Effect of Low Tube Voltage (100 kV) Combined with ASIR-V on the Visualization and Image Quality of the Adamkiewicz Artery: A Comparison with 120 kV Protocol
Abstract
:1. Introduction
2. Materials and Methods
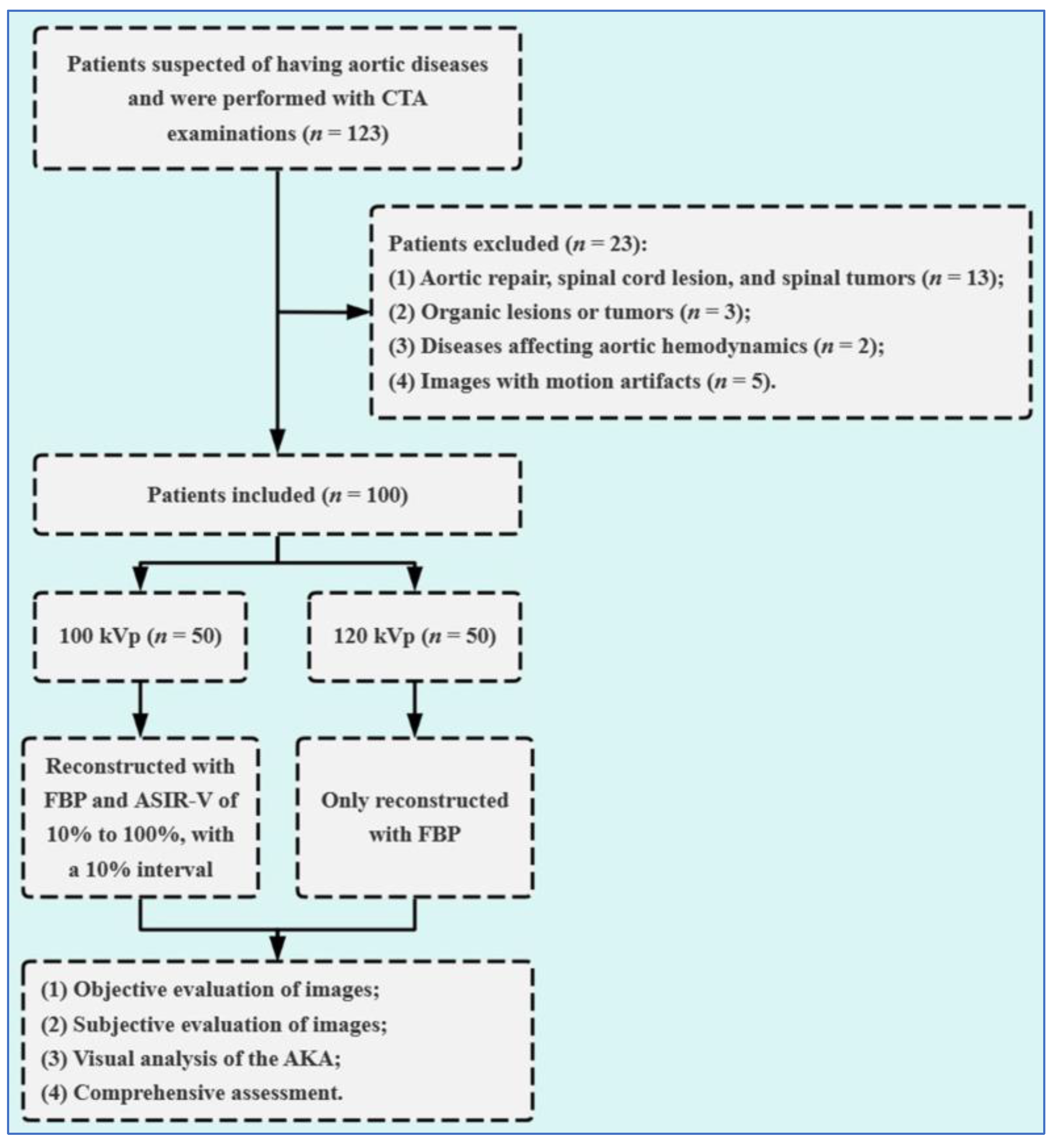
2.1. Study Population
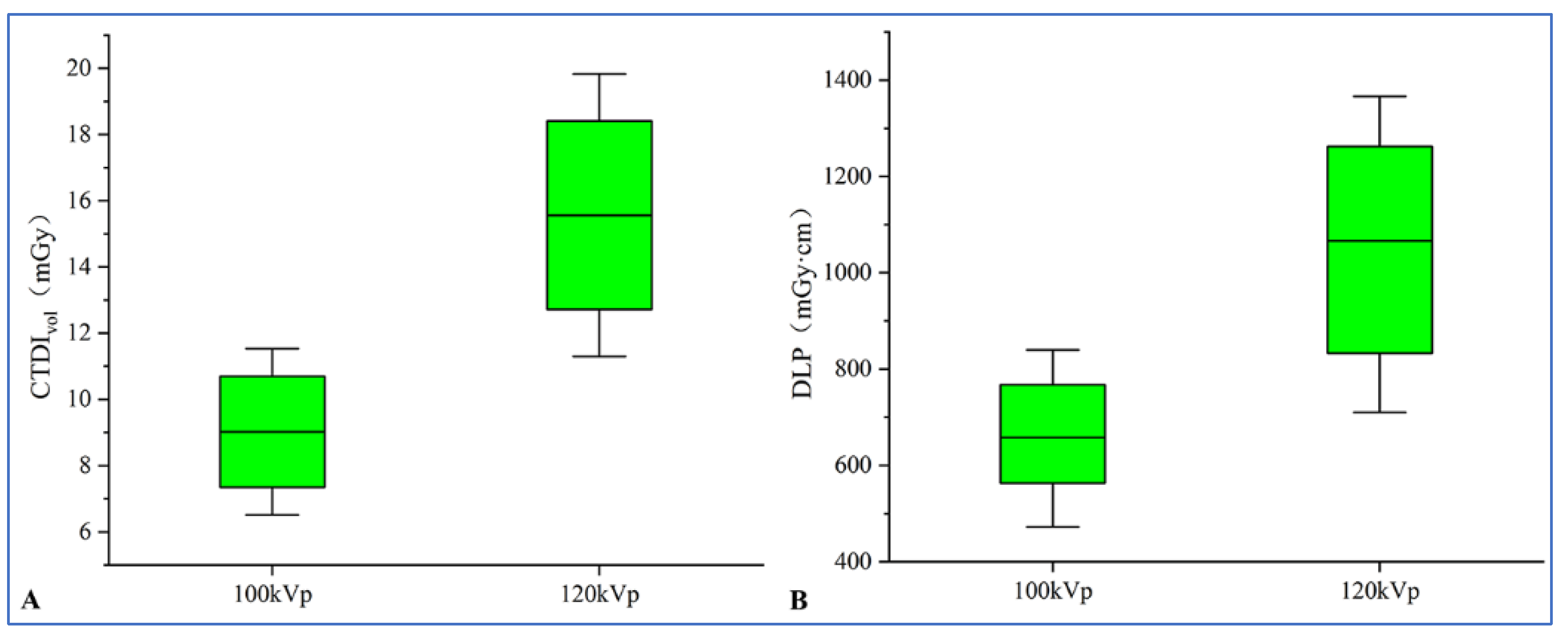
2.2. Data Acquisition
2.3. Image Reconstruction
2.4. Objective Image Analysis
2.5. Subjective Scoring of Image Quality
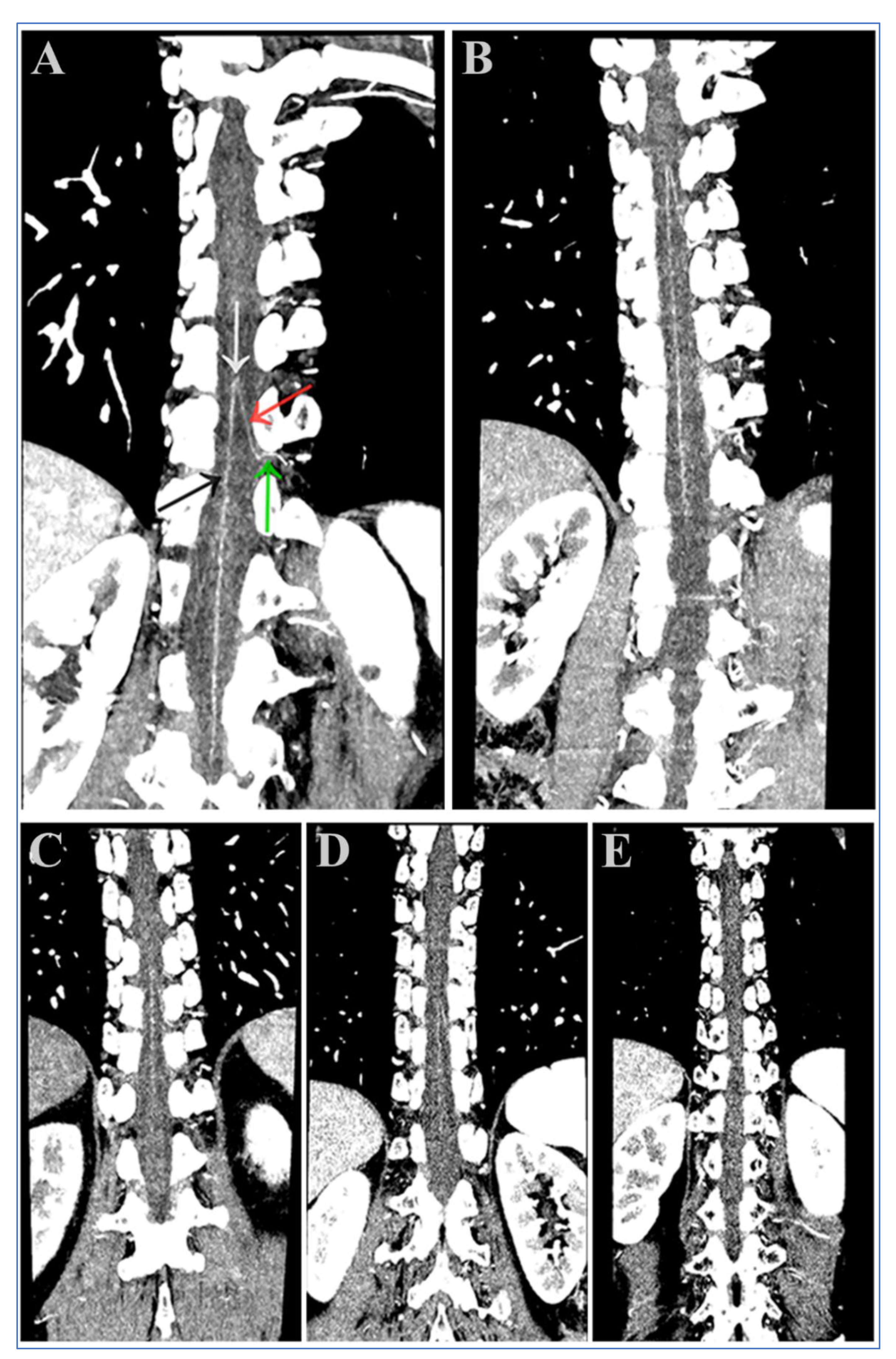
2.6. Visual Assessment of the AKA
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics of Patients
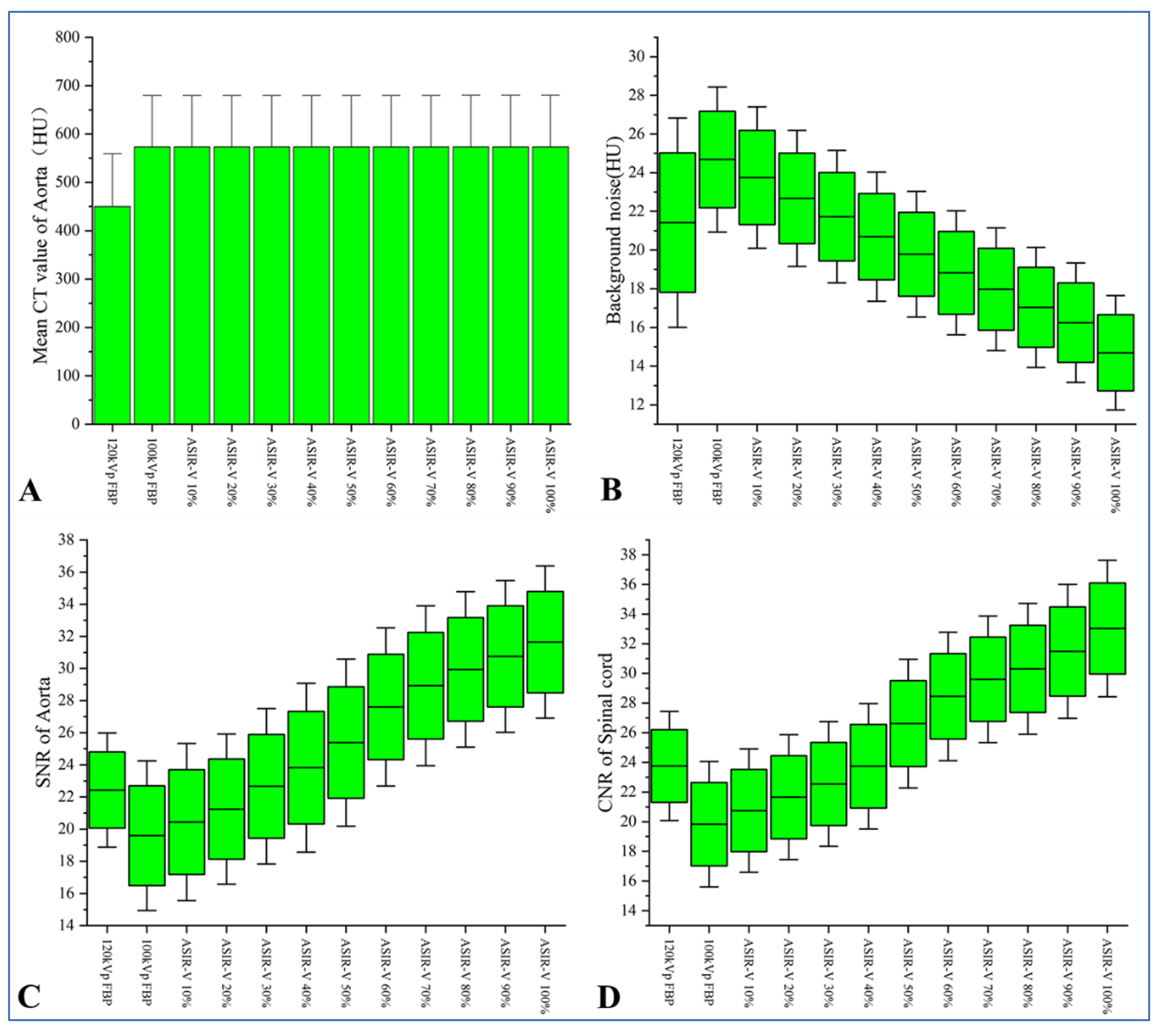
3.2. Objective Evaluation of Images
3.3. Subjective Evaluation of Images
3.4. Visual Analysis of the AKA
3.5. Comprehensive Assessment
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Melissano, G.; Bertoglio, L.; Rinaldi, E.; Leopardi, M.; Chiesa, R. An anatomical review of spinal cord blood supply. J. Cardiovasc. Surg. 2015, 56, 699–706. [Google Scholar]
- Alleyne, C.H., Jr.; Cawley, C.M.; Shengelaia, G.G.; Barrow, D.L. Microsurgical anatomy of the artery of Adamkiewicz and its segmental artery. J. Neurosurg. 1998, 89, 791–795. [Google Scholar] [CrossRef]
- Taterra, D.; Skinningsrud, B.; Pękala, P.A.; Hsieh, W.C.; Cirocchi, R.; Walocha, J.A.; Tubbs, R.S.; Tomaszewski, K.A.; Henry, B.M. Artery of Adamkiewicz: A meta-analysis of anatomical characteristics. Neuroradiology 2019, 61, 869–880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudo, K.; Terae, S.; Asano, T.; Oka, M.; Kaneko, K.; Ushikoshi, S.; Miyasaka, K. Anterior spinal artery and artery of Adamkiewicz detected by using multi-detector row CT. AJNR. Am. J. Neuroradiol. 2003, 24, 13–17. [Google Scholar] [PubMed]
- Yoshioka, K.; Niinuma, H.; Ohira, A.; Nasu, K.; Kawakami, T.; Sasaki, M.; Kawazoe, K. MR angiography and CT angiography of the artery of Adamkiewicz: Noninvasive preoperative assessment of thoracoabdominal aortic aneurysm. Radiographics 2003, 23, 1215–1225. [Google Scholar] [CrossRef]
- Nojiri, J.; Matsumoto, K.; Kato, A.; Miho, T.; Furukawa, K.; Ohtsubo, S.; Itoh, T.; Kudo, S. The Adamkiewicz artery: Demonstration by intra-arterial computed tomographic angiography. Eur. J. Cardiothorac. Surg. 2007, 31, 249–255. [Google Scholar] [CrossRef]
