Characteristics of Rare Inherited Retinal Dystrophies in Adaptive Optics—A Study on 53 Eyes
Abstract
1. Introduction
1.1. Retinitis Pigmentosa
1.2. Characteristics of Stargardt Disease (STGD), Cone Dystrophy (CD), and Cone-Rod Dystrophy (CRD)
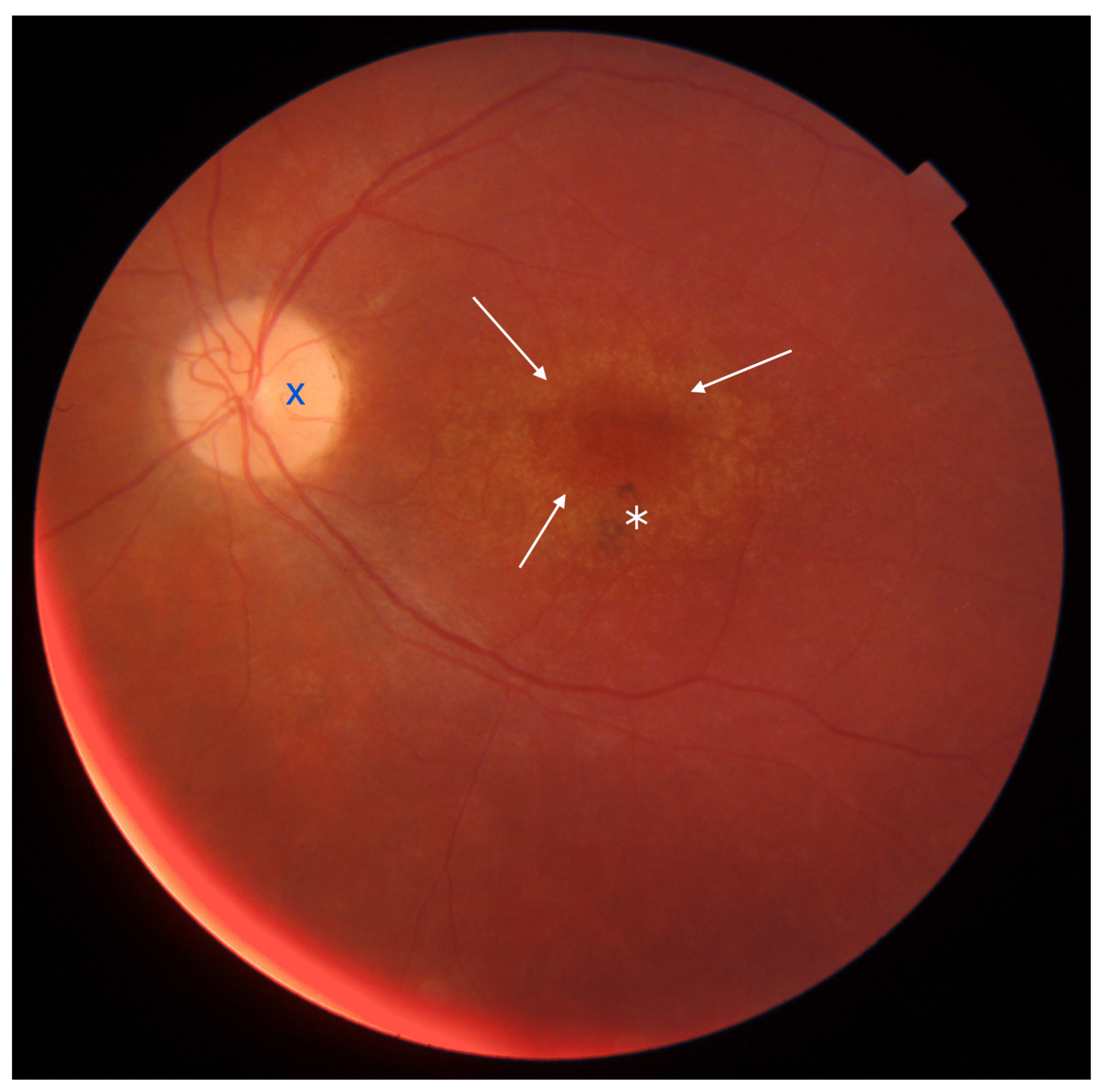
1.2.1. Stargardt Disease (STGD)
1.2.2. Cone-Rod Dystrophy and Cone Dystrophy
1.2.3. Adaptive Optics
1.2.4. Rtx1™
2. Materials and Methods
3. Outcomes
3.1. Differences in Cone Density (DM), Cone Spacing (SM), Cone Regularity (REG), and Voronoi Analysis () between the Study and Control Groups
3.2. Differences in DM, SM, REG, and between the Right Eyes of the Study Group and Controls
3.3. Differences in BCVA, DM, SM, and REG between Right and Left Eyes with IRDs
3.4. Differences in DM and SM among Eyes with CD, CRD, and STGD
3.5. Correlation between Photoreceptor Parameters and BCVA
3.6. Correlation between Photoreceptor Parameters and Age
3.7. Correlation of DM and SM with the Probability of Incomplete Data Acquisition
4. Discussion
4.1. Evaluation of Cones and Rods in IRDs
4.2. Early Diagnosis of IRDs
4.3. Potential for Future Advancement in Adaptive Optics
4.4. The Research Group
4.5. Longitudinal Observation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMD | Age-related macular degeneration |
| AO | Adaptive optics |
| AOFIO | Adaptive optics flood illuminated ophthalmoscope |
| AOSLO | Adaptive optics scanning laser ophthalmoscopy |
| CD | Cone dystrophy |
| CDSR | Cone dystrophy with supernormal rod electroretinogram |
| CRD | Cone-rod dystrophy |
| BCVA | Best-corrected visual acuity |
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of Open Access Journals |
| DM | Cone density [1/mm] |
| FAF | Fundus autofluorescence |
| FFA | Fluorescein angiography |
| HMM | High Magnification Module |
| IQR | Interquartile range |
| IRD | Inherited retinal dystrophy |
| LCA | Leber congenital amaurosis |
| mfERG | Multifocal electroretinography |
| Voronoi analysis of hexagonal cones [%] | |
| RCD | Rod-cone dystrophy |
| REG | Cone regularity [%] |
| RNFL | Retinal nerve fiber layer |
| RP | Retinitis pigmentosa |
| RPE | Retinal pigment epithelium |
| SD | Standard deviation |
| SD-OCT | Spectral-domain optical coherent tomography |
| SM | Cone spacing [m] |
| STGD | Stargardt disease |
| OR | Odds ratio |
References
- Chen, T.C.; Huang, D.S.; Lin, C.W.; Yang, C.H.; Yang, C.M.; Wang, V.Y.; Lin, J.W.; Luo, A.C.; Hu, F.R.; Chen, P.L. Genetic characteristics and epidemiology of inherited retinal degeneration in Taiwan. NPJ Genom. Med. 2021, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, S.D.; Regillo, C.D.; Lam, B.L.; Eliott, D.; Rosenfeld, P.J.; Gregori, N.Z.; Hubschman, J.P.; Davis, J.L.; Heilwell, G.; Spirn, M.; et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: Follow-up of two open-label phase 1/2 studies. Lancet 2015, 385, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.; Lee, M.J.; Choi, J.; Jung, S.Y.; Chong, S.Y.; Sung, J.H.; Shim, S.H.; Song, W.K. Long-term safety and tolerability of subretinal transplantation of embryonic stem cell-derived retinal pigment epithelium in Asian Stargardt disease patients. Br. J. Ophthalmol. 2021, 105, 829–837. [Google Scholar] [CrossRef]
