Abstract
Diagnosis of infection-causing microorganisms with sensitive, rapid, selective and economical diagnostic tests is critical to start the right treatment. With these tests, the spread of infections can be prevented. In addition to that, the detection of antimicrobial resistance also makes a significant contribution to public health. In recent years, different types of diagnostic tests have been developed as alternatives to traditional diagnostic tests used in clinics. In particular, colorimetric tests, which minimize the need for an instrument, have advantages owing to their cost effectiveness, rapid response and naked-eye detection and practical use. In this review, we especially focused on pH indicators and nanomaterial-based colorimetric tests in detection of infection-causing microorganisms and antimicrobial resistance.
1. Introduction
Infectious diseases caused by various pathogens have become an emerging serious health problem. Thus, pathogens like foodborne, waterborne and hospital-acquired ones are among the high-risk groups in terms of both global economy and public health [1,2]. Various diseases caused by bacterial infectious agents are also cited as a cause of mortality and morbidity. In order to prevent the spread of infectious pathogens, accurate, sensitive and rapid diagnosis and the proper treatment methods should be emphasized [3,4,5].
Various traditional methods have been developed and have been in use in the field for the detection and identification of pathogenic bacteria. For instance, methods based on colony counting, culturing, ELISA (enzyme-linked immunosorbent assay) and PCR (polymerase chain reaction) have been actively used in clinics [3,4,5,6,7]. The enzymes are often adapted into the ELISA assay as signal enhancers owing to their substrate-specific catalytic activity. Three enzymes including alkaline phosphatase (ALP), horseradish peroxidase enzyme (HRP) and β-galactosidase are most commonly used in ELISA-based colorimetric immunoassays. The detection principle of ELISA simply relies on oxidation of or reduction in a substrate in the reaction environment. The changes in color type or intensity detected by the naked eye, a spectrometry device or a mobile application on smartphones usually proportionally increase in the target pathogen concentration [8].
The bio-components of microbial biosensors are usually microbial cells, but various microorganisms, tissues, cells and organelles are also included in this sensor group. The detection mechanism of some biosensors depends on amperometric, potentiometric or impedance electrochemical response [9]. These biosensors can be utilized to detect several metabolites such as H2, CO2, NH3 and organic acids found in aerobic organisms [9].
Phenotypic detection methods such as staining and culture have been used as a gold standard method for the pathogens, but a major disadvantage is that the result can be obtained at least in 48 h. Nevertheless, these methods do not provide sufficient information to report antibiotic resistance [10,11,12,13]. The delay in detection of serious infections such as sepsis can adversely influence treatment, which may induce death rate increases by 7.6% for every hour. Although the bacterial culture method is actively used, it must be performed by trained personnel and it cannot provide specific detection of bacteria owing to similarities in metabolic pathways and in phenotypic characteristics of bacteria. However, while molecular detection tests based on the analysis of genomic markers can result in significantly short time for microorganism detection, they often require specialized and expensive equipment and/or expert personnel and interpretation [10].
Conventional diagnostic kits present some challenges including isolation of the pathogen, trained personnel, specialized equipment, long time and high costs necessary to reach a diagnostic conclusion [14,15]. Therefore, there is still high demand for the development of novel methods that are economical, sensitive, specific, user-friendly, fast, and minimize the need for equipment. For these purposes, many different diagnostic tests and methods such as microfluidic devices, electrical sensors and DNA microarrays sensors have been designed [3,16,17,18,19,20,21,22,23]. Additionally, optical biosensors including photoluminescence, fluorescence and colorimetric based have been developed [24,25,26]. Among these, colorimetric biosensors have received considerable attention owing to fast and clear response. With these methods, the target pathogens in patient sample can be rapidly, easily, economically and selectively detected even with the naked eye by color change [27]. For instance, some colorimetric biosensors work based upon enzymatic reaction, and color change can be detected with the naked eye and with a device. Most of the time, colorimetric biosensors do not require an additional complicated equipment owing to visual response. For this purpose, various colorimetric tests have been developed for the diagnosis of pathogenic bacteria and antibiotic resistance.
Multidrug resistance in antibiotic treatment in bacteria is a serious threat that puts community healthcare at risk [28]. For instance, carbapenem resistance, especially in carbapenemase-producing bacteria, play a critical role in multidrug resistance [29,30]. Rapid and sensitive detection of resistant bacteria contributes to rational antibiotic use, protects public health and provides critical information for rapid implementation of epidemic-control measures [30]. Detection of carbapenem resistance may help to select the most suitable antibiotic treatment in the early term in the therapy of Gram-negative bacterial infections. In addition to that, rapid diagnosis of resistance provides advantages in many aspects such as shortening the length of antibiotic use, hospital stay, protecting patient immune system and reducing drug cost [31,32,33]. The active pathway in carbapenem resistance is based on the point that resistant microorganisms degrade the antibiotic by producing the carbapenemase enzyme. There are several analysis procedures for the rapid and sensitive detection of carbapenemase enzyme-release microorganisms through this mechanism, including UV spectrophotometric tests [34], matrix-assisted laser desorption ionization time-of-flight (MALDITOF) technological approaches [35,36,37] and molecular-based methods [38,39,40,41,42]. While the methods mentioned above perform with high sensitivity, specificity and accuracy, they require qualified personnel, are costly and involve time-consuming steps. Furthermore, molecular strategies may not catch carbapenemase-produced genes. As a solution to these problems, biochemical assays have been developed to confirm carbapenemase production and/or the presence of other mechanisms [43,44].
Nordmann and Poirel developed colorimetric tests called “NP Test” and introduced groundbreaking innovations in the sensitive and rapid colorimetric detection of antibiotic resistance. In these tests, a colorimetric response can be observed within 2 h, and it has the potential to be used directly on patient samples such as infected urine and blood cultures. For instance, the Carba NP tests [45] and their derivatives like Blue Carba [46] and β Carba [43] include biochemical reactions and economically and phenotypically verify carbapenemase enzyme release bacteria. The working principle of Carba NP tests is based on the release of carbapenemase enzymes during bacterial growth in bacterial culture (i.e., up to 24–48 h) which hydrolyze carbapenem group drugs such as imipenem or meropenem to produce an acidic environment. Then, a colorimetric response occurs due to the presence of an indicator in Carba NP tests. Thus, indicators such as phenol red or bromine thymol blue change color. Additionally, characteristic inhibitor agents of carbapenemase enzyme activity such as vaborbactam, avibactam or ethylenediamine tetra acetic acid can be included to increase sensitivity [43,47].
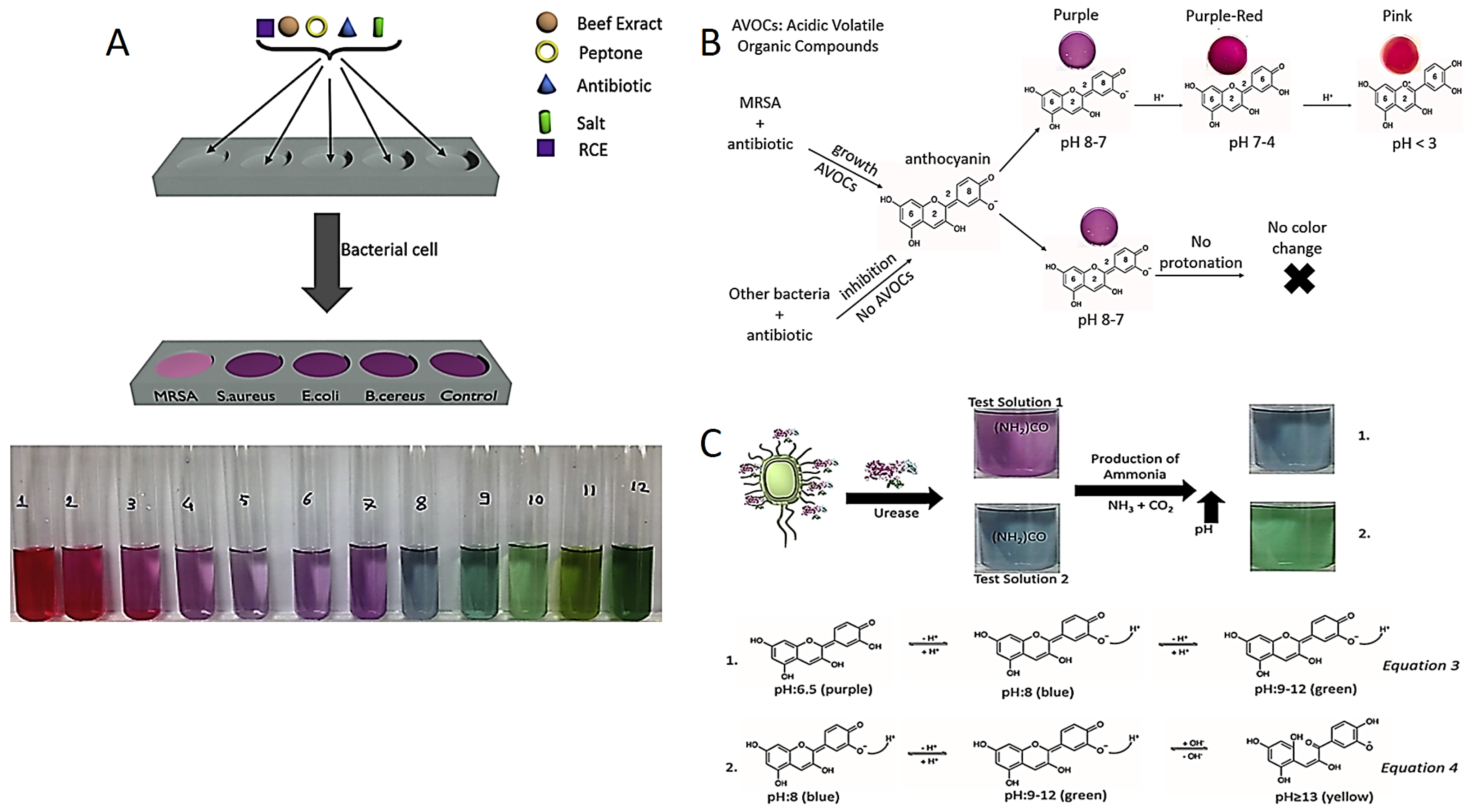
Recently, Celik et al. developed the red cabbage extract (RCE) incorporated media as a novel colorimetric test to diagnose Methicillin-resistant Staphylococcus aureus strains (MRSA). Anthocyanin is a natural pH indicator obtained from RCE which displays various colors in the different pH values, as shown in Figure 1A. For instance, anthocyanin molecules display pink, purple and blue–green colors in acidic, neutral and in alkaline conditions, respectively. Although susceptible bacteria are inhibited in the presence of antibiotics in this method, resistant bacteria continue to grow and release acidic volatile compounds. The color of the test medium changes from purple to pink in acidic liquid media, confirming the presence of MRSA [48].
