J.; Hennerici, M.; Brun, N.C.; Diringer, M.N.; Mayer, S.A.; Begtrup, K.; Steiner, T.; Recombinant Activated Factor VII Intracerebral Hemorrhage Trial Investigators. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology 2006, 66, 1175–1181. [Google Scholar] [CrossRef]
- Kazui, S.; Naritomi, H.; Yamamoto, H.; Sawada, T.; Yamaguchi, T. Enlargement of spontaneous intracerebral hemorrhage. Incidence and time course. Stroke 1996, 27, 1783–1787. [Google Scholar] [CrossRef]
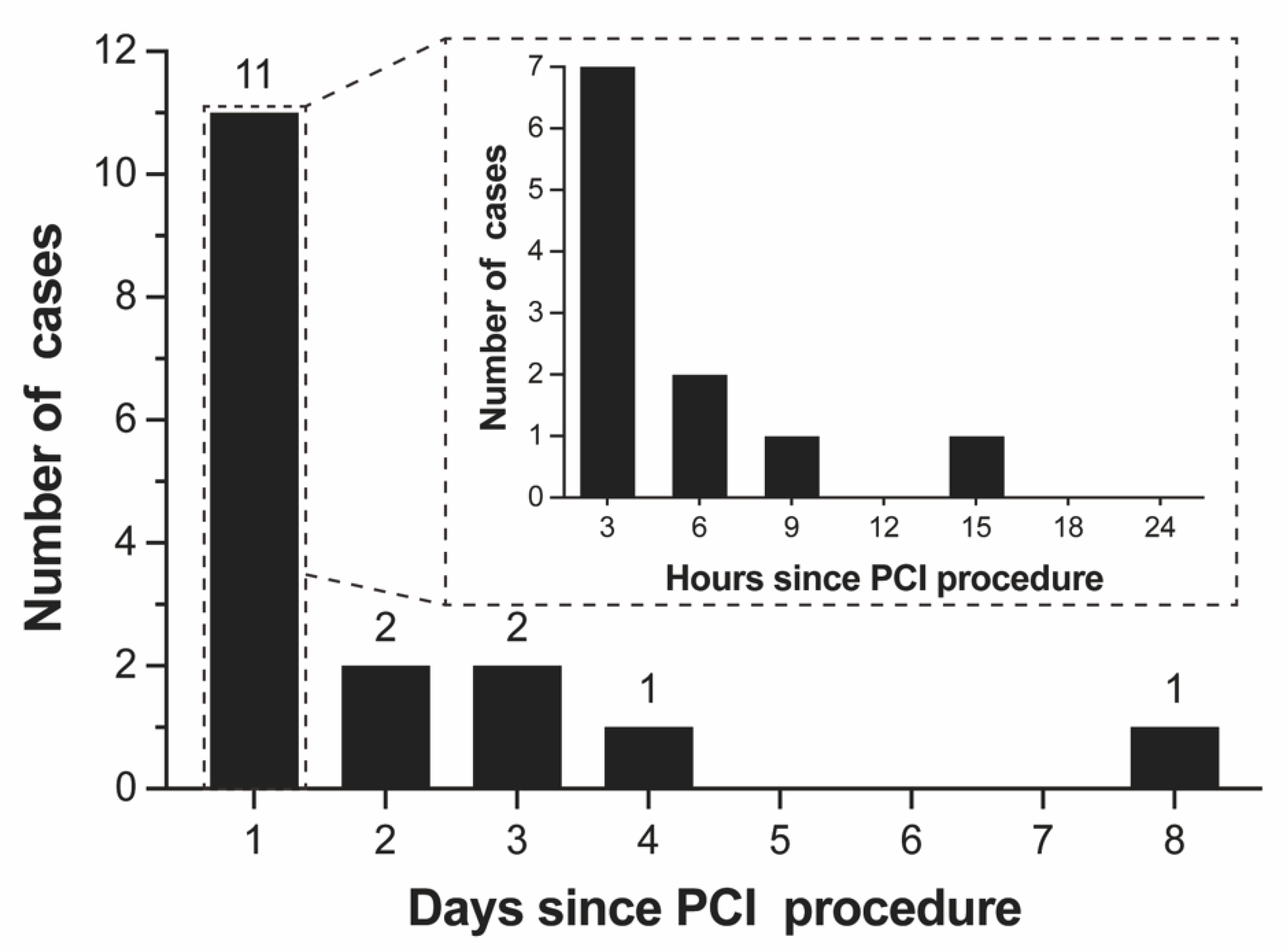
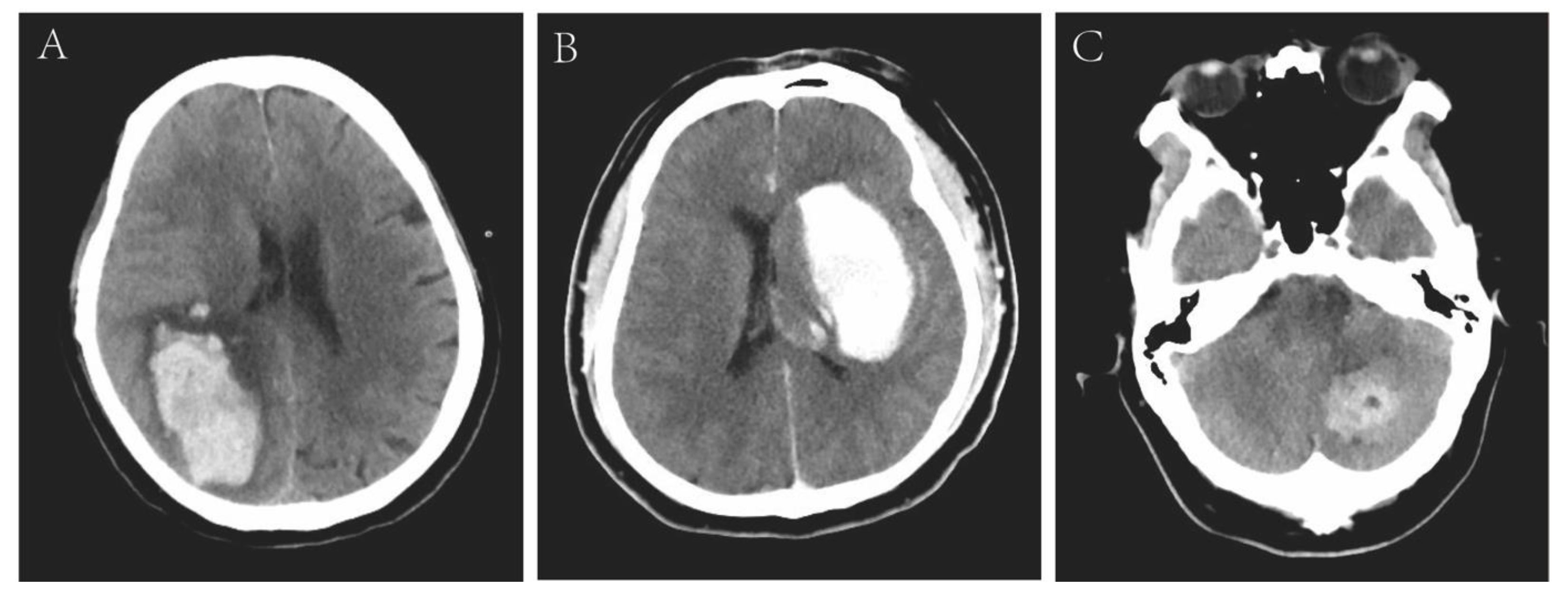
| Variables | Overall (n = 18) | Death Group (n = 13) | Survival Group (n = 5) | p Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 64.56 ± 11.82 | 64.15 ± 11.68 | 65.60 ± 12.60 | 0.976 |
| Male | 13 (72.2) | 9 ((69.2) | 4 (80.0) | 1 |
| BMI, kg/m2 | 25.83 ± 4.39 | 25.95 ± 5.09 | 25.54 ± 2.04 | 1 |
| Medical history | ||||
| Current smoking | 6 (33.3) | 4 (30.8) | 2 (40.0) | 1 |
| Hypertension | 12 (66.7) | 9 (69.2) | 3 (60.0) | 1 |
| Diabetes mellitus | 4 (22.2) | 3 (23.1) | 1 (20.0) | 1 |
| Dyslipidemia | 16 (88.9) | 11 (84.6) | 5 (100) | 1 |
| Anemia | 3 (16.7) | 3 (23.1) | 0 (0) | 0.522 |
| Renal insufficiency | 2 (11.1) | 1 (7.7) | 1 (20.0) | 0.490 |
| Peripheral vascular disease | 3 (16.7) | 3 (23.1) | 0 (0) | 0.522 |
| Prior ischemia stroke/TIA | 2 (11.1) | 2 (15.4) | 0 (0) | 1 |
| Prior MI | 2 (11.1) | 2 (15.4) | 0 (0) | 1 |
| Prior PCI | 5 (27.8) | 4 (30.8) | 1 (20.0) | 1 |
| Diagnosis | 0.636 | |||
| Stable angina | 5 (27.8) | 4 (30.8) | 1 (20.0) | |
| Unstable angina | 9 (50.0) | 7 (53.8) | 2 (40.0) | |
| MI | 4 (22.2) | 2 (15.4) | 2 (40.0) | |
| Procedural characteristics | ||||
| Duration of procedure, mins | 81.33 ± 40.40 | |||
