The Diagnostic Performance of Tumor Stage on MRI for Predicting Prostate Cancer-Positive Surgical Margins: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Literature Search
2.2. Inclusion Criteria
2.3. Exclusion Criteria
2.4. Data Extraction
2.5. Methodologic Quality Assessment
2.6. Data Synthesis and Analysis
3. Results
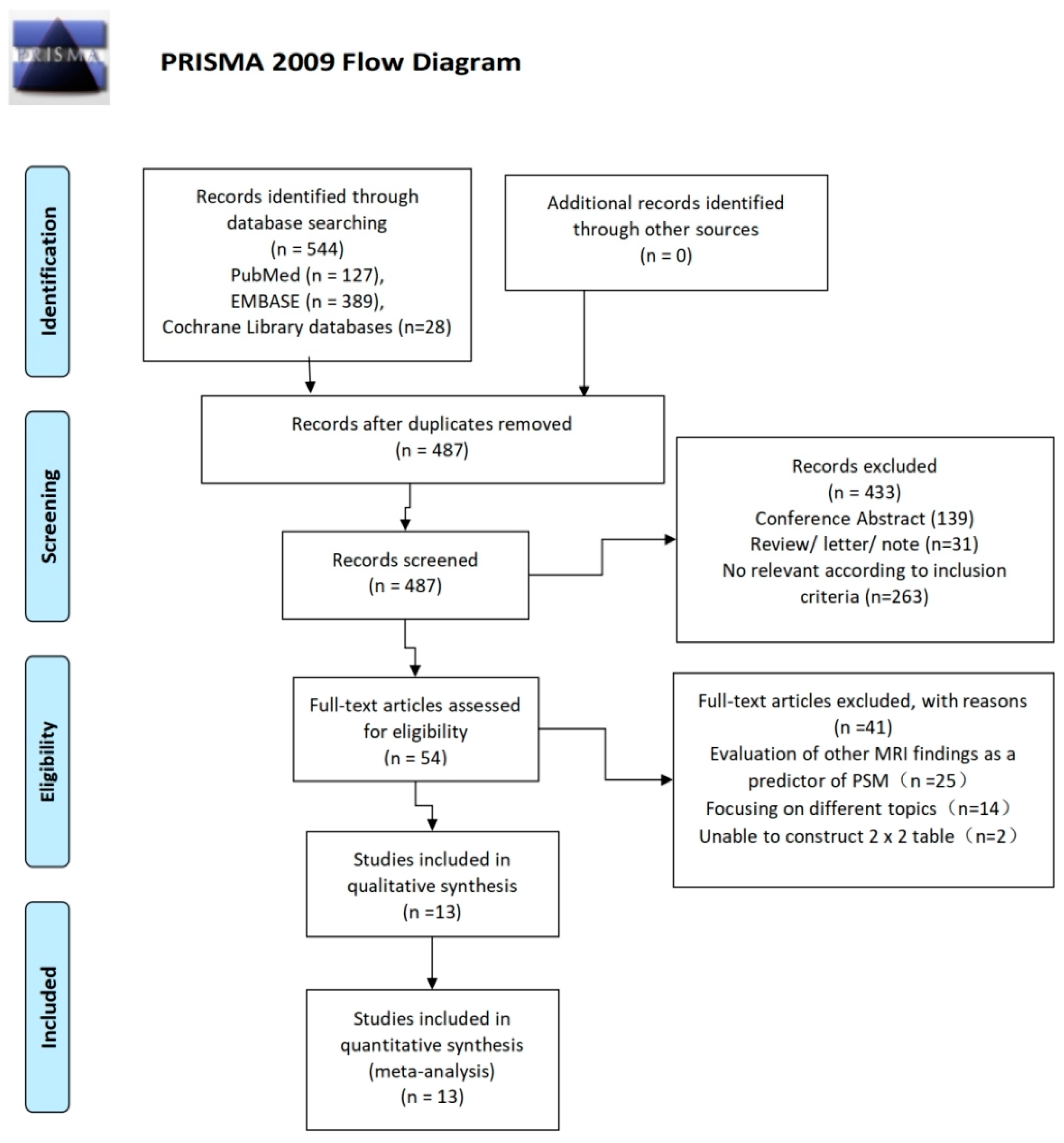
3.1. Literature Search and Article Selection
3.2. Study Characteristics
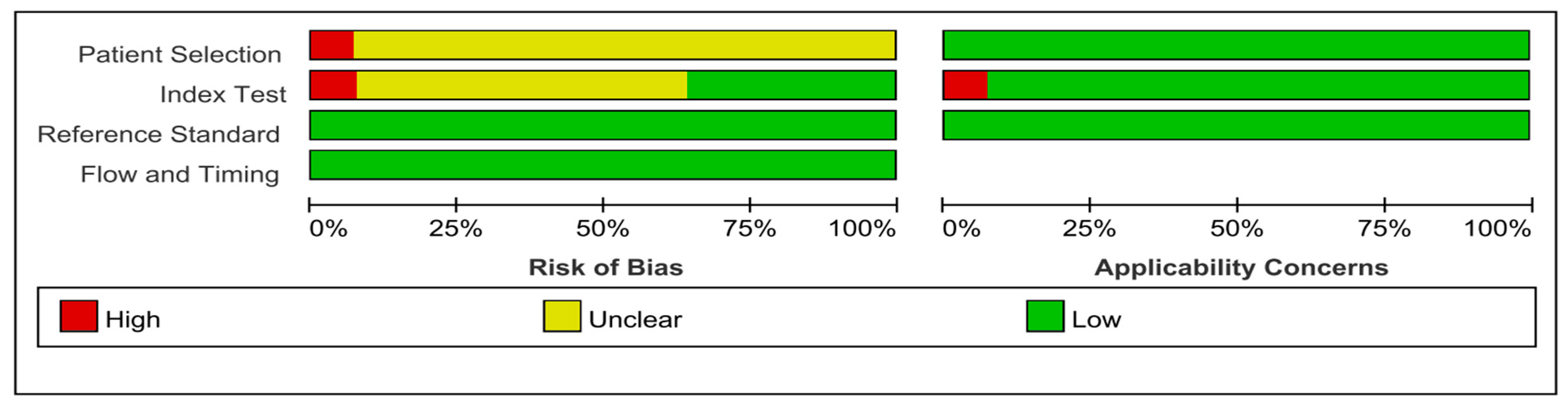
3.3. Quality Assessment
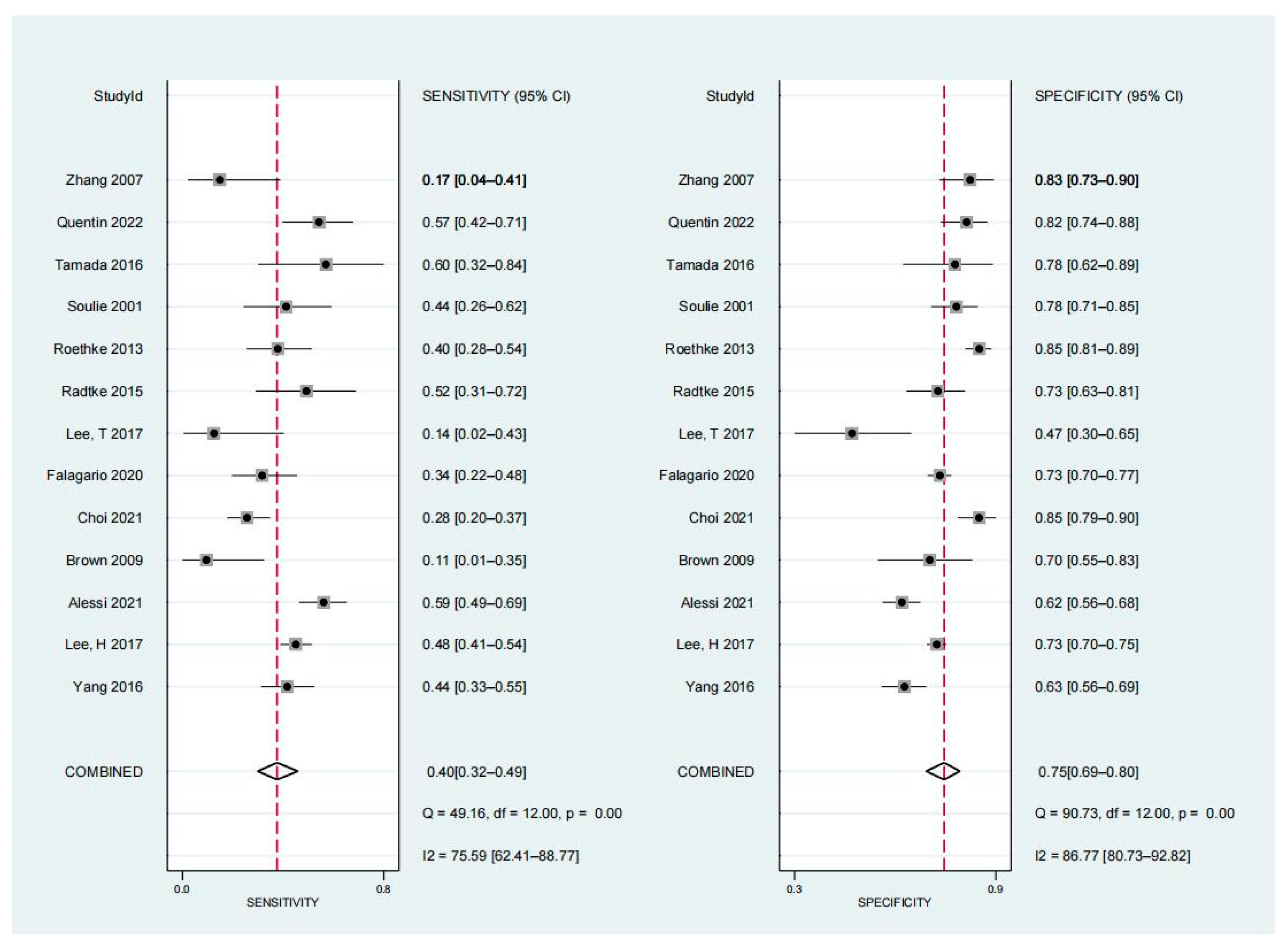
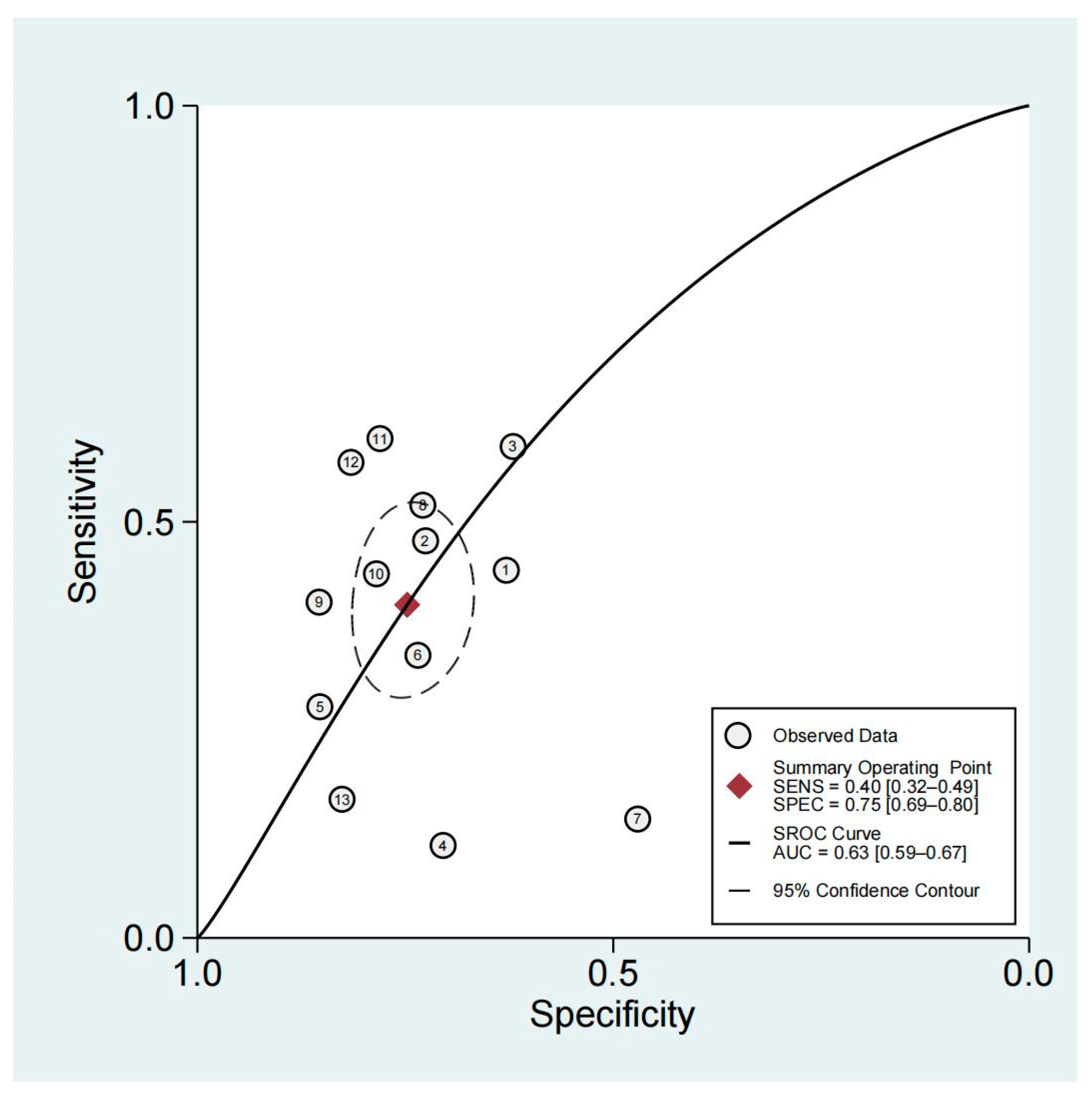
3.4. Diagnostic Performance of Non-Organ-Confined Disease on MRI for the Detection of PSMs
