Physical Functional Ability and Quantitative Assessment of the Multifidus Muscle of the Lumbar Spine in the Elderly
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
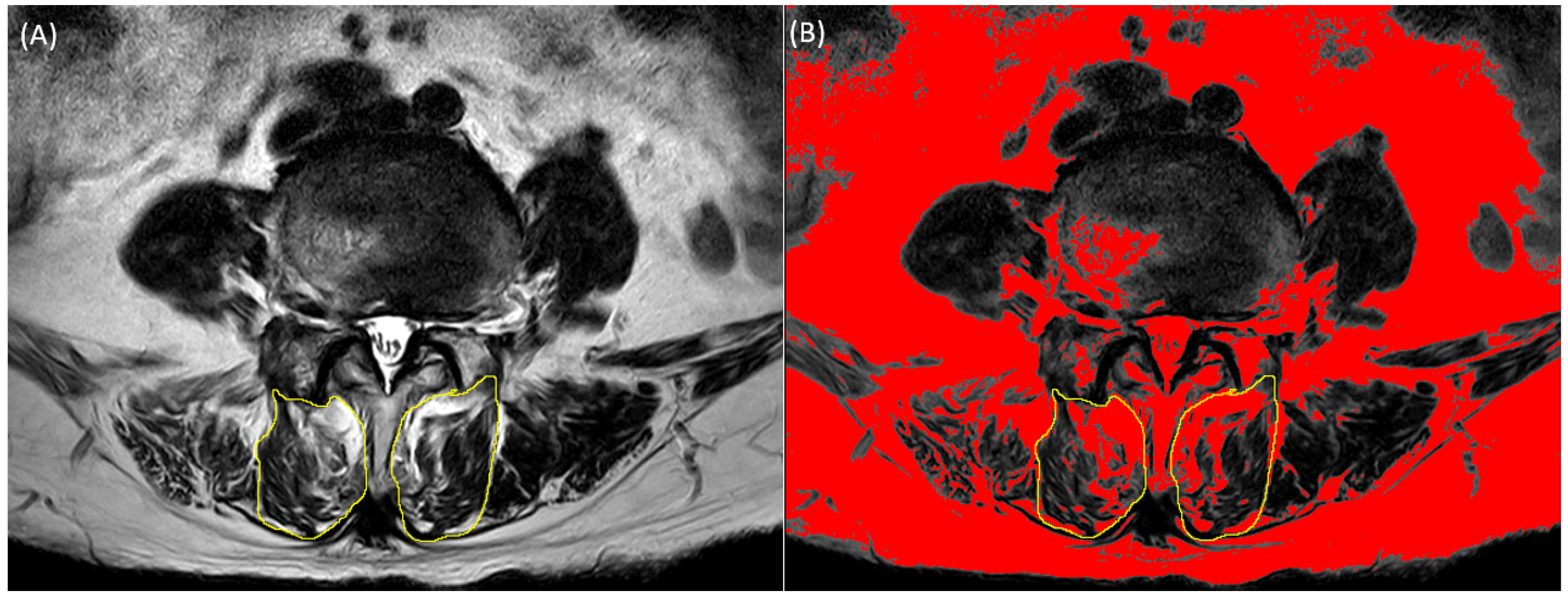
2.2. Multifidus Measurement from Spine MRI
2.3. Pfirrmann Grading System
2.4. Measures
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tournadre, A.; Vial, G.; Capel, F.; Soubrier, M.; Boirie, Y. Sarcopenia. Jt. Bone Spine 2019, 86, 309–314. [Google Scholar] [CrossRef]
- Chen, L.-K.; Woo, J.; Assantachai, P.; Auyeung, T.-W.; Chou, M.-Y.; Iijima, K.; Jang, H.C.; Kang, L.; Kim, M.; Kim, S. Asian Working Group for Sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J. Am. Med. Dir. Assoc. 2020, 21, 300–307.e302. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, K.-Y. Effects of core muscle stability training on the weight distribution and stability of the elderly. J. Phys. Ther. Sci. 2015, 27, 3163–3165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kliziene, I.; Sipaviciene, S.; Klizas, S.; Imbrasiene, D. Effects of core stability exercises on multifidus muscles in healthy women and women with chronic low-back pain. J. Back Musculoskelet. Rehabil. 2015, 28, 841–847. [Google Scholar] [CrossRef]
- Jeon, I.; Kim, S.W.; Yu, D. Paraspinal muscle fatty degeneration as a predictor of progressive vertebral collapse in osteoporotic vertebral compression fractures. Spine J. 2022, 22, 313–320. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, M.; Serrano Sosa, M.; Cattell, R.; Fan, W.; Li, M.; Chen, J.; Gao, M.; Zhou, Q.; Li, S. Fatty infiltration of paraspinal muscles is associated with bone mineral density of the lumbar spine. Arch. Osteoporos. 2019, 14, 99. [Google Scholar] [CrossRef]
- Freeman, M.D.; Woodham, M.A.; Woodham, A.W. The role of the lumbar multifidus in chronic low back pain: A review. PMR 2010, 2, 142–146. [Google Scholar] [CrossRef]
- Wallwork, T.L.; Stanton, W.R.; Freke, M.; Hides, J.A. The effect of chronic low back pain on size and contraction of the lumbar multifidus muscle. Man. Ther. 2009, 14, 496–500. [Google Scholar] [CrossRef]
- Sun, D.; Liu, P.; Cheng, J.; Ma, Z.; Liu, J.; Qin, T. Correlation between intervertebral disc degeneration, paraspinal muscle atrophy, and lumbar facet joints degeneration in patients with lumbar disc herniation. BMC Musculoskelet. Disord. 2017, 18, 167. [Google Scholar] [CrossRef] [Green Version]
- Faur, C.; Patrascu, J.M.; Haragus, H.; Anglitoiu, B. Correlation between multifidus fatty atrophy and lumbar disc degeneration in low back pain. BMC Musculoskelet. Disord. 2019, 20, 414. [Google Scholar] [CrossRef] [Green Version]
- Gómez, J.F.; Curcio, C.-L.; Alvarado, B.; Zunzunegui, M.V.; Guralnik, J. Validity and reliability of the Short Physical Performance Battery (SPPB): A pilot study on mobility in the Colombian Andes. Colomb. Med. 2013, 44, 165–171. [Google Scholar] [CrossRef]