| Selective | 14 (77.8) | 11 (84.6) | 3 (60.0) | 0.533 |
| Emergency | 4 (22.2) | 2 (15.4) | 2 (40.0) | 0.533 |
| Stent implantation | 15 (83.3) | 12 (92.3) | 3 (60.0) | 0.172 |
| Antithrombotic therapy | ||||
| Pre-procedure | 1 | |||
| Aspirin plus clopidogrel | 13 (72.2) | 9 (69.2) | 4 (80.0) | |
| Aspirin plus ticagrelor | 5 (27.8) | 4 (30.8) | 1 (20.0) | |
| During procedure | 1 | |||
| Unfractionated heparin | 16 (88.9) | 11 (84.6) | 5 (100) | |
| Bivalirudin | 2 (11.1) | 2 (15.4) | 0 (0) | |
| Post-procedure | 0.132 | |||
| Tirofiban | 1 (5.6) | 0 (0) | 1 (20.0) | |
| LMWH | 10 (55.6) | 6 (46.2) | 4 (80.0) | |
| Fondaparinux | 2 (11.1) | 2 (15.4) | 0 (0) | |
| Duration of hospitalization, days | 4.74 ± 2.71 | 5.08 ± 3.28 | 4.80 ± 0.84 | 0.782 |
| Variables | Overall (n = 18) | Death Group (n = 13) | Survival Group (n = 5) | p Value |
|---|---|---|---|---|
| Clinical characteristic | ||||
| Initial symptoms | ||||
| Focal neurological signs | 5 (27.8) | 4 (30.8) | 1 (20.0) | 1 |
| Disturbance of consciousness | 8 (44.4) | 8 (61.5) | 0 (0) | 0.036 |
| Time to symptoms after procedure | ||||
| Median time, hours | 7 (2–54) | 3 (1.5–36) | 48 (75–84) | 0.099 |
| Within 12 h | 10 (55.6) | 9 (69.2) | 1 (20.0) | 0.118 |
| More than 12 h | 8 (44.4) | 4 (30.8) | 4 (80.0) | 0.118 |
| Neuroradiologic data | ||||
| Location 1 | 0.109 | |||
| Lobar | 12 (66.7) | 10 (76.9) | 2 (40.0) | |
| Cerebellar | 4 (22.2) | 1 (7.7) | 3 (60.0) | |
| Deep (basal ganglia and thalamus) | 2 (11.1) | 2 (15.4) | 0 (0) | |
| Location 2 | 0.044 | |||
| Infratentorial | 4 (22.2) | 1 (7.7) | 3 (60.0) | |
| Supratentorial | 14 (77.8) | 12 (92.3) | 2 (40.0) | |
| Combined with SAH | 4 (22.2) | 4 (30.8) | 0 (0) | 0.278 |