- Lingam, S.; Liu, Z.; Yang, B.; Wong, W.; Parikh, B.H.; Ong, J.Y.; Goh, D.; Wong, D.S.L.; Tan, Q.S.W.; Tan, G.S.W.; et al. cGMP-grade human iPSC-derived retinal photoreceptor precursor cells rescue cone photoreceptor damage in non-human primates. Stem Cell Res. Ther. 2021, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Girach, A.; Audo, I.; Birch, D.G.; Huckfeldt, R.M.; Lam, B.L.; Leroy, B.P.; Michaelides, M.; Russell, S.R.; Sallum, J.M.F.; Stingl, K.; et al. RNA-based therapies in inherited retinal diseases. Ther. Adv. Ophthalmol. 2022, 14, 25158414221134602. [Google Scholar] [CrossRef]
- John, M.C.; Quinn, J.; Hu, M.L.; Cehajic-Kapetanovic, J.; Xue, K. Gene-agnostic therapeutic approaches for inherited retinal degenerations. Front. Mol. Neurosci. 2022, 15, 1068185. [Google Scholar] [CrossRef] [PubMed]
- Battu, R.; Ratra, D.; Gopal, L. Newer therapeutic options for inherited retinal diseases: Gene and cell replacement therapy. Indian J. Ophthalmol. 2022, 70, 2316–2325. [Google Scholar] [CrossRef]
- Olivares-González, L.; Velasco, S.; Campillo, I.; Rodrigo, R. Retinal Inflammation, Cell Death and Inherited Retinal Dystrophies. Int. J. Mol. Sci. 2021, 22, 2096. [Google Scholar] [CrossRef]
- Pagon, R.A. Retinitis pigmentosa. Surv. Ophthalmol. 1988, 33, 137–177. [Google Scholar] [CrossRef]
- Daiger, S.P.; Sullivan, L.S.; Bowne, S.J. Genes and mutations causing retinitis pigmentosa. Clin. Genet. 2013, 84, 132–141. [Google Scholar] [CrossRef]
- Hamel, C. Retinitis pigmentosa. Orphanet. J. Rare Dis. 2006, 1, 40. [Google Scholar] [CrossRef] [PubMed]
- Maguire, A.M.; Bennett, J.; Aleman, E.M.; Leroy, B.P.; Aleman, T.S. Clinical Perspective: Treating RPE65-Associated Retinal Dystrophy. Mol. Ther. 2021, 29, 442–463. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Edwards, T.L.; O’Hare, F.; Hickey, D.G.; Wang, J.H.; Liu, Z.; Ayton, L.N. Gene therapy for inherited retinal diseases: Progress and possibilities. Clin. Exp. Optom. 2021, 104, 444–454. [Google Scholar] [CrossRef]
- Foote, K.G.; De la Huerta, I.; Gustafson, K.; Baldwin, A.; Zayit-Soudry, S.; Rinella, N.; Porco, T.C.; Roorda, A.; Duncan, J.L. Cone Spacing Correlates With Retinal Thickness and Microperimetry in Patients With Inherited Retinal Degenerations. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1234–1243. [Google Scholar] [CrossRef]
- Vasireddy, V.; Wong, P.; Ayyagari, R. Genetics and molecular pathology of Stargardt-like macular degeneration. Prog. Retin. Eye Res. 2010, 29, 191–207. [Google Scholar] [CrossRef] [PubMed]
- Tanna, P.; Strauss, R.W.; Fujinami, K.; Michaelides, M. Stargardt disease: Clinical features, molecular genetics, animal models and therapeutic options. Br. J. Ophthalmol. 2017, 101, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Ścieżyńska, A.; Oziębło, D.; Ambroziak, A.M.; Korwin, M.; Szulborski, K.; Krawczyński, M.; Stawiński, P.; Szaflik, J.; Szaflik, J.P.; Płoski, R.; et al. Next-generation sequencing of ABCA4: High frequency of complex alleles and novel mutations in patients with retinal dystrophies from Central Europe. Exp. Eye Res. 2016, 145, 93–99. [Google Scholar] [CrossRef]
- Tanna, P.; Kasilian, M.; Strauss, R.; Tee, J.; Kalitzeos, A.; Tarima, S.; Visotcky, A.; Dubra, A.; Carroll, J.; Michaelides, M. Reliability and Repeatability of Cone Density Measurements in Patients With Stargardt Disease and RPGR-Associated Retinopathy. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3608–3615. [Google Scholar] [CrossRef]