- Neuls, P.D.; Clark, T.L.; Van Heuklon, N.C.; Proctor, J.E.; Kilker, B.J.; Bieber, M.E.; Donlan, A.V.; Carr-Jules, S.A.; Neidel, W.H.; Newton, R.A. Usefulness of the Berg Balance Scale to predict falls in the elderly. J. Geriatr. Phys. Ther. 2011, 34, 3–10. [Google Scholar]
- Mehmet, H.; Yang, A.W.; Robinson, S.R. Measurement of hand grip strength in the elderly: A scoping review with recommendations. J. Bodyw. Mov. Ther. 2020, 24, 235–243. [Google Scholar] [CrossRef]
- Sousa-Santos, A.; Amaral, T. Differences in handgrip strength protocols to identify sarcopenia and frailty—A systematic review. BMC Geriatr. 2017, 17, 238. [Google Scholar] [CrossRef] [Green Version]
- Duncan, P.W.; Weiner, D.K.; Chandler, J.; Studenski, S. Functional reach: A new clinical measure of balance. J. Gerontol. 1990, 45, M192–M197. [Google Scholar] [CrossRef]
- Jenkins, M.; Johnson, A.M.; Holmes, J.; Stephenson, F.; Spaulding, S. Predictive validity of the UPDRS postural stability score and the Functional Reach Test, when compared with ecologically valid reaching tasks. Park. Relat. Disord. 2010, 16, 409–411. [Google Scholar] [CrossRef]
- Lee, D.; Kang, M. Correlation between psoas muscle index and degeneration of spinal back muscle in patients with back pain. Healthcare 2021, 9, 1189. [Google Scholar] [CrossRef]
- Hamrick, M.W.; McGee-Lawrence, M.E.; Frechette, D.M. Fatty infiltration of skeletal muscle: Mechanisms and comparisons with bone marrow adiposity. Front. Endocrinol. 2016, 7, 69. [Google Scholar] [CrossRef] [Green Version]
- Kader, D.; Wardlaw, D.; Smith, F. Correlation between the MRI changes in the lumbar multifidus muscles and leg pain. Clin. Radiol. 2000, 55, 145–149. [Google Scholar] [CrossRef] [Green Version]
- Kjaer, P.; Bendix, T.; Sorensen, J.S.; Korsholm, L.; Leboeuf-Yde, C. Are MRI-defined fat infiltrations in the multifidus muscles associated with low back pain? BMC Med. 2007, 5, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shahidi, B.; Hubbard, J.C.; Gibbons, M.C.; Ruoss, S.; Zlomislic, V.; Allen, R.T.; Garfin, S.R.; Ward, S.R. Lumbar multifidus muscle degenerates in individuals with chronic degenerative lumbar spine pathology. J. Orthop. Res. 2017, 35, 2700–2706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, J.K.-F.; Richardson, C.A.; Kippers, V.; Parnianpour, M. Relationship between muscle fiber composition and functional capacity of back muscles in healthy subjects and patients with back pain. J. Orthop. Sports Phys. Ther. 1998, 27, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Richardson, C.; Jull, G. Muscle control–pain control. What exercises would you prescribe? Man. Ther. 1995, 1, 2–10. [Google Scholar] [CrossRef]