| ICH volume, cm3 | 65.83 ± 53.34 | 84.17 ± 50.74 | 18.14 ± 20.85 | 0.001 |
| Small (<30 cm3) | 5 (27.8) | 1 (7.7) | 4 (80.0) | 0.008 |
| Large (≥30 cm3) | 13 (72.2) | 12 (92.3) | 1 (20.0) | 0.008 |
| Brain herniation | 4 (22.2) | 4 (30.8) | 0 (0) | 0.278 |
| Intraventricular hemorrhage | 2 (11.1) | 2 (15.4) | 0 (0) | 1 |
| Midline shift ≥ 10 mm | 6 (33.3) | 6 (46.2) | 0 (0) | 0.114 |
| Treatment | ||||
| Conservative medicine | 17 (94.4) | 13 (100) | 4 (0) | 0.278 |
| Minimally invasive surgery | 1 (5.6) | 0 (0) | 1 (20.0) | 0.278 |
| No. | Age | Gender | Dual Antiplatelet Therapy, Aspirin Plus | During Procedural Anti-Coagulants | Time since PCI, Hours | Onset Symptoms | CT Manifestations | 90-Day Clinical Outcomes | |
|---|---|---|---|---|---|---|---|---|---|
| Bleeding Site | Volume, cm3 | ||||||||
| 1 | 71 | F | clopidogrel | UFH | 192 | DC, FNS | FL | 61 | Died |
| 2 | 75 | F | ticagrelor | UFH | 3 | HA, V | FL, PL | 42 | Died |
| 3 | 52 | M | clopidogrel | UFH | 2 | FNS | BG, TH, EC, IVH | 58 | Died |
| 4 | 60 | M | ticagrelor | UFH | 1 | V, FNS | TL, BG, | 38 | Died |
| 5 | 64 | M | clopidogrel | UFH | 1 | HA, DC | PL, OL, SS | 120 | Died |
| 6 | 44 | M | ticagrelor | UFH | 48 | FNS, V | FL | 113 | Died |
| 7 | 82 | F | clopidogrel | bivalirudin | 2 | HA, DC | TH, BG, CH | 91 | Died |
| 8 | 68 | M | clopidogrel | UFH | 72 | HA, V | CE | 10 | Survived |
| 9 | 73 | M | ticagrelor | UFH | 72 | HA, V | FL, PL, OL | 54 | Survived |
| 10 | 67 | F | clopidogrel | UFH | 3 | HA, V | CE | 11 | Survived |
| 11 | 65 | M | clopidogrel | UFH | 96 | FNS | FL, PL | 2 | Survived |
| 12 | 45 | M | clopidogrel | UFH | 48 | HA | CE | 12 | Died |