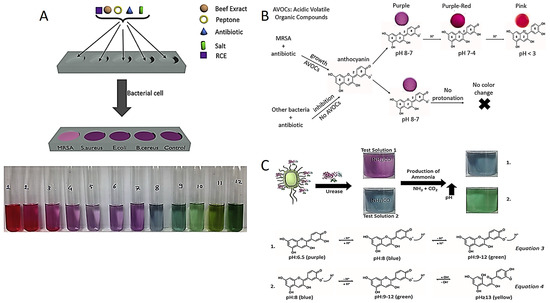
Figure 1.
(A) MRSA diagnostic test components and anthocyanin pH-dependent color change. Reprinted with permission from Ref. [48]. Copyright 2023 Elsevier. (B) Color change in anthocyanin-containing agar in the presence of MRSA. Reprinted with permission from Ref. [49]. Copyright 2023 Elsevier. (C) Detection of H. pylori by colorimetric tests prepared at two different pH and equations for color change mechanisms. Reprinted from Ref. [50].
Celik et al. also reported an anthocyanin-containing test in liquid and agar forms to distinguish MRSA and MRSE (Methicillin-resistant Staphylococcus epidermidis) based on pH change as presented in Figure 1B. Anthocyanin resistance tests have advantages over NO test in terms of sensitivity, detection time, cost effectiveness and biocompatibility of a pH indicator [49,51,52,53]. Researchers have the same strategy for colorimetric detection of urease-positive bacteria. For instance, Helicobacter pylori (H. pylori) colonized in the stomach secretes a specific urease enzyme which converts urea into ammonia (NH3) in in the reaction environment. The NH3 molecules make the reaction environment alkaline, and then the color of the reaction solution turns green from purple due to the deprotonation of anthocyanins shown in Figure 1C.
There are commercial products such as Pyloritek®, CLO test®, Hp One®, Pronto Dry®, and Hp Fast for the detection of H. pylori. While Pyloritek®, CLO test® and Pronto Dry® have phenol red as a pH indicator, Hp One® and Hp Fast® have bromothymol blue. Among these tests, only the CLO test® offers the LOD value of 104 in CFU/mL. However, the LOD value of the anthocyanin test decreases to 1 CFU/mL. In addition to the detection time of Pyloritek®, that of Hp One® and Pronto Dry® is about 1 h, while the CLO test® and Hp Fast® take 4–24 h for detection response. The anthocyanin test produces the colorimetric response in less than 1 h [50,54,55].
Due to the use of a biocompatible pH indicator, anthocyanin, incorporated in these colorimetric phenotypic tests, and a quite economical structure, these colorimetric phenotypic tests have a great potential to be practically used in clinics. Furthermore, anthocyanin-incorporated tests have also been utilized for the detection of urinary tract infection (UTI) pathogens [50,56]. Celik et al. also developed a test for the use in clinical laboratories to detect UTI. They designed a test to detect Proteus mirabilis (P. mirabilis) and Klebsiella pneumonia (K. pneumonia) causing UTI. These UTI bacterias exhibit urease enzyme activity. The urease enzyme hydrolyzes the urea to produce NH3 molecules. NH3 increases the pH of the test solution, in which the anthocyanin molecules in the test solution are deprotonated, then change color in response to the pH change. Commercial tests can detect pH changes using bromothymol blue, phenol red or bromocresol violet as pH indicators. These indicators are synthesized by chemical processes under laboratory conditions and may cause irritation in contact with eyes and skin. They have both corrosive and toxic properties and may cause irritation in the respiratory system when inhaled. For this reason, inhalation of the vapor or the gas form should be avoided. It is recommended to use protective materials to prevent contact with hands, face and eyes. Since anthocyanins, plant-derived pH indicators, are obtained from a plant consumed in daily life, there are no risks such as toxicity and irritation compared to other indicators. Anthocyanins obtained from red cabbage are frequently used in the coloring of food and fruit juices. In addition, regular consumption of food sources rich in anthocyanins is recommended due to their antioxidant effects. Red cabbage grows naturally, is easily accessible and is used as a plant source in antibiotic susceptibility tests due to its high anthocyanin content.
Color image processing techniques are also used for precise analysis of color change. A mobile application has been developed in which an image processing method can be applied in clinical laboratories. The mobile application includes interfaces that can be used directly by the technician. Thus, color changes are analyzed by preventing personal errors. In addition to that, a smartphone platform is printed on a 3D printer to minimize the factors affecting the analysis of the mobile application. With the smartphone platform and mobile application, the color change in the diagnostic test can be analyzed very precisely and quickly [56].
Common techniques such as culturing, microscopic urinalysis, ELISA testing, PCR, MALDI-TOF, FISH, and advanced light scattering are available for the detection of UTI. Although the analysis period of the culture method is quite long (48–72 h) and is used in clinical laboratories, it is the method used as the gold standard [57]. Although microscopic urine analysis is a rapid technique, it has poor sensitivity and specificity. In addition to that, it lacks the ability to detect antimicrobial resistance [58]. The ELISA test is a method that enables the indirect qualitative colorimetric detection of pathogens based on antigen–antibody combinations and can provide results in 2–3 h. However, a long process is required for the application of the method. In terms of sensitivity and specificity, it is evaluated as insufficient in the literature [59]. PCR is a method that provides results based on the amplification of specific genes (known to be specific to certain bacteria) from total genomic DNA extracted from urine samples, and is a sensitive and specific analysis method that can provide results in 5–6 h. Requirement of special probes for all pathogens, extensive and long preparation procedure and lack of quantitative data are the major disadvantages of the PCR method. In addition to that, the PCR method requires expensive devices and expert personnel [57,60]. In the detection of pathogens with the MALDI-TOF method, charged molecules are formed by ionization, separated according to the mass/charge ratio, and detected and measured using the TOF mass analyzer. Although it provides fast, precise and specific results, it is an expensive method. Sample preparation is difficult and interpretation of results is more complex than that of other methods [61,62]. In the FISH method, microscopic detection of microorganisms is performed by using fluorescently labeled nucleic acid probes hybridized to complementary targets. Although it is fast, sensitive and specific, its biggest disadvantage is the requirement of special probes for all pathogens [63]. In the forward light scattering method, bacterial growth is detected by forward light scattering based on changes, and the detection time is 90 min. It is cheap and requires a small amount of samples, and it is not able to identify the type of pathogen [64].
Nanoparticle (NPs)-based colorimetric biosensors including gold NPs (Au NPs), Ag/Au core-shell NPs, and quantum dots such as CdSe/ZnS have also been in use with biological elements [65,66,67,68,69,70]. Colorimetric detection methods based on NPs can be divided into three groups: (i) peroxidase-like activity of NPs, (ii) dispersion and aggregation of the NPs, and (iii) destabilization of the NPs.