- Kawaharada, N.; Morishita, K.; Fukada, J.; Yamada, A.; Muraki, S.; Hyodoh, H.; Abe, T. Thoracoabdominal or descending aortic aneurysm repair after preoperative demonstration of the Adamkiewicz artery by magnetic resonance angiography. Eur. J. Cardiothorac. Surg. 2002, 21, 970–974. [Google Scholar] [CrossRef] [Green Version]
- Ogino, H.; Sasaki, H.; Minatoya, K.; Matsuda, H.; Yamada, N.; Kitamura, S. Combined use of adamkiewicz artery demonstration and motor-evoked potentials in descending and thoracoabdominal repair. Ann. Thorac. Surg. 2006, 82, 592–596. [Google Scholar] [CrossRef]
- Tuleasca, C.; Herbrecht, A.; Knafo, S.; Aghakhani, N.; Parker, F. The relevance of the artery of Adamkiewicz for microsurgical resection of spinal tumors-short overview and case series: Technical note. Neurochirurgie 2020, 66, 378–382. [Google Scholar] [CrossRef]
- Bley, T.A.; Duffek, C.C.; François, C.J.; Schiebler, M.L.; Acher, C.W.; Mell, M.; Grist, T.M.; Reeder, S.B. Presurgical localization of the artery of Adamkiewicz with time-resolved 3.0-T MR angiography. Radiology 2010, 255, 873–881. [Google Scholar] [CrossRef] [Green Version]
- Mizushima, S.; Mine, T.; Abe, M.; Sekine, T.; Fujii, M.; Hayashi, H.; Ikeda, S.; Happoh, S.; Takashi, Y.; Kumita, S.I. Comparison of Slow-Infusion Magnetic Resonance Angiography with Sequential K-Space Filling and Computed Tomography Angiography to Detect the Adamkiewicz Artery. Ann. Vasc. Surg. 2023. [Google Scholar] [CrossRef] [PubMed]
- Shimoyama, S.; Nishii, T.; Watanabe, Y.; Kono, A.K.; Kagawa, K.; Takahashi, S.; Sugimura, K. Advantages of 70-kV CT Angiography for the Visualization of the Adamkiewicz Artery: Comparison with 120-kV Imaging. AJNR. Am. J. Neuroradiol. 2017, 38, 2399–2405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishii, T.; Kotoku, A.; Hori, Y.; Matsuzaki, Y.; Watanabe, Y.; Morita, Y.; Kono, A.K.; Matsuda, H.; Fukuda, T. Filtered back projection revisited in low-kilovolt computed tomography angiography: Sharp filter kernel enhances visualization of the artery of Adamkiewicz. Neuroradiology 2019, 61, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Tanaka, R.; Takagi, H.; Ueyama, Y.; Sugawara, T.; Chiba, T.; Arakita, K.; Schuijf, J.D. Systematic evaluation of collateral pathways to the artery of Adamkiewicz using computed tomography. Eur. J. Cardiothorac. Surg. 2018, 54, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Piola, F.P.F.; Nogueira-Barbosa, M.H.; Maranho, D.A.C.; Martins, A.A.; Barbosa, M.F.; Herrero, C.F.P.S. Identification of the Artery of Adamkiewicz Using Multidetector Computed Tomography Angiography (MCTA). Rev. Bras. Ortop. 