- Kong, X.; Fujinami, K.; Strauss, R.W.; Munoz, B.; West, S.K.; Cideciyan, A.V.; Michaelides, M.; Ahmed, M.; Ervin, A.M.; Schönbach, E.; et al. Visual Acuity Change Over 24 Months and Its Association With Foveal Phenotype and Genotype in Individuals With Stargardt Disease: ProgStar Study Report No. 10. JAMA Ophthalmol. 2018, 136, 920–928. [Google Scholar] [CrossRef]
- Schönbach, E.M.; Strauss, R.W.; Ibrahim, M.A.; Janes, J.L.; Birch, D.G.; Cideciyan, A.V.; Sunness, J.S.; Muñoz, B.; Ip, M.S.; Sadda, S.R.; et al. Faster Sensitivity Loss around Dense Scotomas than for Overall Macular Sensitivity in Stargardt Disease: ProgStar Report No. 14. Am. J. Ophthalmol. 2020, 216, 219–225. [Google Scholar] [CrossRef]
- Tanna, P.; Georgiou, M.; Strauss, R.W.; Ali, N.; Kumaran, N.; Kalitzeos, A.; Fujinami, K.; Michaelides, M. Cross-Sectional and Longitudinal Assessment of the Ellipsoid Zone in Childhood-Onset Stargardt Disease. Transl. Vis. Sci. Technol. 2019, 8, 1. [Google Scholar] [CrossRef]
- Strauss, R.W.; Muñoz, B.; Ho, A.; Jha, A.; Michaelides, M.; Cideciyan, A.V.; Audo, I.; Birch, D.G.; Hariri, A.H.; Nittala, M.G.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence in the Retrospective Progression of Stargardt Disease Study (ProgStar Report No. 9). JAMA Ophthalmol. 2017, 135, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Strauss, R.W.; Kong, X.; Ho, A.; Jha, A.; West, S.; Ip, M.; Bernstein, P.S.; Birch, D.G.; Cideciyan, A.V.; Michaelides, M.; et al. Progression of Stargardt Disease as Determined by Fundus Autofluorescence Over a 12-Month Period: ProgStar Report No. 11. JAMA Ophthalmol. 2019, 137, 1134–1145. [Google Scholar] [CrossRef] [PubMed]
- Fujinami, K.; Lois, N.; Mukherjee, R.; McBain, V.A.; Tsunoda, K.; Tsubota, K.; Stone, E.M.; Fitzke, F.W.; Bunce, C.; Moore, A.T.; et al. A longitudinal study of Stargardt disease: Quantitative assessment of fundus autofluorescence, progression, and genotype correlations. Investig. Ophthalmol. Vis. Sci. 2013, 54, 8181–8190. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ratnam, K.; Sundquist, S.M.; Lujan, B.; Ayyagari, R.; Gudiseva, V.H.; Roorda, A.; Duncan, J.L. Cone photoreceptor abnormalities correlate with vision loss in patients with Stargardt disease. Investig. Ophthalmol. Vis. Sci. 2011, 52, 3281–3292. [Google Scholar] [CrossRef]
- Song, H.; Rossi, E.A.; Latchney, L.; Bessette, A.; Stone, E.; Hunter, J.J.; Williams, D.R.; Chung, M. Cone and rod loss in Stargardt disease revealed by adaptive optics scanning light ophthalmoscopy. JAMA Ophthalmol. 2015, 133, 1198–1203. [Google Scholar] [CrossRef]
- Scoles, D.; Sulai, Y.N.; Langlo, C.S.; Fishman, G.A.; Curcio, C.A.; Carroll, J.; Dubra, A. In vivo imaging of human cone photoreceptor inner segments. Investig. Ophthalmol. Vis. Sci. 2014, 55, 4244–4251. [Google Scholar] [CrossRef]
- Song, H.; Latchney, L.; Williams, D.; Chung, M. Fluorescence adaptive optics scanning laser ophthalmoscope for detection of reduced cones and hypoautofluorescent spots in fundus albipunctatus. JAMA Ophthalmol. 2014, 132, 1099–1104. [Google Scholar] [CrossRef]
- Al-Khuzaei, S.; Shah, M.; Foster, C.R.; Yu, J.; Broadgate, S.; Halford, S.; Downes, S.M. The role of multimodal imaging and vision function testing in ABCA4-related retinopathies and their relevance to future therapeutic interventions. Ther. Adv. Ophthalmol. 2021, 13, 25158414211056384. [Google Scholar] [CrossRef]