2. Colorimetric Detection Based on Peroxidase-like Nature of NPs
Enzymes have been preferred as indication transducers in colorimetric detection methods due to their high sensitivity and specificity against certain substrates [71,72]. However, the use of enzymes in biosensors has been limited due to their high cost and short lifetime [73]. Various NPs with enzyme-like properties have been used as an alternative to enzymes [74,75,76]. Magnetic NPs, carbon nanotubes (CNTs), Au NP, graphene oxide (GO) and platinum (Pt) NP have been designed for the detection of pathogens via the peroxidase-like effect (Figure 2A). The OH− radical can oxidize substrates and provide a colorimetric response. NPs conjugated with various targeting biomolecules such as DNA probes, DNA aptamers and antibodies are preferred in the specific and colorimetric detection of microorganisms via peroxidase-like activity [77,78,79,80,81]. For instance, antibody-conjugated Pt NP was developed for the colorimetric detection of Escherichia coli [78]. As a consequence of the peroxidase enzyme activity of Pt NP, the colorless TMB (3,3′,5,5′-Tetramethylbenzidine) molecule was oxidized and turned blue. In another study, Fe3O4 NP was functionalized with an aptamer for specific and colorimetric detection of Salmonella typhimurium (Figure 2B) [79]; similarly, colorless TMB was oxidized to a blue-color product [80,81]. The single-stranded DNA (ssDNA) aptamer was adsorbed on the positively charged surface of Fe3O4NPs; then, the aptamer was detached from Fe3O4 NPs in the presence of the pathogen and for binding to the pathogen. The naked Fe3O4 NPs oxidized TMB with peroxidase-like activity to form a blue-color TMBox (Figure 2B).
The GO and CNTs have attracted great interest for biosensor applications owing to their high conductive properties [82,83,84]. It was observed that GO, CNT, Au NPs@ GO and Au NPs@CNT act as Fenton reagents and show a highly sensitive colorimetric response in the presence of H2O2. For instance, the influenza A (H3N2) virus was sensitively detected using Au NPs@CNT nanocomposites (NCs). The haemagglutinin (HA) monoclonal antibodies were bound to Au NPs@CNT NC to selectively recognize influenza A (H3N2) and show peroxidase-like activity (Figure 2C) [85]. In another study, norovirus was sensitively and specifically detected by enhancing the peroxidase enzymatic activity of Au NPs@ GO NCs (Figure 2C) [86].
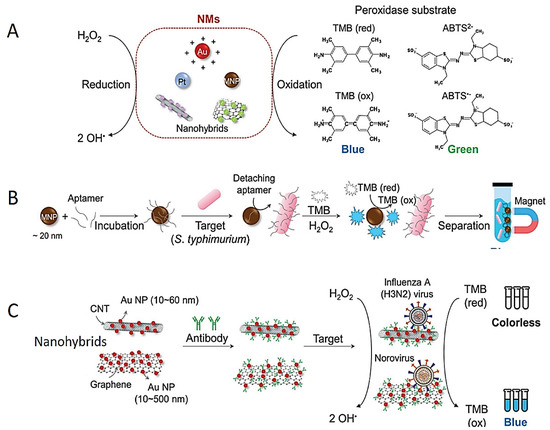
Figure 2.
Schematic designs of colorimetric tests based on peroxidase-like activities of the NPs. (A) Scheme of peroxidase-like activity of different NPs. (B) Scheme of S. Typhimurium detection test using aptamer conjugated Fe3O4 NPs. (C) Colorimetric detection of influenza (H3N2) virus using Au NPs@CNTs NCs. Reprinted from Ref. [87].
Figure 2.
Schematic designs of colorimetric tests based on peroxidase-like activities of the NPs. (A) Scheme of peroxidase-like activity of different NPs. (B) Scheme of S. Typhimurium detection test using aptamer conjugated Fe3O4 NPs. (C) Colorimetric detection of influenza (H3N2) virus using Au NPs@CNTs NCs. Reprinted from Ref. [87].
3. Colorimetric Detection Based on Dispersion and Aggregation of NPs
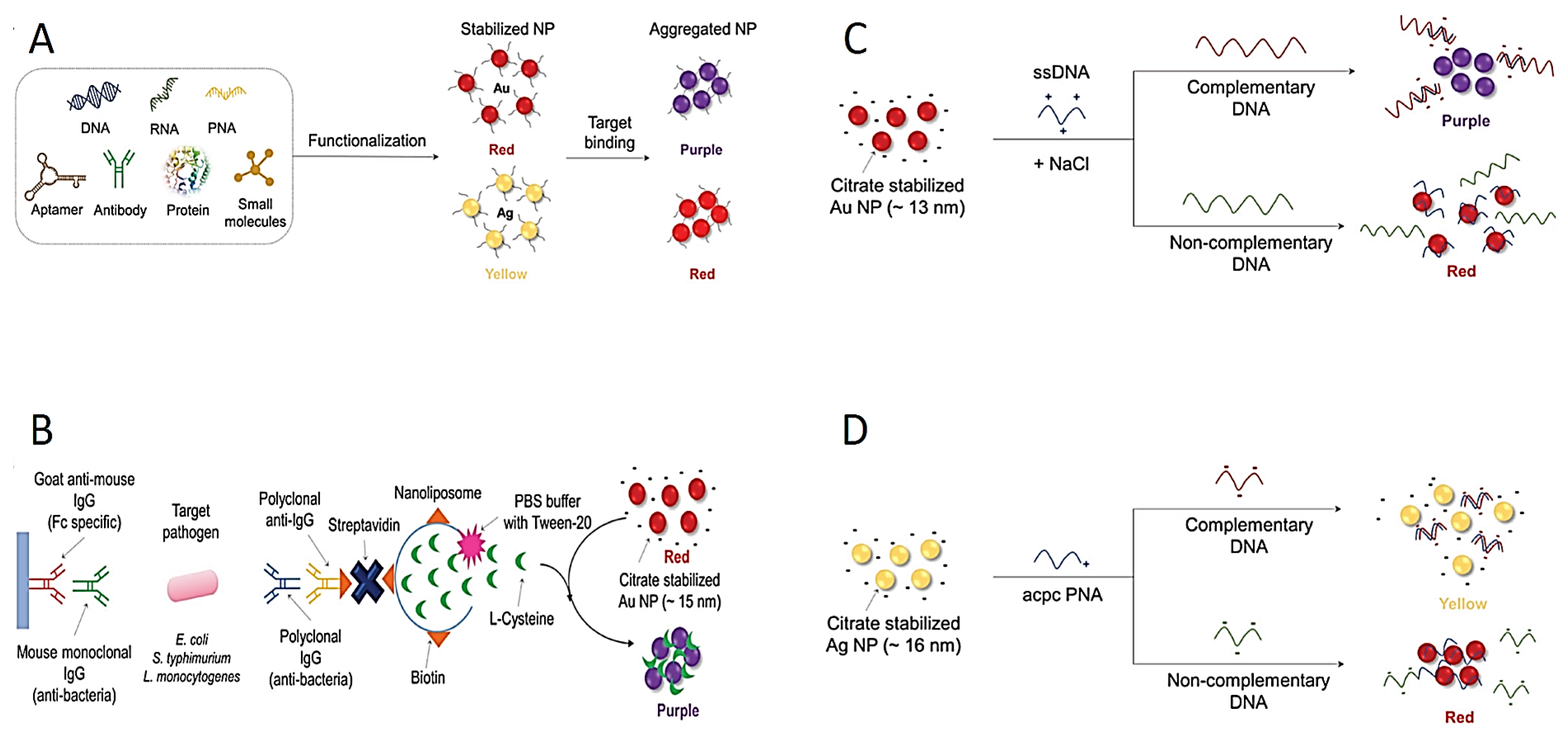
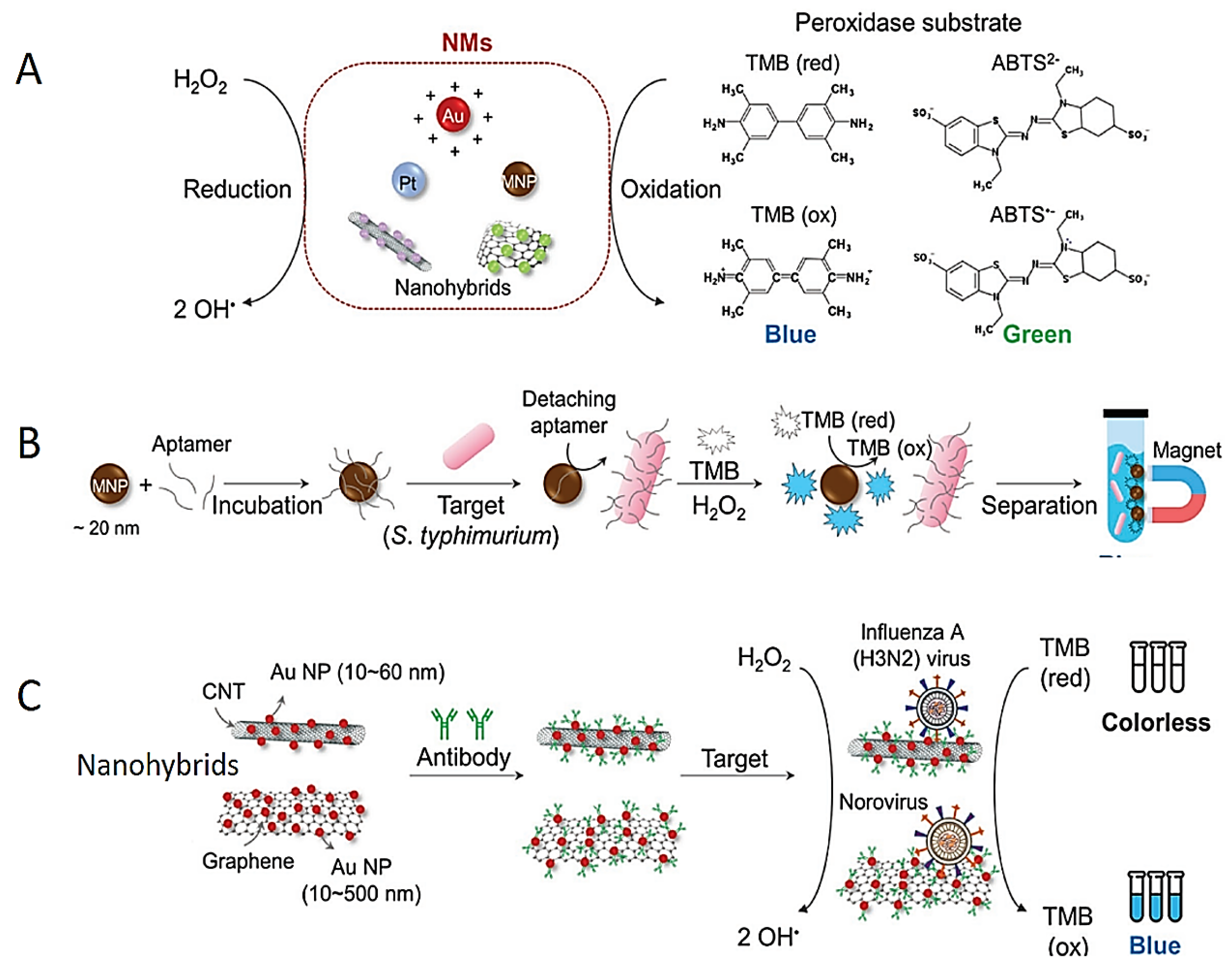
Because of the extraordinary optic properties of plasmonic NPs (especially Ag and Au NPs), the direct use of these NPs in colorimetric diagnostic tests has also been presented as a new approach. The changes on the surface of the NPs may lead to dispersion or aggregation, both of which may induce color changes [66]. These color changes are observed by naked eye and spectrophotometer. The color change in NPs provides a practical and easy platform in colorimetric tests for the detection of pathogens (Figure 3A). The Ag and Au NPs are most frequently preferred for monitoring color changes due to their unique plasmonic properties. For instance, while colloidal Au NPs in solutions produce a red color, a blue–purple color appears due to the aggregation of Au NPs in the solution. Similarly, the Ag NP solution is usually of a yellow color, and when the particles are aggregated, the color of the Ag NP solution becomes red.