2020, 55, 70–74. [Google Scholar]
- Hino, T.; Kamitani, T.; Sagiyama, K.; Yamasaki, Y.; Matsuura, Y.; Tsutsui, S.; Sakai, Y.; Furuyama, T.; Yabuuchi, H. Detectability of the artery of Adamkiewicz on computed tomography angiography of the aorta by using ultra-high-resolution computed tomography. Jpn. J. Radiol. 2020, 38, 658–665. [Google Scholar] [CrossRef]
- Kubota, Y.; Yokota, H.; Mukai, H.; Horikoshi, T.; Ota, J.; Okafuji, Y.; Ueda, H.; Matsumiya, G.; Uno, T. Low-tube-voltage CT assessment of Adamkiewicz artery: Precise comparison between 100-kVp- and 120-kVp protocols. Eur. J. Radiol. 2019, 111, 56–61. [Google Scholar] [CrossRef]
- Jiang, J.M.; Miao, L.; Liang, X.; Liu, Z.H.; Zhang, L.; Li, M. The Value of Deep Learning Image Reconstruction in Improving the Quality of Low-Dose Chest CT Images. Diagnostics 2022, 12, 2560. [Google Scholar] [CrossRef]
- Goodenberger, M.H.; Wagner-Bartak, N.A.; Gupta, S.; Liu, X.; Yap, R.Q.; Sun, J.; Tamm, E.P.; Jensen, C.T. Computed Tomography Image Quality Evaluation of a New Iterative Reconstruction Algorithm in the Abdomen (Adaptive Statistical Iterative Reconstruction-V) a Comparison With Model-Based Iterative Reconstruction, Adaptive Statistical Iterative Reconstruction, and Filtered Back Projection Reconstructions. J. Comput. Assist. Tomogr. 2018, 42, 184–190. [Google Scholar]
- Chen, C.W.; Chen, P.A.; Chou, C.C.; Fu, J.H.; Wang, P.C.; Hsu, S.H.; Lai, P.H. Combination of Adaptive Statistical Iterative Reconstruction-V and Lower Tube Voltage During Craniocervical Computed Tomographic Angiography Yields Better Image Quality with a Reduced Radiation Dose. Acad. Radiol. 2019, 26, e233–e240. [Google Scholar] [CrossRef]