- Song, H.; Rossi, E.A.; Yang, Q.; Granger, C.E.; Latchney, L.R.; Chung, M.M. High-Resolution Adaptive Optics in Vivo Autofluorescence Imaging in Stargardt Disease. JAMA Ophthalmol. 2019, 137, 603–609. [Google Scholar] [CrossRef]
- Vincent, A.; Wright, T.; Garcia-Sanchez, Y.; Kisilak, M.; Campbell, M.; Westall, C.; Héon, E. Phenotypic characteristics including in vivo cone photoreceptor mosaic in KCNV2-related “cone dystrophy with supernormal rod electroretinogram”. Investig. Ophthalmol. Vis. Sci. 2013, 54, 898–908. [Google Scholar] [CrossRef]
- Gill, J.S.; Georgiou, M.; Kalitzeos, A.; Moore, A.T.; Michaelides, M. Progressive cone and cone-rod dystrophies: Clinical features, molecular genetics and prospects for therapy. Br. J. Ophthalmol. 2019, 103, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Thiadens, A.A.H.J.; Phan, T.M.L.; Zekveld-Vroon, R.C.; Leroy, B.P.; van den Born, L.I.; Hoyng, C.B.; Klaver, C.C.W.; Roosing, S.; Pott, J.W.R.; van Schooneveld, M.J.; et al. Clinical course, genetic etiology, and visual outcome in cone and cone-rod dystrophy. Ophthalmology 2012, 119, 819–826. [Google Scholar] [CrossRef] [PubMed]
- Wolfing, J.I.; Chung, M.; Carroll, J.; Roorda, A.; Williams, D.R. High-resolution retinal imaging of cone-rod dystrophy. Ophthalmology 2006, 113, 1019.e1. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Doble, N.; Hardy, J.L.; Jones, S.M.; Keltner, J.L.; Olivier, S.S.; Werner, J.S. In vivo imaging of the photoreceptor mosaic in retinal dystrophies and correlations with visual function. Investig. Ophthalmol. Vis. Sci. 2006, 47, 2080–2092. [Google Scholar] [CrossRef]
- Roorda, A.; Zhang, Y.; Duncan, J.L. High-resolution in vivo imaging of the RPE mosaic in eyes with retinal disease. Investig. Ophthalmol. Vis. Sci. 2007, 48, 2297–2303. [Google Scholar] [CrossRef]
- Duncan, J.L.; Zhang, Y.; Gandhi, J.; Nakanishi, C.; Othman, M.; Branham, K.E.H.; Swaroop, A.; Roorda, A. High-resolution imaging with adaptive optics in patients with inherited retinal degeneration. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3283–3291. [Google Scholar] [CrossRef]
- Song, H.; Rossi, E.A.; Stone, E.; Latchney, L.; Williams, D.; Dubra, A.; Chung, M. Phenotypic diversity in autosomal-dominant cone-rod dystrophy elucidated by adaptive optics retinal imaging. Br. J. Ophthalmol. 2018, 102, 136–141. [Google Scholar] [CrossRef]
- Bensinger, E.; Rinella, N.; Saud, A.; Loumou, P.; Ratnam, K.; Griffin, S.; Qin, J.; Porco, T.C.; Roorda, A.; Duncan, J.L. Loss of Foveal Cone Structure Precedes Loss of Visual Acuity in Patients With Rod-Cone Degeneration. Investig. Ophthalmol. Vis. Sci. 2019, 60, 3187–3196. [Google Scholar] [CrossRef]
- Samelska, K.; Kupis, M.; Zaleska-Żmijewska, A.; Szaflik, J.P. Adaptive optics imaging in the most common inherited retinal degenerations. Klin. Ocz. 2021, 123, 74–79. [Google Scholar] [CrossRef]
- Gill, J.S.; Moosajee, M.; Dubis, A.M. Cellular imaging of inherited retinal diseases using adaptive optics. Eye 2019, 33, 1683–1698. [Google Scholar] [CrossRef]
- Dreher, A.W.; Bille, J.F.; Weinreb, R.N. Active optical depth resolution improvement of the laser tomographic scanner. Appl. Opt. 1989, 28, 804–808. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Williams, D.R.; Miller, D.T. Supernormal vision and high-resolution retinal imaging through adaptive optics. J. Opt. Soc. Am. A Opt. Image Sci. Vis. 1997, 14, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, M.; Serrao, S.; Devaney, N.; Parravano, M.; Lombardo, G. Adaptive optics technology for high-resolution retinal imaging. Sensors 2012, 13, 334–366. [Google Scholar] [CrossRef] [PubMed]