Figure 3.
Schematic explanation of colorimetric diagnostic tests based on dispersion and aggregation of NPs. (A) Scheme of aggregation of different-molecule-integrated NPs. (B) Signal-amplified detection of pathogens using protein functionalized nanoliposomes. (C) Schematic design of detection of the virus DNA using aggregation of Au NP in the existence of dsDNA. (D) Schematic design of detection of the Middle East respiratory syndrome coronavirus DNA using redispersion–aggregation mechanism of Ag NP. Reprinted from Ref. [87].
The surface of these NPs can be conjugated with RNA, DNA and/or antibodies for a selective detection of targets (Figure 3A). For instance, pathogen-specific antibodies are conjugated to the surface of Au NPs; then, the Au NPs come together on the surface of the target pathogen. Thus, color change occurs due to the aggregation of Au NPs, and colorimetric response is observed by the naked eye [74,88,89,90,91]. Furthermore, nanoliposome NPs have been used as a signal amplification tool for the detection of foodborne pathogens (Figure 3B) [92]. Mouse monoclonal antibody-conjugated nanoliposome NPs detect certain pathogens by containing cysteine molecules that induce the aggregation of Au NPs. Mouse monoclonal antibodies molecularly recognize the pathogens such as E. coli O157:H7, S. typhimurium and Listeria monocytogenes (L. monocytogenes); subsequently, polyclonal antibodies bind to target pathogens. However, this multi-step process is a serious disadvantage for industrial production. The complex and time-consuming preparation process limits its applicability to commercial kits. To address this issue, the use of unmodified NPs was aimed to facilitate the commercialization of colorimetric assays [3]. The thiol-Au binding method developed for this purpose has been widely used as a DNA conjugation method for enhanced binding potency [93,94]. Therefore, unmodified DNA adsorption on unmodified Au NPs has been used for the detection of various pathogens [95]. For instance, the DNA of Cyprinid herpesvirus-3 pathogen was successfully identified based on the aggregation using citrate-stabilized Au NP (Figure 3C) [96].
Furthermore, single-stranded and double-stranded DNA molecules can be distinguished by producing different responses in the colorimetric test in the presence of NaCl because of their distinct electrostatic interactions [97]. Single-stranded DNA (ssDNA) can be adsorbed on the surface of Au NPs, and no aggregation occurs. However, the double-stranded DNA (dsDNA) cannot be adsorbed on the surface of Au NPs owing to the blockage of pi electrons. Based on this, in the presence of herpesvirus-3 DNA, the ssDNA probe reacts with herpesvirus-3 DNA to form the dsDNA structure, then leading to the assembling of Au NPs, providing a colorimetric result. A similar method was used to detect double-stranded DNA of the S. typhimurium pathogen using Au NPs [98].
In another study, pyrrolidinyl peptide nucleic acid known as acpc PNA was bonded on (2S)-aminocyclopentane-(1S)-carboxylic acid to detect Mycobacterium tuberculosis, human papillomavirus and Middle East respiratory syndrome coronavirus in the presence of Ag NPs (Figure 3D) [99]. The acpc PNA probe induced the aggregation of Ag NPs, leading to the color changing from yellow to red due to electrostatic interactions. When PNAs bind to target DNAs, Ag NPs are stabilized in the test environment. There exists a mention that this mechanism has been used in the improvement of paper-based analytical instruments owing to its easy integration, affordability and portability [100,101,102]. Carbapenem-resistant bacterial strains including Rhodopseudomonas, Acetobacter aceti, Staphylococcus and E. coli were detected colorimetrically using unmodified Au NPs and Ag NPs [103].