- Li, W.; Diao, K.; Wen, Y.; Shuai, T.; You, Y.; Zhao, J.; Liao, K.; Lu, C.; Yu, J.; He, Y.; et al. High-strength deep learning image reconstruction in coronary CT angiography at 70-kVp tube voltage significantly improves image quality and reduces both radiation and contrast doses. Eur. Radiol. 2022, 32, 2912–2920. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Hu, Z.; Li, D.; Liu, Z.; Wei, D.; Jia, Y.; Yu, N.; Yu, Y.; Lei, Y.; et al. Reducing Radiation Dose and Improving Image Quality in CT Portal Venography Using 80 kV and Adaptive Statistical Iterative Reconstruction-V in Slender Patients. Acad. Radiol. 2020, 27, 233–243. [Google Scholar] [CrossRef]
- Han, W.K.; Na, J.C.; Park, S.Y. Low-dose CT angiography using ASiR-V for potential living renal donors: A prospective analysis of image quality and diagnostic accuracy. Eur. Radiol. 2020, 30, 798–805. [Google Scholar] [CrossRef]
- Nijenhuis, R.J.; Jacobs, M.J.; Jaspers, K.; Reinders, M.; van Engelshoven, J.M.; Leiner, T.; Backes, W.H. Comparison of magnetic resonance with computed tomography angiography for preoperative localization of the Adamkiewicz artery in thoracoabdominal aortic aneurysm patients. J. Vasc. Surg. 2007, 45, 677–685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, J.; Kitagawa, K.; Nagata, M.; Yamazaki, A.; Nagasawa, N.; Sakuma, H. Model-based iterative reconstruction for multi-detector row CT assessment of the Adamkiewicz artery. Radiology 2014, 270, 282–291. [Google Scholar] [CrossRef] [PubMed]
- Yin, X.R.; Xia, W.; Yao, Z.W.; He, H.J.; Feng, X.Y. The Initial Exploration of Adamkiewicz Artery Computed Tomographic Angiography With Monochromatic Reconstruction of Gemstone Spectral Imaging. J. Comput. Assist. Tomogr. 2016, 40, 820–826. [Google Scholar] [CrossRef]
- Nakayama, Y.; Awai, K.; Yanaga, Y.; Nakaura, T.; Funama, Y.; Hirai, T.; Yamashita, Y. Optimal contrast medium injection protocols for the depiction of the Adamkiewicz artery using 64-detector CT angiography. Clin. Radiol. 2008, 63, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, K.; Tanaka, R.; Takagi, H.; Ueyama, Y.; Kikuchi, K.; Chiba, T.; Arakita, K.; Schuijf, J.D.; Saito, Y. Ultra-high-resolution CT angiography of the artery of Adamkiewicz: A feasibility study. Neuroradiology 2018, 60, 109–115. [Google Scholar] [CrossRef]
- Utsunomiya, D.; Yamashita, Y.; Okumura, S.; Urata, J. Demonstration of the Adamkiewicz artery in patients with descending or thoracoabdominal aortic aneurysm: Optimization of contrast-medium application for 64-detector-row CT angiography. Eur. Radiol. 2008, 18, 2684–2690. [Google Scholar] [CrossRef]