- Davidson, B.; Kalitzeos, A.; Carroll, J.; Dubra, A.; Ourselin, S.; Michaelides, M.; Bergeles, C. Automatic Cone Photoreceptor Localisation in Healthy and Stargardt Afflicted Retinas Using Deep Learning. Sci. Rep. 2018, 8, 7911. [Google Scholar] [CrossRef]
- Zaleska-Żmijewska, A.; Wawrzyniak, Z.M.; Ulińska, M.; Szaflik, J.; Dąbrowska, A.; Szaflik, J.P. Human photoreceptor cone density measured with adaptive optics technology (rtx1 device) in healthy eyes: Standardization of measurements. Medicine 2017, 96, e7300. [Google Scholar] [CrossRef]
- Polans, J.; Keller, B.; Carrasco-Zevallos, O.M.; LaRocca, F.; Cole, E.; Whitson, H.E.; Lad, E.M.; Farsiu, S.; Izatt, J.A. Wide-field retinal optical coherence tomography with wavefront sensorless adaptive optics for enhanced imaging of targeted regions. Biomed. Opt. Express 2017, 8, 16–37. [Google Scholar] [CrossRef] [PubMed]
- Wynne, N.; Heitkotter, H.; Woertz, E.N.; Cooper, R.F.; Carroll, J. Comparison of Cone Mosaic Metrics From Images Acquired With the SPECTRALIS High Magnification Module and Adaptive Optics Scanning Light Ophthalmoscopy. Transl. Vis. Sci. Technol. 2022, 11, 19. [Google Scholar] [CrossRef]
- Zaleska-Żmijewska, A.; Wawrzyniak, Z.M.; Dąbrowska, A.; Szaflik, J.P. Adaptive Optics (rtx1) High-Resolution Imaging of Photoreceptors and Retinal Arteries in Patients with Diabetic Retinopathy. J. Diabetes Res. 2019, 2019, 9548324. [Google Scholar] [CrossRef]
- Zaleska-Żmijewska, A.; Piątkiewicz, P.; Śmigielska, B.; Sokołowska-Oracz, A.; Wawrzyniak, Z.M.; Romaniuk, D.; Szaflik, J.; Szaflik, J.P. Retinal Photoreceptors and Microvascular Changes in Prediabetes Measured with Adaptive Optics (rtx1™): A Case-Control Study. J. Diabetes Res. 2017, 2017, 4174292. [Google Scholar] [CrossRef]
- Rosenbaum, D.; Mattina, A.; Koch, E.; Rossant, F.; Gallo, A.; Kachenoura, N.; Paques, M.; Redheuil, A.; Girerd, X. Effects of age, blood pressure and antihypertensive treatments on retinal arterioles remodeling assessed by adaptive optics. J. Hypertens. 2016, 34, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Meixner, E.; Michelson, G. Measurement of retinal wall-to-lumen ratio by adaptive optics retinal camera: A clinical research. Graefes Arch. Clin. Exp. Ophthalmol. 2015, 253, 1985–1995. [Google Scholar] [CrossRef] [PubMed]
- Zaleska-Żmijewska, A.; Wawrzyniak, Z.; Kupis, M.; Szaflik, J.P. The Relation between Body Mass Index and Retinal Photoreceptor Morphology and Microvascular Changes Measured with Adaptive Optics (rtx1) High-Resolution Imaging. J. Ophthalmol. 2021, 2021, 6642059. [Google Scholar] [CrossRef]
- Boretsky, A.; Khan, F.; Burnett, G.; Hammer, D.X.; Ferguson, R.D.; van Kuijk, F.; Motamedi, M. In vivo imaging of photoreceptor disruption associated with age-related macular degeneration: A pilot study. Lasers Surg. Med. 2012, 44, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Ratnam, K.; Carroll, J.; Porco, T.C.; Duncan, J.L.; Roorda, A. Relationship between foveal cone structure and clinical measures of visual function in patients with inherited retinal degenerations. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5836–5847. [Google Scholar] [CrossRef]