4. Colorimetric Detection Based on Destabilization of NPs
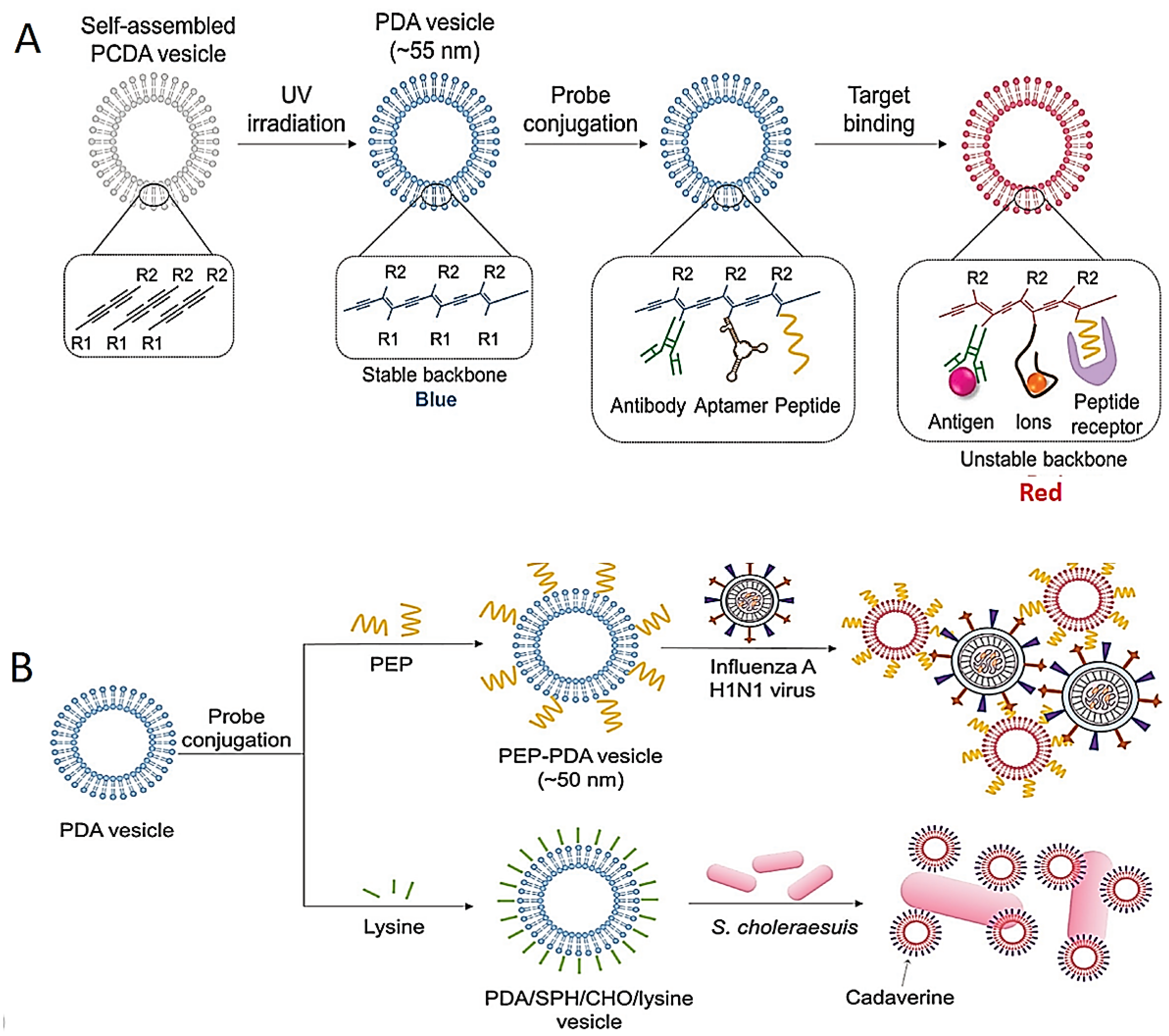
Various tests for the colorimetric identification of pathogens have also been developed based on the modification and stabilization of the NPs. The most frequently used NPs are polydiacetylene (PDA)-based structures with interesting chromatic features [91,104]. Blue-color PDA structures are synthesized by 1,4 photopolymerization of self-assembled diacetylene monomers. The specific aptamer-, antibody- and/or peptide-conjugated PDA structures for the detection of pathogens are shown in Figure 4A. For instance, the color of the peptide-bound PDA vesicle system changes from blue to red; this was used for the identification of the influenza H1N1 virus (Figure 4B) [105].
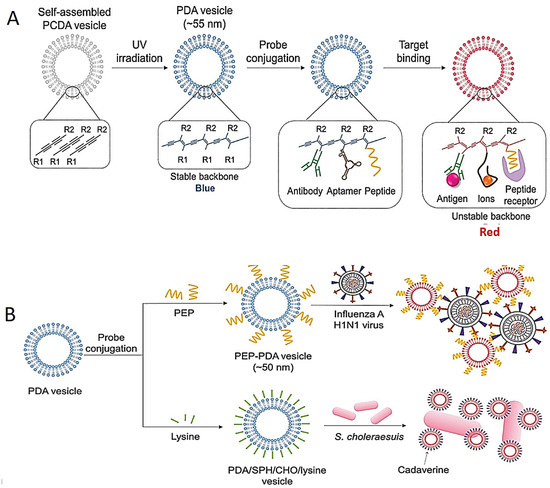
Figure 4.
Schematic images of colorimetric test based on destabilization of NP structures. (A) Image of color-changing of PDA structures by their structure transition on target interactions. (B) Schematic illustrations of identification of influenza H1N1 virus and Salmonella choleraesuis with PEP–PDA and PDA/SPH/CHO/lysine structure, respectively. Reprinted from Ref. [87].
In another study, the PDA/sphingomyelin (SPH)/cholesterol (CHO)/lysine structure was used as a colorimetric test for the identification of Salmonella choleraesuis in chicken (Figure 4B) [106]. Although highly sensitive detection could be achieved using the PDA vesicle in this study, long incubation time (almost 48 h) was needed.
5. Conclusions and Future Perspectives
This review presents recent studies on the identification of various pathogens by different types of colorimetric diagnostic tests. NPs with unique electrical, optical and catalytic features have been used for specific and sensitive detection of microorganisms. Nevertheless, there are still some challenges for NP-based colorimetric diagnostic tests in terms of stability, need of expertise and cost.
There is a high demand for the development of economical, rapid and sensitive colorimetric diagnostic tests. In particular, metals such as Ag, Au and Pt NPs have superior optical, catalytic and electrical properties but may not be economical enough for industrial production and large-scale applications [107,108]. In addition to that, controlling the size and shape of NPs is a crucial point for sensitivity and selectivity, because chemical, physical and biological properties including catalytic properties are directly dependent on the size and shape of NPs [67,85,86,109,110,111]. For instance, smaller-size Au NPs or Au NPs@GO NCs have high surface area and consequently show increase in peroxidase-like activities [86].
Various pH-indicator-based phenotypic and colorimetric tests have been developed for rapid, economical, sensitive and accurate detection of infection-causing microorganisms and antimicrobial resistance with the naked eye. While these colorimetric tests do not require any complicated and expensive devices, and even expert personnel to use, they are considered to be portable and point-of-care (POC) tests.
Author Contributions
C.C. as a first author and I.O. as a corresponding author provided predominant contributions in writing—original draft and review and editing, I.O. provided supervision, project administration and funding acquisition. G.K., Z.C. and N.I. made contributions in writing—original draft and review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This work is financially supported by grants awarded from the Erciyes University Scientific Research Office (TDK-2021 10943).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Global Burden of Foodborne Diseases, WHO, Geneva, Switzerland. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/199350/9789241565165_eng.pdf (accessed on 24 May 2023).
- WHO. Pandemic and Epidemic Diseases. Available online: https://openwho.org/courses/pandemic-epidemic-diseases (accessed on 19 May 2023).
- Lin, T.L.; Chang, P.H.; Chen, I.L.; Lai, W.H.; Chen, Y.J.; Li, W.F.; Wang, C.C. Risk factors and mortality associated with multi-drug-resistant Gram-negative bacterial infection in adult patients following abdominal surgery. J. Hosp. Infect. 2022, 119, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Santoso, P.; Sung, M.; Hartantri, Y.; Andriyoko, B.; Sugianli, A.K.; Alisjahbana, B.; Soeroto, A.Y. MDR pathogens organisms as risk factor of mortality in secondary pulmonary bacterial infections among COVID-19 patients: Observational studies in two referral hospitals in West Java, Indonesia. Int. J. Gen. Med. 2022, 15, 4741–4751. [Google Scholar] [CrossRef] [PubMed]
- Vidic, J.; Manzano, M.; Chang, C.M.; Jaffrezic-Renault, N. Advanced biosensors for detection of pathogens related to livestock and poultry. Vet. Res. 2017, 48, 11. [Google Scholar] [CrossRef] [PubMed]
- Lifson, M.A.; Ozen, M.O.; Inci, F.; Wang, S.; Inan, H.; Baday, M.; Demirci, U. Advances in biosensing strategies for HIV-1 detection, diagnosis, and therapeutic monitoring. Adv. Drug Deliv. Rev. 2016, 103, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; O’Garra, A.; Sher, A.; Wack, A. Host-directed immunotherapy of viral and bacterial infections: Past, present and future. Nat. Rev. Immunol. 2023, 23, 121–133. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Hao, Y.; Deng, D.; Xia, N. Nanomaterials-based colorimetric immunoassays. Nanomaterials 2019, 9, 316. [Google Scholar] [CrossRef]
- Su, L.; Jia, W.; Hou, C.; Lei, Y. Microbial biosensors: A review. Biosens. Bioelectron. 2011, 26, 1788–1799. [Google Scholar] [CrossRef]
- Váradi, L.; Luo, J.L.; Hibbs, D.E.; Perry, J.D.; Anderson, R.J.; Orenga, S.; Groundwater, P.W. Methods for the detection and identification of pathogenic bacteria: Past, present, and future. Chem. Soc. Rev. 2017, 46, 4818–4832. [Google Scholar] [CrossRef]
- Park, K.S. Nucleic acid aptamer-based methods for diagnosis of infections. Biosens. Bioelectron. 2018, 102, 179–188. [Google Scholar] [CrossRef]
- Suaifan, G.A.; Alhogail, S.; Zourob, M. Rapid and low-cost biosensor for the detection of Staphylococcus aureus. Biosens. Bioelectron. 2017, 90, 230–237. [Google Scholar] [CrossRef]
- Frei, A.; Verderosa, A.D.; Elliott, A.G.; Zuegg, J.; Blaskovich, M.A. Metals to combat antimicrobial resistance. Nat. Rev. Chem. 2023, 7, 202–224. [Google Scholar] [CrossRef] [PubMed]
- Trinh, K.T.L.; Chae, W.R.; Lee, N.Y. Recent advances in the fabrication strategies of paper-based microfluidic devices for rapid detection of bacteria and viruses. Microchem. J. 2022, 180, 107548. [Google Scholar] [CrossRef]
- Zamani, M.; Furst, A.L. Electricity, chemistry and biomarkers: An elegant and simple package: The potential of electrochemical biosensors for developing novel point-of-care diagnostics. EMBO Rep. 2022, 23, e55096. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Zhang, S.; Link, A.J.; McAlpine, M.C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 19207–19212. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, R.; Li, Y. Electrochemical biosensors for rapid detection of Escherichia coli O157: H7. Talanta 2017, 162, 511–522. [Google Scholar] [CrossRef]