- Amako, M.; Yamamoto, Y.; Nakamura, K.; Tobinaga, S.; Nakamura, E.; Hosokawa, Y.; Ohno, T.; Akashi, H.; Aoyagi, S.; Tanaka, H. Preoperative visualization of the artery of Adamkiewicz by dual-phase CT angiography in patients with aortic aneurysm. Kurume. Med. J. 2011, 58, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Tanabe, Y.; Kido, T.; Kurata, A.; Kouchi, T.; Hosokawa, T.; Nishiyama, H.; Kawaguchi, N.; Kido, T.; Uetani, T.; Mochizuki, T. Impact of Knowledge-Based Iterative Model Reconstruction on Image Quality and Hemodynamic Parameters in Dynamic Myocardial Computed Tomography Perfusion Using Low-Tube-Voltage Scan: A Feasibility Study. J. Comput. Assist. Tomogr. 2019, 43, 811–816. [Google Scholar] [PubMed]
- Hou, P.; Feng, X.; Liu, J.; Wang, X.; Jiang, Y.; Dong, L.; Gao, J. Low Tube Voltage and Iterative Model Reconstruction in Follow-up CT Angiography After Thoracic Endovascular Aortic Repair: Ultra-low Radiation Exposure and Contrast Medium Dose. Acad. Radiol. 2018, 25, 494–501. [Google Scholar] [PubMed]
- Wei, L.; Li, S.; Gao, Q.; Liu, Y.; Ma, X. Use of low tube voltage and low contrast agent concentration yields good image quality for aortic CT angiography. Clin. Radiol. 2016, 71, e1–e6. [Google Scholar] [CrossRef]
- Chen, L.H.; Jin, C.; Li, J.Y.; Wang, G.L.; Jia, Y.J.; Duan, H.F.; Pan, N.; Guo, J. Image quality comparison of two adaptive statistical iterative reconstruction (ASiR.; ASiR-V) algorithms and filtered back projection in routine liver CT. Br. J. Radiol. 2018, 91, 20170655. [Google Scholar] [CrossRef]
- Ren, Z.; Zhang, X.; Hu, Z.; Li, D.; Liu, Z.; Wei, D.; Jia, Y.; Yu, N.; Yu, Y.; Lei, Y.; et al. Application of Adaptive Statistical Iterative Reconstruction-V With Combination of 80 kV for Reducing Radiation Dose and Improving Image Quality in Renal Computed Tomography Angiography for Slim Patients. Acad. Radiol. 2019, 26, e324–e332. [Google Scholar] [CrossRef] [PubMed]
- Benz, D.C.; Gräni, C.; Mikulicic, F.; Vontobel, J.; Fuchs, T.A.; Possner, M.; Clerc, O.F.; Stehli, J.; Gaemperli, O.; Pazhenkottil, A.P.; et al. Adaptive Statistical Iterative Reconstruction-V: Impact on Image Quality in Ultralow-Dose Coronary Computed Tomography Angiography. J. Comput. Assist. Tomogr. 2016, 40, 958–963. [Google Scholar] [CrossRef] [Green Version]