- Muthiah, M.N.; Gias, C.; Chen, F.K.; Zhong, J.; McClelland, Z.; Sallo, F.B.; Peto, T.; Coffey, P.J.; da Cruz, L. Cone photoreceptor definition on adaptive optics retinal imaging. Br. J. Ophthalmol. 2014, 98, 1073–1079. [Google Scholar] [CrossRef]
- Sahel, J.A.; Grieve, K.; Pagot, C.; Authié, C.; Mohand-Said, S.; Paques, M.; Audo, I.; Becker, K.; Chaumet-Riffaud, A.E.; Azoulay, L.; et al. Assessing Photoreceptor Status in Retinal Dystrophies: From High-Resolution Imaging to Functional Vision. Am. J. Ophthalmol. 2021, 230, 12–47. [Google Scholar] [CrossRef] [PubMed]
- Palejwala, N.V.; Gale, M.J.; Clark, R.F.; Schlechter, C.; Weleber, R.G.; Pennesi, M.E. Insights into autosomal dominant stargardt-like macular dystrophy through multimodality diagnostic imaging. Retina 2016, 36, 119–130. [Google Scholar] [CrossRef]
- Ito, N.; Kameya, S.; Gocho, K.; Hayashi, T.; Kikuchi, S.; Katagiri, S.; Gekka, T.; Yamaki, K.; Takahashi, H.; Tsuneoka, H. Multimodal imaging of a case of peripheral cone dystrophy. Doc. Ophthalmol. 2015, 130, 241–251. [Google Scholar] [CrossRef][Green Version]
- Kubota, D.; Gocho, K.; Kikuchi, S.; Akeo, K.; Miura, M.; Yamaki, K.; Takahashi, H.; Kameya, S. CEP250 mutations associated with mild cone-rod dystrophy and sensorineural hearing loss in a Japanese family. Ophthalmic Genet. 2018, 39, 500–507. [Google Scholar] [CrossRef]
- Morgan, J.I.W.; Chui, T.Y.P.; Grieve, K. Twenty-five years of clinical applications using adaptive optics ophthalmoscopy [Invited]. Biomed. Opt. Express 2023, 14, 387–428. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Roorda, A.; Duncan, J.L. Advances in imaging of Stargardt disease. Adv. Exp. Med. Biol. 2010, 664, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Razeen, M.M.; Cooper, R.F.; Langlo, C.S.; Goldberg, M.R.; Wilk, M.A.; Han, D.P.; Connor, T.B.J.; Fishman, G.A.; Collison, F.T.; Sulai, Y.N.; et al. Correlating Photoreceptor Mosaic Structure to Clinical Findings in Stargardt Disease. Transl. Vis. Sci. Technol. 2016, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- Gale, M.J.; Harman, G.A.; Chen, J.; Pennesi, M.E. Repeatability of Adaptive Optics Automated Cone Measurements in Subjects With Retinitis Pigmentosa and Novel Metrics for Assessment of Image Quality. Transl. Vis. Sci. Technol. 2019, 8, 17. [Google Scholar] [CrossRef]
- Bergeles, C.; Dubis, A.M.; Davidson, B.; Kasilian, M.; Kalitzeos, A.; Carroll, J.; Dubra, A.; Michaelides, M.; Ourselin, S. Unsupervised identification of cone photoreceptors in non-confocal adaptive optics scanning light ophthalmoscope images. Biomed. Opt. Express 2017, 8, 3081–3094. [Google Scholar] [CrossRef]
- Esengönül, M.; Marta, A.; Beirão, J.; Pires, I.M.; Cunha, A. A Systematic Review of Artificial Intelligence Applications Used for Inherited Retinal Disease Management. Medicina 2022, 58, 504. [Google Scholar] [CrossRef]
- Williams, D.R.; Burns, S.A.; Miller, D.T.; Roorda, A. Evolution of adaptive optics retinal imaging [Invited]. Biomed. Opt. Express 2023, 14, 1307–1338. [Google Scholar] [CrossRef]
- Tuten, W.S.; Vergilio, G.K.; Young, G.J.; Bennett, J.; Maguire, A.M.; Aleman, T.S.; Brainard, D.H.; Morgan, J.I.W. Visual Function at the Atrophic Border in Choroideremia Assessed with Adaptive Optics Microperimetry. Ophthalmol. Retin. 2019, 3, 888–899. [Google Scholar] [CrossRef]