- Ahn, J.H.; Choi, S.J.; Im, M.; Kim, S.; Kim, C.H.; Kim, J.Y.; Choi, Y.K. Charge and dielectric effects of biomolecules on electrical characteristics of nanowire FET biosensors. Appl. Phys. Lett. 2017, 111, 113701. [Google Scholar] [CrossRef]
- Yoo, S.M.; Baek, Y.K.; Shin, S.H.R.; Kim, J.H.; Jung, H.T.; Choi, Y.K.; Lee, S.Y. Single walled carbon nanotube-based electrical biosensor for the label-free detection of pathogenic bacteria. J. Nanosci. Nanotechnol. 2016, 16, 6520–6525. [Google Scholar] [CrossRef]
- Huh, Y.S.; Park, T.J.; Lee, E.Z.; Hong, W.H.; Lee, S.Y. Development of a fully integrated microfluidic system for sensing infectious viral disease. Electrophoresis 2008, 29, 2960–2969. [Google Scholar] [CrossRef] [PubMed]
- Yoo, S.M.; Keum, K.C.; Yoo, S.Y.; Choi, J.Y.; Chang, K.H.; Yoo, N.C.; Lee, S.Y. Development of DNA microarray forpathogen detection. Biotechnol. Bioprocess Eng. 2004, 9, 93–99. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, Y.; Jiang, K.; Wang, J.; White, W.L.; Yang, S.; Lu, J. Rapid detection of Listeria monocytogenes in food by biofunctionalized magnetic nanoparticle based on nuclear magnetic resonance. Food Control 2017, 71, 110–116. [Google Scholar] [CrossRef]
- Idil, N.; Hedström, M.; Denizli, A.; Mattiasson, B. Whole cell based microcontact imprinted capacitive biosensor for the detection of Escherichia coli. Biosens. Bioelectron. 2017, 87, 807–815. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing single molecules and single nanoparticles by surface-enhanced Raman scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Kim, D.K.; Park, T.J.; Lee, S.J.; Lee, S.Y. Label-free optical diagnosis of hepatitis B virus with genetically engineered fusion proteins. Talanta 2010, 82, 803–809. [Google Scholar] [CrossRef] [PubMed]
- Park, T.J.; Lee, S.J.; Kim, D.K.; Heo, N.S.; Park, J.Y.; Lee, S.Y. Development of label-free optical diagnosis for sensitive detection of influenza virus with genetically engineered fusion protein. Talanta 2012, 89, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Ajay Piriya, V.S.; Printo, J.; KirubaDaniel, S.G.G.; Susithra, L.; Takatoshi, K.; Sivakumar, M. Colorimetric sensors for rapid detection of various analytes. Mater. Sci. Eng. C 2017, 78, 1231–1245. [Google Scholar] [CrossRef]
- Sadek, M.; Bouvier, M.; Kerbol, A.; Poirel, L.; Nordmann, P. Evaluation of novel immunological rapid test (KNIVO Detection K-Set) for rapid detection of carbapenemase producers in multidrug-resistant gram negatives. Diagn. Microbiol. Infect. Dis. 2023, 104, 115761. [Google Scholar] [CrossRef]
- Campos-Madueno, E.I.; Moser, A.I.; Keller, P.M.; Perreten, V.; Poirel, L.; Nordmann, P.; Endimiani, A. Evaluation of Phenotypic Tests to Detect Extended-Spectrum β-Lactamase (ESBL)-Producing Klebsiella oxytoca Complex Strains. J. Clin. Microbiol. 2023, 61, e01706-22. [Google Scholar] [CrossRef]
- Nordmann, P.; Poirel, L. Development of Rapid Diagnostic Tests and Screening Culture Media by the NARA for Detection of Multidrug Resistance; NARA: Fribourg, Switzerland, 2022. [Google Scholar]
- Lee, Y.L.; Chen, H.M.; Hii, M.; Hsueh, P.R. Carbapenemase-producing Enterobacterales infections: Recent advances in diagnosis and treatment. Inter. J. Antimic. Agents 2022, 59, 106528. [Google Scholar] [CrossRef]
- Rao, M.R.; Chandrashaker, P.; Mahale, R.P.; Shivappa, S.G.; Gowda, R.S.; Chitharagi, V.B. Detection of carbapenemase production in Enterobacteriaceae and Pseudomonas species by carbapenemase Nordmann–Poirel test. J. Lab. Physicians 2019, 11, 107–110. [Google Scholar] [CrossRef]
- Zhong, H.; Wu, M.L.; Feng, W.J.; Huang, S.F.; Yang, P. Accuracy and applicability of different phenotypic methods for carbapenemase detection in Enterobacteriaceae: A systematic review and meta-analysis. J. Glob. Antimicrob. Resist. 2020, 21, 138–147. [Google Scholar] [CrossRef]
- Bernabeu, S.; Poirel, L.; Nordmann, P. Spectrophotometry-based detection of carbapenemase producers among Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2012, 74, 88–90. [Google Scholar] [CrossRef] [PubMed]
- Hrabák, J.; Walková, R.; Studentová, V.; Chudácková, E.; Bergerová, T. Carbapenemase activity detection by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J. Clin. Microbiol. 2011, 49, 3222–3227. [Google Scholar] [CrossRef] [PubMed]
- Kempf, M.; Bakour, S.; Flaudrops, C.; Berrazeg, M.; Brunel, J.M.; Drissi, M.; Mesli, E.; Touati, A.; Rolain, J.M. Rapid detection of carbapenem resistance in Acinetobacter baumannii using matrix-assisted laser desorption ionization-time of flight mass spectrometry. PLoS ONE 2012, 7, e31676. [Google Scholar] [CrossRef]
- Lee, W.; Chung, H.S.; Lee, Y.; Yong, D.; Jeong, S.H.; Lee, K.; Chong, Y. Comparison of matrix-assisted laser desorption ionization-time-of-flight mass spectrometry assay with conventional methods for detection of IMP-6, VIM-2, NDM-1, SIM-1, KPC-1, OXA-23, and OXA-51 carbapenemase-producing Acinetobacter spp., Pseudomonas aeruginosa, and Klebsiella pneumoniae. Diagn. Microbiol. Infect. Dis. 2013, 77, 227–230. [Google Scholar] [PubMed]
- Poirel, L.; Walsh, T.R.; Cuvillier, V.; Nordmann, P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn. Microbiol. Infect. Dis. 2011, 70, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Woodford, N.; Ellington, M.J.; Coelho, J.M.; Turton, J.F.; Ward, M.E.; Brown, S.; Amyes, S.G.; Livermore, D.M. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int. J. Antimicrob. Agents. 2006, 27, 351–353. [Google Scholar] [CrossRef]
- Hofko, M.; Mischnik, A.; Kaase, M.; Zimmermann, S.; Dalpke, A.H. Detection of carbapenemases by real-time PCR and melt curve analysis on the BD Max system. J. Clin. Microbiol. 2014, 52, 1701–1704. [Google Scholar] [CrossRef]
- Cuzon, G.; Naas, T.; Bogaerts, P.; Glupczynski, Y.; Nordmann, P. Evaluation of a DNA microarray for the rapid detection of extended-spectrum β-lactamases (TEM, SHV and CTX-M), plasmid-mediated cephalosporinases (CMY-2-like, DHA, FOX, ACC-1, ACT/MIR and CMY-1-like/MOX) and carbapenemases (KPC, OXA-48, VIM, IMP and NDM). J. Antimicrob. Chemother. 2012, 67, 1865–1869. [Google Scholar] [CrossRef]
- Dortet, L.; Bréchard, L.; Cuzon, G.; Poirel, L.; Nordmann, P. Strategy for rapid detection of carbapenemase-producing Enterobacteriaceae. Antimicrob. Agents Chemother. 2014, 58, 2441–2445. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing. EUCAST Guidelines for Detection of Resistance Mechanisms and Specific Resistances of Clinical and/or Epidemiological Importance. Version 2.0.2017. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Resistance_mechanisms/EUCAST_detection_of_resistance_mechanisms_170711.pdf (accessed on 24 May 2023).
- Centers for Disease Control and Prevention. Facility Guidance for Control of Carbapenem-Resistant Enterobacteriaceae—November 2015 Update. Available online: https://www.cdc.gov/hai/pdfs/cre/CRE-guidance-508.pdf (accessed on 24 May 2023).