| 13 | 65 | F | clopidogrel | UFH | 24 | DC | FL, SS | 70 | Died |
| 14 | 44 | M | ticagrelor | UFH | 2 | DC | FL, TL, SS, CH | 92 | Died |
| 15 | 78 | M | clopidogrel | UFH | 12 | V | CE, CH | 9 | Survived |
| 16 | 67 | M | ticagrelor | UFH | 8 | DC | PL, TL, OL | 94 | Died |
| 17 | 77 | M | clopidogrel | bivalirudin | 6 | DC | FL, SS, EC, IVH | 217 | Died |
| 18 | 63 | M | clopidogrel | UFH | 6 | DC | TL, CH | 82 | Died |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Sui, Y.-G.; Wang, B.-C.; Xu, Y.-L.; Wu, N.-Q.; Wu, Y.-J.; Li, J.-J.; Qian, J. Intracranial Hemorrhage in Hospitalized Patients Following Percutaneous Coronary Intervention: A Large Cohort Analysis from a Single Center. Diagnostics 2023, 13, 2422. https://doi.org/10.3390/diagnostics13142422
Yang C, Sui Y-G, Wang B-C, Xu Y-L, Wu N-Q, Wu Y-J, Li J-J, Qian J. Intracranial Hemorrhage in Hospitalized Patients Following Percutaneous Coronary Intervention: A Large Cohort Analysis from a Single Center. Diagnostics. 2023; 13(14):2422. https://doi.org/10.3390/diagnostics13142422
Chicago/Turabian StyleYang, Cheng, Yong-Gang Sui, Bin-Cheng Wang, Yan-Lu Xu, Na-Qiong Wu, Yong-Jian Wu, Jian-Jun Li, and Jie Qian. 2023. "Intracranial Hemorrhage in Hospitalized Patients Following Percutaneous Coronary Intervention: A Large Cohort Analysis from a Single Center" Diagnostics 13, no. 14: 2422. https://doi.org/10.3390/diagnostics13142422
APA StyleYang, C., Sui, Y.-G., Wang, B.-C., Xu, Y.-L., Wu, N.-Q., Wu, Y.-J., Li, J.-J., & Qian, J. (2023). Intracranial Hemorrhage in Hospitalized Patients Following Percutaneous Coronary Intervention: A Large Cohort Analysis from a Single Center. Diagnostics, 13(14), 2422. https://doi.org/10.3390/diagnostics13142422