- Chung, S.; Lee, J.; Yoon, J. Effects of stabilization exercise using a ball on mutifidus cross-sectional area in patients with chronic low back pain. J. Sports Sci. Med. 2013, 12, 533. [Google Scholar]
- Rezazadeh, F.; Taheri, N.; Okhravi, S.M.; Hosseini, S.M. The relationship between cross-sectional area of multifidus muscle and disability index in patients with chronic non-specific low back pain. Musculoskelet. Sci. Pract. 2019, 42, 1–5. [Google Scholar] [CrossRef]
- Cho, S.-M.; Kim, S.-H.; Ha, S.-K.; Kim, S.-D.; Lim, D.-J.; Cha, J.; Kim, B.-J. Paraspinal muscle changes after single-level posterior lumbar fusion: Volumetric analyses and literature review. BMC Musculoskelet. Disord. 2020, 21, 73. [Google Scholar] [CrossRef]
- Ahsan, K.; Khan, S.I.; Zaman, N.; Ahmed, N.; Montemurro, N.; Chaurasia, B. Fusion versus nonfusion treatment for recurrent lumbar disc herniation. J. Craniovertebral Junction Spine 2021, 12, 44. [Google Scholar] [CrossRef]
- Blackburn, T.; Guskiewicz, K.M.; Petschauer, M.A.; Prentice, W.E. Balance and joint stability: The relative contributions of proprioception and muscular strength. J. Sport Rehabil. 2000, 9, 315–328. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.W.; Bhattacharyya, N. Balance disorders in the elderly: Epidemiology and functional impact. Laryngoscope 2012, 122, 1858–1861. [Google Scholar] [CrossRef]
- Redfern, M.S.; Yardley, L.; Bronstein, A.M. Visual influences on balance. J. Anxiety Disord. 2001, 15, 81–94. [Google Scholar] [CrossRef]
- Taekema, D.G.; Gussekloo, J.; Maier, A.B.; Westendorp, R.G.; de Craen, A.J. Handgrip strength as a predictor of functional, psychological and social health. A prospective population-based study among the oldest old. Age Ageing 2010, 39, 331–337. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, K.; Imagama, S.; Ando, K.; Tsushima, M.; Machino, M.; Ota, K.; Tanaka, S.; Morozumi, M.; Kanbara, S.; Ishiguro, N. Weakness of grip strength reflects future locomotive syndrome and progression of locomotive risk stage: A 10-year longitudinal cohort study. Mod. Rheumatol. 2020, 30, 573–579. [Google Scholar] [CrossRef]
- Rantanen, T.; Guralnik, J.M.; Foley, D.; Masaki, K.; Leveille, S.; Curb, J.D.; White, L. Midlife hand grip strength as a predictor of old age disability. JAMA 1999, 281, 558–560. [Google Scholar] [CrossRef] [Green Version]
- Clark, N.; Voight, M.L.; Campbell, A.M.; Pierce, S.; Sells, P.; Cook, R.; Henley, C.; Schiller, L. The relationship between segmental rolling ability and lumbar multifidus activation time. Int. J. Sports Phys. Ther. 2017, 12, 921. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | N = 57 |
|---|---|
| Age (y) | 73.89 (6.09) |
| Gender (n, % male) | 23 (40.35) |
| Height (kg) | 1.57 (0.08) |
| Weight (m) | 60.03 (9.56) |
| BMI (kg/m2) | 24.21 (2.98) |
| SPPB | 9.39 (1.93) |
| BBS | 52.42 (2.98) |