- Nordmann, P.; Poirel, L.; Dortet, L. Rapid detection of carbapenemase-producing Enterobacteriaceae. Emerg. Infect. Dis. 2012, 18, 1503–1507. [Google Scholar] [CrossRef]
- Pires, J.; Novais, A.; Peixe, L. Blue-Carba, an easy biochemical test for detection of diverse carbapenemase producers directly from bacterial cultures. J. Clin. Microbiol. 2013, 51, 4281–4283. [Google Scholar] [CrossRef] [PubMed]
- Lutgring, J.D.; Limbago, B.M. The problem of carbapenemase-producing-carbapenem-resistant-Enterobacteriaceae detection. J. Clin. Microbiol. 2016, 54, 529–534. [Google Scholar] [CrossRef]
- Celik, C.; Ildiz, N.; Kaya, M.Z.; Kilic, A.B.; Ocsoy, I. Preparation of natural indicator incorporated media and its logical use as a colorimetric biosensor for rapid and sensitive detection of Methicillin-resistant Staphylococcus aureus. Anal. Chim. Acta 2020, 1128, 80–89. [Google Scholar] [CrossRef] [PubMed]
- Celik, C.; Ildiz, N.; Sagiroglu, P.; Atalay, M.A.; Yazici, C.; Ocsoy, I. Preparation of nature inspired indicator based agar for detection and identification of MRSA and MRSE. Talanta 2020, 219, 121292. [Google Scholar] [CrossRef]
- Celik, C.; Can Sezgin, G.; Kocabas, U.G.; Gursoy, S.; Ildiz, N.; Tan, W.; Ocsoy, I. Novel anthocyanin-based colorimetric assay for the rapid, sensitive, and quantitative detection of helicobacter pylori. Anal. Chem. 2021, 93, 6246–6253. [Google Scholar] [CrossRef] [PubMed]
- Rudresh, S.M.; Ravi, G.S.; Sunitha, L.; Hajira, S.N.; Kalaiarasan, E.; Harish, B.N. Simple, rapid, and cost-effective modified Carba NP test for carbapenemase detection among Gram-negative bacteria. J. Lab. Physicians 2017, 9, 303–307. [Google Scholar] [CrossRef]
- Shinde, S.; Gupta, R.; Raut, S.S.; Nataraj, G.; Mehta, P.R. Carba NP as a simpler, rapid, cost-effective, and a more sensitive alternative to other phenotypic tests for detection of carbapenem resistance in routine diagnostic laboratories. J. Lab. Physicians 2017, 9, 100–103. [Google Scholar] [CrossRef]
- Bir, R.; Mohapatra, S.; Kumar, A.; Tyagi, S.; Sood, S.; Das, B.K.; Kapil, A. Comparative evaluation of in-house Carba NP test with other phenotypic tests for rapid detection of carbapenem-resistant Enterobacteriaceae. J. Clin. Lab. 2019, 33, e22652. [Google Scholar] [CrossRef]
- Jamaludin, S.; Mustaffa, N.; Hamzah, N.A.C.; Aziz, S.H.S.A.; Lee, Y.Y. Diagnostic accuracy of reused ProntoDry® test and CLOtest® in the detection of Helicobacter pylori infection. BMC Gastroenterol. 2015, 15, 101. [Google Scholar] [CrossRef]
- Cagnoni, M.; Pagnini, C.; Crovaro, M.; Aucello, A.; Urgesi, R.; Pallotta, L.; Graziani, M.G. Evaluation of Accuracy and Feasibility of a New-Generation Ultra-Rapid Urease Test for Detection of Helicobacter pylori Infection. Gastroin. Disord. 2022, 4, 205–213. [Google Scholar] [CrossRef]
- Celik, C.; Demir, N.Y.; Duman, M.; Ildiz, N.; Ocsoy, I. Red cabbage extract-mediated colorimetric sensor for swift, sensitive and economic detection of urease-positive bacteria by naked eye and Smartphone platform. Sci. Rep. 2023, 13, 2056. [Google Scholar] [CrossRef] [PubMed]
- Altobelli, E.; Mohan, R.; Mach, K.E.; Sin, M.L.Y.; Anikst, V.; Buscarini, M.; Wong, P.K.; Gau, V.; Banaei, N.; Liao, J.C. Integrated biosensor assay for rapid uropathogen identification and phenotypic antimicrobial susceptibility testing. Eur. Urol. Focus 2017, 3, 293–299. [Google Scholar] [CrossRef]
- Kurup, R.; Leich, M. Comparison of urine analysis using manual method and sedimentation method. West Indian Med. J. 2012, 61, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Gan, S.D.; Patel, K.R. Enzyme immunoassay and enzyme-linked immunosorbent assay. J. Investig. Dermatol. 2013, 133, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Singh, P.; Mustapha, A. High-resolution melt curve PCR assay for specific detection of E. coli O157:H7 in beef. Food Control 2018, 86, 275–282. [Google Scholar] [CrossRef]
- Veron, L.; Mailler, S.; Girard, V.; Muller, B.H.; L’Hostis, G.; Ducruix, C.; Lesenne, A.; Richez, A.; Rostaing, H.; Lanet, V.; et al. Rapid urine preparation prior to identification of uropathogens by MALDI-TOF MS. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1787–1795. [Google Scholar] [CrossRef]
- Sauget, M.; Valot, B.; Bertrand, X.; Hocquet Can, D. MALDI-TOF mass spectrometry reasonably type bacteria? Trends Microbiol. 2017, 25, 447–455. [Google Scholar] [CrossRef]
- Ma, S.; Tang, Y.; Liu, J.; Wu, J. Visible paper chip immunoassay for rapid determination of bacteria in water distribution system. Talanta 2014, 120, 135–140. [Google Scholar] [CrossRef]
- Mo, M.; Yang, Y.; Zhang, F.; Jing, W.; Iriya, R.; Popovich, J.; Wang, S.; Grys, T.; Haydel, S.E.; Tao, N. Rapid antimicrobial susceptibility testing of patient urine samples using large volume free-solution light scattering microscopy. Anal. Chem. 2019, 91, 10164–10171. [Google Scholar] [CrossRef]
- Yuan, J.; Gaponik, N.; Eychmüller, A. Application of Polymer Quantum Dot Enzyme Hybrids in the Biosensor Development and Test Paper Fabrication. Anal. Chem. 2012, 84, 5047–5052. [Google Scholar] [CrossRef]
- Kelly, K.L.; Coronado, E.; Zhao, L.L.; Schatz, G.C. The optical properties of metal nanoparticles: The influence of size, shape, and dielectric environment. J. Phys. Chem. B 2003, 107, 668–677. [Google Scholar] [CrossRef]
- Ray, P.C. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem. Rev. 2010, 110, 5332–5365. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Qin, W. Applications of nanomaterials in potentiometric sensors. TrAC Trends Anal. Chem. 2013, 51, 79–86. [Google Scholar] [CrossRef]
- Rowland, C.E.; Brown, C.W., III; Delehanty, J.B.; Medintz, I.L. Nanomaterial-based sensors for the detection of biOlogical threat agents. Mater. Today 2016, 19, 464–477. [Google Scholar] [CrossRef]
- Mustafa, F.; Hassan, R.Y.; Andreescu, S. Multifunctional nanotechnology-enabled sensors for rapid capture and detection of pathogens. Sensors 2017, 17, 2121. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Oh, B.K. A rapid and sensitive immunoassay for detection of E. coli O157: H7 using multienzyme—Au nanoparticle complex. BioChip J. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Ren, J.; Miyoshi, D.; Sugimoto, N.; Qu, X. Label-free colorimetric and quantitative detection of cancer marker protein using noncrosslinking aggregation of Au/Ag nanoparticles induced by target-specific peptide probe. Biosens. Bioelectron. 2011, 26, 4804–4809. [Google Scholar] [CrossRef]
- Yang, H.H.; Zhang, S.Q.; Chen, X.L.; Zhuang, Z.X.; Xu, J.G.; Wang, X.R. Magnetite-containing spherical silica nanoparticles for biocatalysis and bioseparations. Anal. Chem. 2004, 76, 1316–1321. [Google Scholar] [CrossRef]
- Verma, M.S.; Rogowski, J.L.; Jones, L.; Gu, F.X. Colorimetric biosensing of pathogens using gold nanoparticles. Biotechnol. Adv. 2015, 33, 666–680. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Yan, X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Dong, Y.L.; Zhang, H.G.; Rahman, Z.U.; Su, L.; Chen, X.J.; Hu, J.; Chen, X.G. Graphene oxide–Fe 3 O 4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale 2012, 4, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Andler, S.M.; Goddard, J.M.; Nugen, S.R.; Rotello, V.M. Integrating recognition elements with nanomaterials for bacteria sensing. Chem. Soc. Rev. 2017, 46, 1272–1283. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.; Lee, S.; Ahn, M.M.; Kang, I.S.; Park, K.H.; Jeon, S. Colorimetric detection of pathogenic bacteria using platinum-coated magnetic nanoparticle clusters and magnetophoretic chromatography. Anal. Chim. Acta 2015, 883, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jeong, H.Y.; Kim, M.I.; Park, T.J. Colorimetric detection system for Salmonella typhimurium based on peroxidase-like activity of magnetic nanoparticles with DNA aptamers. J. Nanomater. 2015, 2015, 527126. [Google Scholar] [CrossRef]