- Roshandel, D.; Heath Jeffery, R.C.; Charng, J.; Sampson, D.M.; McLenachan, S.; Mackey, D.A.; Chen, F.K. Short-Term Parafoveal Cone Loss Despite Preserved Ellipsoid Zone in Rod Cone Dystrophy. Transl. Vis. Sci. Technol. 2021, 10, 11. [Google Scholar] [CrossRef]
- Ziccardi, L.; Giannini, D.; Lombardo, G.; Serrao, S.; Dell’Omo, R.; Nicoletti, A.; Bertelli, M.; Lombardo, M. Multimodal Approach to Monitoring and Investigating Cone Structure and Function in an Inherited Macular Dystrophy. Am. J. Ophthalmol. 2015, 160, 301–312. [Google Scholar] [CrossRef]
- Potic, J.; Bergin, C.; Giacuzzo, C.; Daruich, A.; Pournaras, J.A.; Kowalczuk, L.; Behar-Cohen, F.; Konstantinidis, L.; Wolfensberger, T.J. Changes in visual acuity and photoreceptor density using adaptive optics after retinal detachment repair. Retina 2020, 40, 376–386. [Google Scholar] [CrossRef] [PubMed]








| Study Group () | Control Group () | |
|---|---|---|
| Age | ||
| Mean (SD) | ||
| Median (IQR) | 44 (35–54) | 47.5 (42.25–55.25) |
| Range | 19–73 | 31–59 |
| Sex | ||
| Female | ||
| Male | ||
| Diagnosis | ||
| CD | - | |
| CRD | - | |
| STGD | - | |
| Eye | ||
| Right | 28 | 14 |
| Left | 25 | 0 |
| BCVA | ||
| Mean (SD) | ||
| Median (IQR) | 0.07 (0.05–0.16) | |
| Range | 0.01–0.7 |
| Study Group () | Control Group () | p-Value (U Mann–Whitney) | |
|---|---|---|---|
| DM | |||
| Mean (SD) | 10,111.33 (3198.77) | 25,656.42 (2132.93) | |
| Median (IQR) | 10,228.25 (7943.67–12,341.25) | 24,961.54 (24,046.79–27,320.94) | |
| Range | 3830–16,341.25 | 22,977.75–29,455.25 | |
| SM | |||
| Mean (SD) | |||
| Median (IQR) | 10.91 (9.92–12.24) | 7 (6.68–7.13) | |
| Range | 8.59–35.08 | 6.42–7.3 | |
| REG | |||
| Mean (SD) | |||
| Median (IQR) | 86.09 (80.81–88.96) | 91.25 (89.64–92.18) | |
| Range | 48.28–96.77 | 87.81–94.07 | |
| Mean (SD) | |||
| Median (IQR) | 43.5 (40.5–48) | 48.88 (48.18–49.58) | |
| Range | 27.65–73.75 | 41.8–53.27 |
| Study Group () | Control Group () | p-Value (U Mann–Whitney) | |
|---|---|---|---|
| Mean DM [1/mm] (SD) | |||
| DM_T | 10,893.92 (6038.18) | 26,729.98 (2058.61) | |
| DM_N | 25,585.69 (2153.57) | ||
| DM_S | 25,386.9 (2768.69) | ||
| DM_I | 10,159.14 (4408.24) | 24,923.12 (3023.91) | |
| Mean SM m] (SD) | |||
| SM_T | |||
| SM_N | |||
| SM_S | |||
| SM_I | |||
| Mean REG [%] (SD) | |||
| REG_T | |||
| REG_N | |||
| REG_S | |||
| REG_I | |||
| Mean [%] (SD) | |||
| _T | |||
| _N | |||
| _S | |||
| _I |
| Study Group () | Control Group () | p-Value (U Mann–Whitney) | |
|---|---|---|---|
| DM | 10,154.52 (3641.81) | 25,656.42 (2132.93) | |
| SM | |||
| REG | |||
| Right Eye () | Left Eye () | p-Value (Test) | |
|---|---|---|---|
| BCVA | |||
| Mean (SD) | (t-test) | ||
| Median (IQR) | 0.06 (0.04–0.2) | 0.05 (0.04–0.12) | |
| Range | 0.01–0.8 | 0.01–0.8 | |
| DM | |||
| Mean (SD) | (t-test) | ||
| Median (IQR) | 9396.5 (8420.12–12,993.88) | 10,480.5 (6807–12,074.25) | |
| Range | 3830–15,499.88 | 4584.33–16,341.25 | |
| SM | |||
| Mean (SD) | (Wilcoxon) | ||
| Median (IQR) | 11.31 (9.91–12.11) | 10.53 (10.06–13.21) | |
| Range | 8.85–35.08 | 8.59–21.18 | |
| REG | |||
| Mean (SD) | (Wilcoxon) | ||
| Median (IQR) | 85.66 (78.39–88.39) | 86.17 (84.48–88.96) | |
| Range | 60.66–96.77 | 66.67–95.84 |
| CD () | CRD () | STGD () | p-Value (Kruskal–Wallis) | |
|---|---|---|---|---|
| DM | 16,209.66 (8024.64) | |||
| SM | ||||
| REG | ||||