| Grip (kg) | 24.03 (7.06) |
| FRT (cm) | 19.56 (7.22) |
| Psoas index (cm2/m2) | 7.80 (2.17) |
| Multifidus (cm2) | Functional Multifidus (cm2) | Ratio (%) | ||||
|---|---|---|---|---|---|---|
| Group | F (p-Value) | F (p-Value) | F (p-Value) | |||
| L2-3 | 727.58 ± 184.28 a | 66.141 | 546.04 ± 179.91 a | 30.112 | 74.17 ± 10.85 b | 3.287 |
| L3-4 | 1058.75 ± 301.98 b | (<0.001) | 793.58 ± 317.03 b | (<0.001) | 73.58 ± 11.58 ab | (0.022) |
| L4-5 | 1455.5 ± 360.06 c | 1038.02 ± 358.49 c | 70.54 ± 11.39 ab | |||
| L5-S1 | 1550.94 ± 484.79 c | 1079.81 ± 440.78 c | 68.27 ± 11.83 a | |||
| Total | 1200.26 ± 478.62 | 865.77 ± 398.06 | 71.63 ± 11.60 | |||
| L2-3 | L3-4 | L4-5 | L5-S1 | |||||
|---|---|---|---|---|---|---|---|---|
| Multifidus CSA | r | p-Value | r | p-Value | r | p-Value | r | p-Value |
| SPPB | 0.048 | 0.727 | 0.144 | 0.284 | 0.213 | 0.112 | 0.065 | 0.632 |
| BBS | 0.177 | 0.077 | 0.179 | 0.182 | 0.275 | 0.039 * | 0.061 | 0.654 |
| Grip | 0.405 | 0.002 * | 0.583 | <0.001 * | 0.588 | <0.001 * | 0.393 | 0.003 * |
| FRT | 0.119 | 0.119 | 0.273 | 0.040 * | 0.181 | 0.177 | 0.228 | 0.088 |
| Psoas index | 0.383 | 0.004 * | 0.406 | 0.002 * | 0.454 | <0.001 * | 0.285 | 0.032 * |
| Functional CSA | r | p | r | p | r | p | r | p |
| SPPB | 0.186 | 0.169 | 0.269 | 0.043 * | 0.300 | 0.024 * | 0.198 | 0.139 |
| BBS | 0.138 | 0.138 | 0.238 | 0.074 | 0.290 | 0.028 * | 0.141 | 0.295 |
| Grip | 0.572 | <0.001 * | 0.687 | <0.001 * | 0.612 | <0.001 * | 0.520 | <0.001 * |
| FRT | 0.254 | 0.059 | 0.311 | 0.019 * | 0.325 | 0.014 * | 0.342 | 0.009 * |
| Psoas index | 0.491 | <0.001 * | 0.463 | <0.001 * | 0.471 | <0.001 * | 0.296 | 0.026 * |
| Ratio | r | p | r | p | r | p | r | p |
| SPPB | 0.284 | 0.034 * | 0.367 | 0.005 * | 0.339 | 0.010 * | 0.362 | 0.006 * |
| BBS | 0.143 | 0.292 | 0.218 | 0.103 | 0.213 | 0.111 | 0.213 | 0.057 |
| Grip | 0.530 | <0.001 * | 0.580 | <0.001 * | 0.331 | 0.012 * | 0.331 | <0.001 * |
| FRT | 0.366 | 0.006 * | 0.278 | 0.036 * | 0.373 | 0.004 * | 0.373 | 0.002 * |
| Psoas index | 0.392 | 0.003 * | 0.393 | 0.002 * | 0.271 | 0.042 * | 0.409 | 0.002 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yun, J.H.; Lee, D.G. Physical Functional Ability and Quantitative Assessment of the Multifidus Muscle of the Lumbar Spine in the Elderly. Diagnostics 2023, 13, 2423. https://doi.org/10.3390/diagnostics13142423
Yun JH, Lee DG. Physical Functional Ability and Quantitative Assessment of the Multifidus Muscle of the Lumbar Spine in the Elderly. Diagnostics. 2023; 13(14):2423. https://doi.org/10.3390/diagnostics13142423
Chicago/Turabian StyleYun, Jung Hae, and Dong Gyu Lee. 2023. "Physical Functional Ability and Quantitative Assessment of the Multifidus Muscle of the Lumbar Spine in the Elderly" Diagnostics 13, no. 14: 2423. https://doi.org/10.3390/diagnostics13142423