- Machida, H.; Tanaka, I.; Fukui, R.; Kita, K.; Shen, Y.; Ueno, E.; Suzuki, S. Improved delineation of the anterior spinal artery with model-based iterative reconstruction in CT angiography: A clinical pilot study. AJR. Am. J. Roentgenol. 2013, 200, 442–446. [Google Scholar] [CrossRef]
- Gatti, M.; Marchisio, F.; Fronda, M.; Rampado, O.; Faletti, R.; Bergamasco, L.; Ropolo, R.; Fonio, P. Adaptive Statistical Iterative Reconstruction-V Versus Adaptive Statistical Iterative Reconstruction: Impact on Dose Reduction and Image Quality in Body Computed Tomography. J. Comput. Assist. Tomogr. 2018, 42, 191–196. [Google Scholar] [CrossRef]
- Lee, S.; Kwon, H.; Cho, J. The Detection of Focal Liver Lesions Using Abdominal CT: A Comparison of Image Quality Between Adaptive Statistical Iterative Reconstruction V and Adaptive Statistical Iterative Reconstruction. Acad. Radiol. 2016, 23, 1532–1538. [Google Scholar]
- Halama, D.; Merkel, H.; Werdehausen, R.; Gaber, K.; Schob, S.; Quäschling, U.; Ziganshyna, S.; Hoffmann, K.T.; Lindner, D.; Richter, C. Reference Values of Cerebral Artery Diameters of the Anterior Circulation by Digital Subtraction Angiography: A Retrospective Study. Diagnostics 2022, 12, 2471. [Google Scholar] [CrossRef]

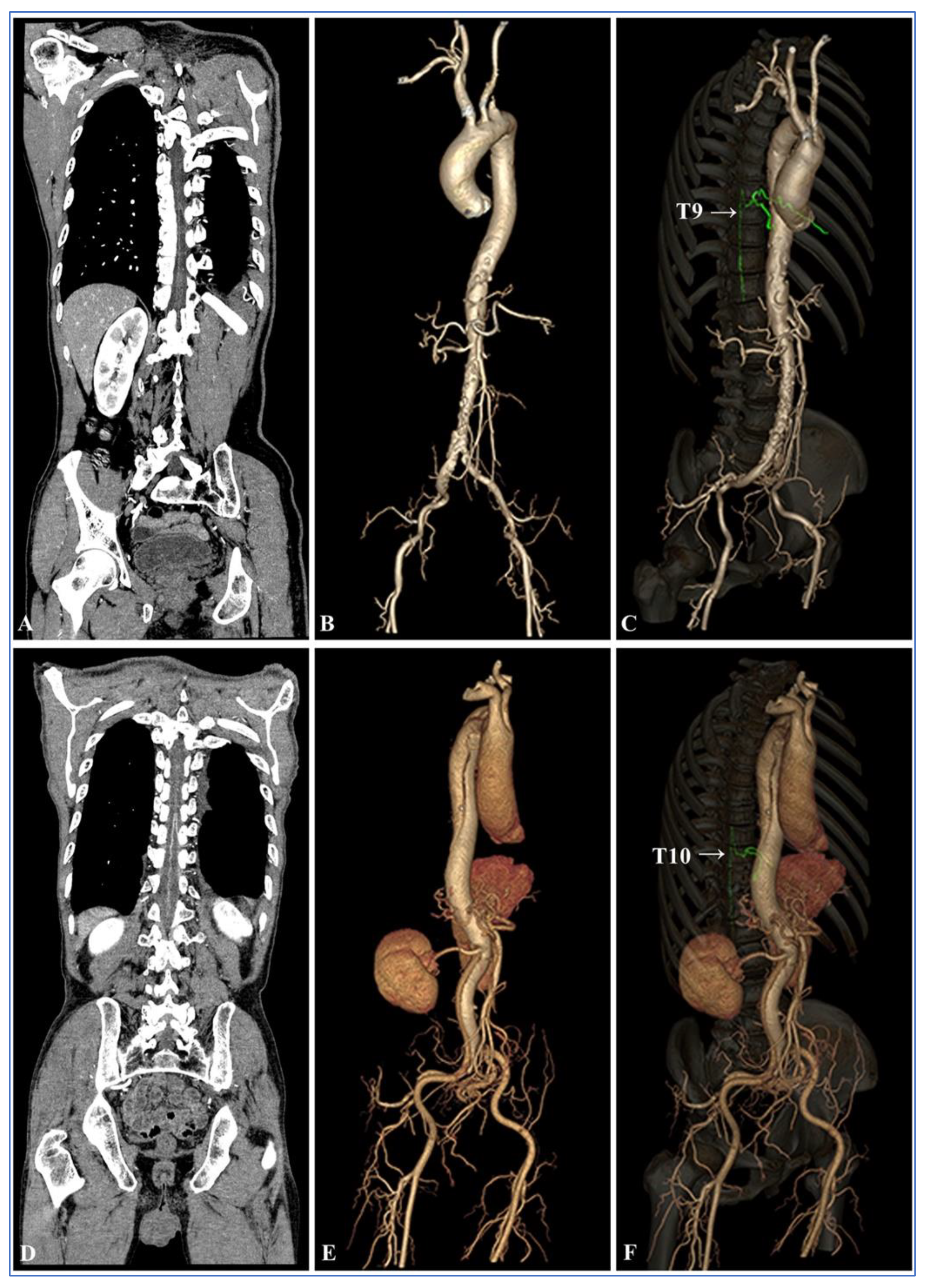


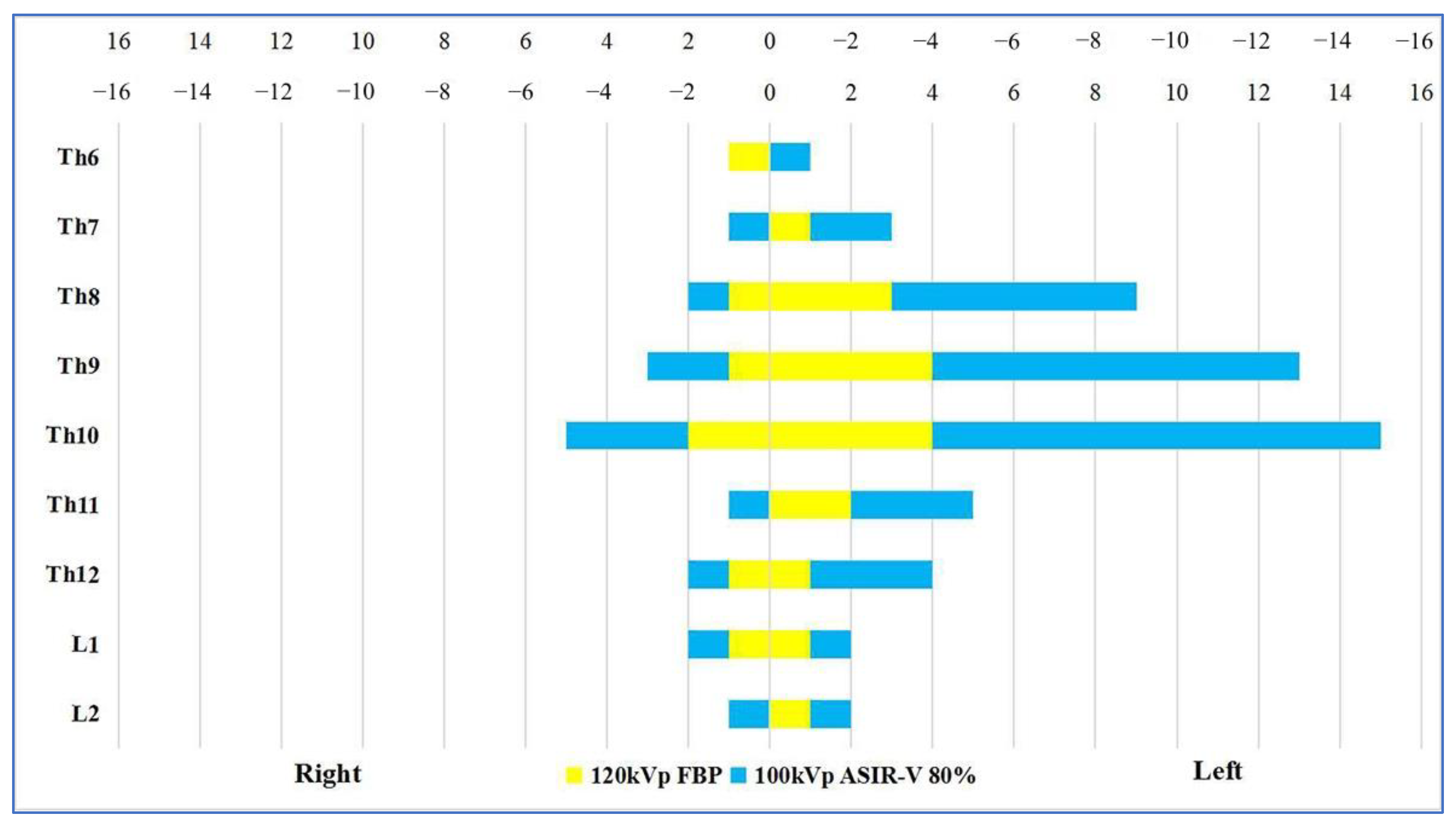
| Items | Group A (n = 50) | Group B (n = 50) | p Value |
|---|---|---|---|
| Age (years) | 65.91 ± 11.19 | 65.78 ± 10.69 | 0.928 |
| Height (cm) | 164.84 ± 6.77 | 163.33 ± 6.53 | 0.284 |
| Weight (kg) | 61.62 ± 7.82 | 61.53 ± 6.40 | 0.953 |
| Males and Females | 28/22 | 30/20 | 0.840 |
| Aortic Condition, n (%) | |||
| AD | 15 (30.0) | 12 (24.0) | 0.653 |
| AA | 11 (22.0) | 13 (26.0) | 0.815 |
| IMH | 12 (24.0) | 9 (18.0) | 0.624 |
| PAU | 7 (14.0) | 10 (20.0) | 0.595 |
| Normal | 5 (10.0) | 6 (12.0) | 0.749 |
| Groups | Image Reconstruction Methods | CTAorta (HU) | Background Noise (HU) | SNRAorta | CNRSpinal cord |
|---|---|---|---|---|---|
| Group B | 120 kV FBP | 446.36 ± 71.41 | 21.42 ± 3.61 | 22.43 ± 2.37 | 23.76 ± 2.45 |
| Group A | 100 kV FBP | 573.86 ± 77.01 | 24.68 ± 2.50 | 19.59 ± 3.10 | 19.83 ± 2.81 |
| 100 kV ASIR-V 10% | 573.82 ± 77.02 | 23.75 ± 2.43 | 20.44 ± 3.26 | 20.74 ± 2.77 | |