| CD () | CRD () | STGD () | p-Value (Kruskal–Wallis) | |
|---|---|---|---|---|
| REG_T | ||||
| Mean (SD) | 0.91 (0.05) | 0.89 (0.05) | 0.76 (0.2) | |
| Median (IQR) | 0.92 (0.86–0.95) | 0.91 (0.88–0.92) | 0.85 (0.75–0.88) | |
| Range | 0.84–0.96 | 0.82–0.94 | 0.33–0.94 | |
| REG_N | 0.953 | |||
| Mean (SD) | 0.85 (0.12) | 0.86 (0.04) | 0.87 (0.08) | |
| Median (IQR) | 0.85 (0.84–0.87) | 0.87 (0.85–0.89) | 0.85 (0.81–0.92) | |
| Range | 0.67–1 | 0.8–0.89 | 0.75–1 | |
| REG_S | 0.681 | |||
| Mean (SD) | 0.87 (0.07) | 0.8 (0.14) | 0.81 (0.14) | |
| Median (IQR) | 0.87 (0.86–0.89) | 0.85 (0.76–0.89) | 0.87 (0.74–0.88) | |
| Range | 0.77–0.96 | 0.6–0.9 | 0.5–1 | |
| REG_I | 0.511 | |||
| Mean (SD) | 0.87 (0.04) | 0.87 (0.03) | 0.85 (0.07) | |
| Median (IQR) | 0.89 (0.86–0.89) | 0.88 (0.86–0.89) | 0.85 (0.81–0.88) | |
| Range | 0.8–0.9 | 0.82–0.9 | 0.71–1 |
| Correlation Coefficient (r) | p-Value | |
|---|---|---|
| DM | ||
| SM | ||
| REG | ||
| Correlation Coefficient (r) | p-Value | |
|---|---|---|
| Right eyes | ||
| DM | ||
| SM | ||
| REG | ||
| Left eyes | ||
| DM | ||
| SM | ||
| REG | ||
| Correlation Coefficient (r) | p-Value | |
|---|---|---|
| Study group | ||
| DM | ||
| SM | ||
| REG | ||
| Control group | ||
| DM | ||
| SM | ||
| REG | ||
| Incomplete Data () | Complete Data () | p-Value (Test) | ||
|---|---|---|---|---|
| Mean age (SD) | ||||
| (t-test) | ||||
| Sex | ||||
| Male | ||||
| Female | (chi-squared) | |||
| Diagnosis | ||||
| CD | ||||
| CRD | (Fisher) | |||
| STGD | ||||
| BCVA | ||||
| Mean (SD) | 0.1 (0.12) | 0.15 (0.19) | 0.309 | |
| Median (IQR) | 0.05 (0.04–0.11) | 0.1 (0.04–0.2) | (U Mann–Whitney) | |
| Range | 0.01–0.4 | 0.01–0.8 | ||
| DM | ||||
| Mean (SD) | 9667.5 (3092.92) | 10,673.83 (3263.13) | 0.284 | |
| Median (IQR) | 9180 (8083–10,990.75) | 10,228.25 (8593–13,400.5) | (U Mann–Whitney) | |
| Range | 5292.75–15,499.88 | 3830–15,499.88 | ||
| SM | ||||
| Mean (SD) | 13.28 (5.93) | 11.99 (4.67) | 0.103 | |
| Median (IQR) | 11.39 (10.91–13.89) | 10.91 (9.76–11.99) | (U Mann–Whitney) | |
| Range | 9.04–35.08 | 8.85–35.08 | ||
| REG | ||||
| Mean (SD) | 84.19 (7.3) | 82.82 (7.59) | 0.889 | |
| Median (IQR) | 85.66 (77.46–88.31) | 85.66 (79.05–88.47) | (U Mann–Whitney) | |
| Range | 72.81–96.77 | 60.66–91.31 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samelska, K.; Szaflik, J.P.; Guszkowska, M.; Kurowska, A.K.; Zaleska-Żmijewska, A. Characteristics of Rare Inherited Retinal Dystrophies in Adaptive Optics—A Study on 53 Eyes. Diagnostics 2023, 13, 2472. https://doi.org/10.3390/diagnostics13152472
Samelska K, Szaflik JP, Guszkowska M, Kurowska AK, Zaleska-Żmijewska A. Characteristics of Rare Inherited Retinal Dystrophies in Adaptive Optics—A Study on 53 Eyes. Diagnostics. 2023; 13(15):2472. https://doi.org/10.3390/diagnostics13152472
Chicago/Turabian StyleSamelska, Katarzyna, Jacek Paweł Szaflik, Maria Guszkowska, Anna Katarzyna Kurowska, and Anna Zaleska-Żmijewska. 2023. "Characteristics of Rare Inherited Retinal Dystrophies in Adaptive Optics—A Study on 53 Eyes" Diagnostics 13, no. 15: 2472. https://doi.org/10.3390/diagnostics13152472
APA StyleSamelska, K., Szaflik, J. P., Guszkowska, M., Kurowska, A. K., & Zaleska-Żmijewska, A. (2023). Characteristics of Rare Inherited Retinal Dystrophies in Adaptive Optics—A Study on 53 Eyes. Diagnostics, 13(15), 2472. https://doi.org/10.3390/diagnostics13152472