- Park, K.S.; Kim, M.I.; Cho, D.Y.; Park, H.G. Label-free colorimetric detection of nucleic acids based on target-induced shielding against the peroxidase-mimicking activity of magnetic nanoparticles. Small 2011, 7, 1521–1525. [Google Scholar] [CrossRef]
- Woo, M.A.; Kim, M.I.; Jung, J.H.; Park, K.S.; Seo, T.S.; Park, H.G. A novel colorimetric immunoassay utilizing the peroxidase mimicking activity of magnetic nanoparticles. Int. J. Mol. Sci. 2013, 14, 9999–10014. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Julkapli, N.M.; Yehye, W.A.; Basirun, W.J.; Bhargava, S.K. Graphene–gold nanoparticles hybrid—Synthesis, functionalization, and application in a electrochemical and surface-enhanced raman scattering biosensor. Materials 2016, 9, 406. [Google Scholar] [CrossRef]
- González, V.J.; Martín-Alberca, C.; Montalvo, G.; García-Ruiz, C.; Baselga, J.; Terrones, M.; Martin, O. Carbon nanotube-Cu hybrids enhanced catalytic activity in aqueous media. Carbon 2014, 78, 10–18. [Google Scholar] [CrossRef]
- Guo, S.; Sun, S. FePt nanoparticles assembled on graphene as enhanced catalyst for oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 2492–2495. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Kim, J.; Suzuki, T.; Lee, J.; Park, E.Y. Enhanced catalytic activity of gold nanoparticle-carbon nanotube hybrids for influenza virus detection. Biosens. Bioelectron. 2016, 85, 503–508. [Google Scholar] [CrossRef]
- Ahmed, S.R.; Takemeura, K.; Li, T.C.; Kitamoto, N.; Tanaka, T.; Suzuki, T.; Park, E.Y. Size-controlled preparation of peroxidase-like graphene-gold nanoparticle hybrids for the visible detection of norovirus-like particles. Biosens. Bioelectron. 2017, 87, 558–565. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Hwang, J.H.; Lee, S.Y. Recent Trends in Nanomaterials-Based Colorimetric Detection of Pathogenic Bacteria and Viruses. Small Methods 2018, 2, 1700351. [Google Scholar] [CrossRef] [PubMed]
- Quinten, M.; Kreibig, U. Optical properties of aggregates of small metal particles. Surf. Sci. 1986, 172, 557–577. [Google Scholar] [CrossRef]
- Verdoodt, N.; Basso, C.R.; Rossi, B.F.; Pedrosa, V.A. Development of a rapid and sensitive immunosensor for the detection of bacteria. Food Chem. 2017, 221, 1792–1796. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Wei, W.; Zhao, H.; Zhou, Z.; Zhang, Y.; Liu, S. Colorimetric detection of influenza A virus using antibody-functionalized gold nanoparticles. Analyst 2015, 140, 3989–3995. [Google Scholar] [CrossRef]
- Qiu, S.; Lin, Z.; Zhou, Y.; Wang, D.; Yuan, L.; Wei, Y.; Chen, G. Highly selective colorimetric bacteria sensing based on protein-capped nanoparticles. Analyst 2015, 140, 1149–1154. [Google Scholar] [CrossRef] [PubMed]
- Bui, M.P.N.; Ahmed, S.; Abbas, A. Single-digit pathogen and attomolar detection with the naked eye using liposome-amplified plasmonic immunoassay. Nano Lett. 2015, 15, 6239–6246. [Google Scholar] [CrossRef]
- Veigas, B.; Pedrosa, P.; Carlos, F.F.; Mancio-Silva, L.; Grosso, A.R.; Fortunato, E.; Baptista, P.V. One nanoprobe, two pathogens: Gold nanoprobes multiplexing for point-of-care. J. Nanobiotechnol. 2015, 13, 48. [Google Scholar] [CrossRef]
- Shafiee, H.; Asghar, W.; Inci, F.; Yuksekkaya, M.; Jahangir, M.; Zhang, M.H.; Demirci, U. Paper and flexible substrates as materials for biosensing platforms to detect multiple biotargets. Sci. Rep. 2015, 5, 8719. [Google Scholar] [CrossRef]
- Li, H.; Rothberg, L.J. Label-free colorimetric detection of specific sequences in genomic DNA amplified by the polymerase chain reaction. J. Am. Chem. Soc. 2004, 126, 10958–10961. [Google Scholar] [CrossRef]
- Saleh, M.; El-Matbouli, M. Rapid detection of Cyprinid herpesvirus-3 (CyHV-3) using a gold nanoparticle-based hybridization assay. J. Virol. Methods 2015, 217, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Ke, C.; Humeniuk, M.; Hanna, S.; Marszalek, P.E. Direct measurements of base stacking interactions in DNA by single-molecule atomic-force spectroscopy. Phys. Rev. Lett. 2007, 99, 018302. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Song, L.; Zhou, N.; Xia, Y.; Wang, Z. A novel aptasensor for the colorimetric detection of S. typhimurium based on gold nanoparticles. Int. J. Food Microbiol. 2017, 245, 1–5. [Google Scholar] [CrossRef]
- Teengam, P.; Siangproh, W.; Tuantranont, A.; Vilaivan, T.; Chailapakul, O.; Henry, C.S. Multiplex paper-based colorimetric DNA sensor using pyrrolidinyl peptide nucleic acid-induced AgNPs aggregation for detecting MERS-CoV, MTB, and HPV oligonucleotides. Anal. Chem. 2017, 89, 5428–5435. [Google Scholar] [CrossRef]
- Martinez, A.W.; Phillips, S.T.; Butte, M.J.; Whitesides, G.M. Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew. Chem. 2007, 119, 1340–1342. [Google Scholar] [CrossRef]
- Cate, D.M.; Adkins, J.A.; Mettakoonpitak, J.; Henry, C.S. Recent developments in paper-based microfluidic devices. Anal. Chem. 2015, 87, 19–41. [Google Scholar] [CrossRef] [PubMed]
- Yetisen, A.K.; Akram, M.S.; Lowe, C.R. based microfluidic point-of-care diagnostic devices. Lab Chip 2013, 13, 2210–2251. [Google Scholar] [CrossRef]
- Li, D.; Dong, Y.; Li, B.; Wu, Y.; Wang, K.; Zhang, S. Colorimetric sensor array with unmodified noble metal nanoparticles for naked-eye detection of proteins and bacteria. Analyst 2015, 140, 7672–7677. [Google Scholar] [CrossRef]
- Jung, Y.K.; Kim, T.W.; Kim, J.; Kim, J.M.; Park, H.G. Universal colorimetric detection of nucleic acids based on polydiacetylene (PDA) liposomes. Adv. Func. Mater. 2008, 18, 701–708. [Google Scholar] [CrossRef]
- Song, S.; Ha, K.; Guk, K.; Hwang, S.G.; Choi, J.M.; Kang, T.; Lim, E.K. Colorimetric detection of influenza A (H1N1) virus by a peptide-functionalized polydiacetylene (PEP-PDA) nanosensor. RSC Adv. 2016, 6, 48566–48570. [Google Scholar] [CrossRef]
- De Oliveira, T.V.; de FF Soares, N.; de Andrade, N.J.; Silva, D.J.; Medeiros, E.A.A.; Badaró, A.T. Application of PCDA/SPH/CHO/Lysine vesicles to detect pathogenic bacteria in chicken. Food Chem. 2015, 172, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhao, G.; Dou, W. Blue silica nanoparticle-based colorimetric immunoassay for detection of Salmonella pullorum. Anal. Methods 2015, 7, 8647–8654. [Google Scholar] [CrossRef]
- Bagwe, R.P.; Yang, C.; Hilliard, L.R.; Tan, W. Optimization of dye-doped silica nanoparticles prepared using a reverse microemulsion method. Langmuir 2004, 20, 8336–8342. [Google Scholar] [CrossRef] [PubMed]
- An, K.; Somorjai, G.A. Size and shape control of metal nanoparticles for reaction selectivity in catalysis. Chem. Cat. Chem. 2012, 4, 1512–1524. [Google Scholar] [CrossRef]
- Xu, S.; Ouyang, W.; Xie, P.; Lin, Y.; Qiu, B.; Lin, Z.; Guo, L. Highly uniform gold nanobipyramids for ultrasensitive colorimetric detection of influenza virus. Anal. Chem. 2017, 89, 1617–1623. [Google Scholar] [CrossRef]
- Karakoti, A.S.; Shukla, R.; Shanker, R.; Singh, S. Surface functionalization of quantum dots for biological applications. Adv. Colloid İnterface Sci. 2015, 215, 28–45. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).