| 100 kV ASIR-V 20% | 573.83 ± 77.03 | 22.67 ± 2.34 | 21.24 ± 3.12 | 21.65 ± 2.81 | |
| 100 kV ASIR-V 30% | 573.83 ± 77.04 | 21.72 ± 2.28 | 22.66 ± 3.23 | 22.54 ± 2.80 | |
| 100 kV ASIR-V 40% | 573.84 ± 77.05 | 20.69 ± 2.23 | 23.83 ± 3.50 | 23.75 ± 2.82 | |
| 100 kV ASIR-V 50% | 573.84 ± 77.06 | 19.78 ± 2.17 | 25.39 ± 3.47 | 26.61 ± 2.89 | |
| 100 kV ASIR-V 60% | 573.82 ± 77.10 | 18.82 ± 2.14 | 27.60 ± 3.28 | 28.45 ± 2.88 | |
| 100 kV ASIR-V 70% | 573.82 ± 77.11 | 17.97 ± 2.11 | 28.93 ± 3.32 | 29.60 ± 2.85 | |
| 100 kV ASIR-V 80% | 573.83 ± 77.15 | 17.04 ± 2.07 | 29.94 ± 3.23 | 30.31 ± 2.94 | |
| 100 kV ASIR-V 90% | 573.87 ± 77.16 | 16.25 ± 2.06 | 30.75 ± 3.15 | 31.48 ± 3.01 | |
| 100 kV ASIR-V 100% | 573.95 ± 77.18 | 14.69 ± 1.97 | 31.65 ± 3.16 | 33.03 ± 3.07 | |
| H Value | 84.786 | 386.666 | 391.562 | 439.635 | |
| p value | <0.001 | <0.001 | <0.001 | <0.001 |
| Groups | Image Reconstruction Methods | Radiologist A | Radiologist B | Kappa | p Value |
|---|---|---|---|---|---|
| Group B | 120 kV FBP | 3.78 ± 0.48 | 3.80 ± 0.50 | 0.909 | <0.001 |
| Group A | 100 kV FBP | 3.06 ± 0.24 | 3.08 ± 0.27 | 0.807 | <0.001 |
| 100 kV ASIR-V 10% | 3.10 ± 0.30 | 3.08 ± 0.27 | 0.685 | <0.001 | |
| 100 kV ASIR-V 20% | 3.12 ± 0.33 | 3.14 ± 0.35 | 0.719 | <0.001 | |
| 100 kV ASIR-V 30% | 3.26 ± 0.44 | 3.28 ± 0.45 | 0.703 | <0.001 | |
| 100 kV ASIR-V 40% | 3.52 ± 0.50 | 3.56 ± 0.50 | 0.789 | <0.001 | |
| 100 kV ASIR-V 50% | 3.80 ± 0.40 | 3.84 ± 0.42 | 0.875 | <0.001 | |
| 100 kV ASIR-V 60% | 4.16 ± 0.37 | 4.12 ± 0.33 | 0.887 | <0.001 | |
| 100 kV ASIR-V 70% | 4.54 ± 0.49 | 4.50 ± 0.42 | 0.699 | <0.001 | |
| 100 kV ASIR-V 80% | 4.96 ± 0.20 | 4.94 ± 0.24 | 0.847 | <0.001 | |
| 100 kV ASIR-V 90% | 4.48 ± 0.50 | 4.46 ± 0.48 | 0.679 | <0.001 | |
| 100 kV ASIR-V 100% | 4.32 ± 0.47 | 4.30 ± 0.46 | 0.815 | <0.001 | |
| H Value | 414.257 | 405.879 | - | - | |
| p Value | <0.001 | <0.001 | - | - |
| Groups | Image Reconstruction Methods | Visualization Scores of the Adamkiewicz Arteries | Hairpin Curve | Branching Level | ||||
|---|---|---|---|---|---|---|---|---|
| 1 = Poor | 2 = Fair | 3 = Good | 4 = Fine | 5 = Excellent | ||||
| Group B | 120 kV FBP | 12 (24.0%) | 14 (28.0%) | 11 (22.0%) | 9 (18.0%) | 4 (8.0%) | 38 (76.0%) | 24 (48.0%) |
| Group A | 100 kV FBP | 5 (10.0%) | 9 (18.0%) | 16 (32.0%) | 12 (24.0%) | 8 (16.0%) | 45 (90.0%) | 36 (72.0%) |
| 100 kV ASIR-V 10% | 5 (10.0%) | 9 (18.0%) | 16 (32.0%) | 12 (24.0%) | 8 (16.0%) | 45 (90.0%) | 36 (72.0%) | |
| 100 kV ASIR-V 20% | 5 (10.0%) | 8 (16.0%) | 16 (32.0%) | 13 (26.0%) | 8 (16.0%) | 45 (90.0%) | 37 (74.0%) | |
| 100 kV ASIR-V 30% | 4 (8.0%) | 7 (14.0%) | 14 (28.0%) | 16 (32.0%) | 9 (18.0%) | 46 (92.0%) | 39 (78.0%) | |
| 100 kV ASIR-V 40% | 4 (8.0%) | 7 (14.0%) | 13 (26.0%) | 17 (34.0%) | 9 (18.0%) | 46 (92.0%) | 39 (78.0%) | |
| 100 kV ASIR-V 50% | 4 (8.0%) | 6 (12.0%) | 12 (24.0%) | 18 (36.0%) | 10 (20.0%) | 46 (92.0%) | 40 (80.0%) | |
| 100 kV ASIR-V 60% | 3 (6.0%) | 5 (10.0%) | 10 (20.0%) | 19 (38.0%) | 13 (26.0%) | 47 (94.0%) | 42 (84.0%) | |
| 100 kV ASIR-V 70% | 3 (6.0%) | 4 (8.0%) | 10 (20.0%) | 20 (40.0%) | 13 (26.0%) | 47 (94.0%) | 43 (86.0%) | |
| 100 kV ASIR-V 80% | 2 (4.0%) | 3 (6.0%) | 9 (18.0%) | 22 (44.0%) | 14 (28.0%) | 48 (96.0%) | 45 (90.0%) | |
| 100 kV ASIR-V 90% | 2 (4.0%) | 3 (6.0%) | 7 (14.0%) | 24 (48.0%) | 14 (28.0%) | 48 (96.0%) | 45 (90.0%) | |
| 100 kV ASIR-V 100% | 2 (4.0%) | 3 (6.0%) | 6 (12.0%) | 24 (48.0%) | 15 (30.0%) | 48 (96.0%) | 45 (90.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, J.; Ma, T.; Yan, J.; Wu, S.; Yan, G.; Li, H.; Li, Y.; Zhao, L.; Fan, X.; McClure, M.A.; et al. Effect of Low Tube Voltage (100 kV) Combined with ASIR-V on the Visualization and Image Quality of the Adamkiewicz Artery: A Comparison with 120 kV Protocol. Diagnostics 2023, 13, 2495. https://doi.org/10.3390/diagnostics13152495
Deng J, Ma T, Yan J, Wu S, Yan G, Li H, Li Y, Zhao L, Fan X, McClure MA, et al. Effect of Low Tube Voltage (100 kV) Combined with ASIR-V on the Visualization and Image Quality of the Adamkiewicz Artery: A Comparison with 120 kV Protocol. Diagnostics. 2023; 13(15):2495. https://doi.org/10.3390/diagnostics13152495
Chicago/Turabian StyleDeng, Jiantao, Ting Ma, Jing Yan, Siyi Wu, Gaowu Yan, Hongwei Li, Yong Li, Linwei Zhao, Xiaoping Fan, Morgan A. McClure, and et al. 2023. "Effect of Low Tube Voltage (100 kV) Combined with ASIR-V on the Visualization and Image Quality of the Adamkiewicz Artery: A Comparison with 120 kV Protocol" Diagnostics 13, no. 15: 2495. https://doi.org/10.3390/diagnostics13152495