3.5. Subgroup Analysis and Meta-Regression
3.6. Publication Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Tewari, A.; Sooriakumaran, P.; Bloch, D.A.; Seshadri-Kreaden, U.; Hebert, A.E.; Wiklund, P. Positive surgical margin and perioperative complication rates of primary surgical treatments for prostate cancer: A systematic review and meta-analysis comparing retropubic, laparoscopic, and robotic prostatectomy. Eur. Urol. 2012, 62, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Tan, P.H.; Cheng, L.; Srigley, J.R.; Griffiths, D.; Humphrey, P.A.; van der Kwast, T.H.; Montironi, R.; Wheeler, T.M.; Delahunt, B.; Egevad, L.; et al. International Society of Urological Pathology (ISUP) Consensus Conference on Handling and Staging of Radical Prostatectomy Specimens. Working group 5: Surgical margins. Mod. Pathol. 2011, 24, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Cao, K.; Han, T.; Zhang, Y.-F.; Zhang, G.-T.; Xu, L.-F.; Lian, H.-B.; Li, X.-G.; Guo, H.-Q. Perineural invasion status, Gleason score and number of positive cores in biopsy pathology are predictors of positive surgical margin following laparoscopic radical prostatectomy. Asian J. Androl. 2017, 19, 468–472. [Google Scholar] [CrossRef]
- Yossepowitch, O.; Briganti, A.; Eastham, J.A.; Epstein, J.; Graefen, M.; Montironi, R.; Touijer, K. Positive surgical margins after radical prostatectomy: A systematic review and contemporary update. Eur. Urol. 2014, 65, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Bolla, M.; van Poppel, H.; Tombal, B.; Vekemans, K.; Da Pozzo, L.; de Reijke, T.M.; Verbaeys, A.; Bosset, J.-F.; van Velthoven, R.; Colombel, M.; et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012, 380, 2018–2027. [Google Scholar] [CrossRef]
- Vis, A.N.; Schröder, F.H.; van der Kwast, T.H. The actual value of the surgical margin status as a predictor of disease progression in men with early prostate cancer. Eur. Urol. 2006, 50, 258–265. [Google Scholar] [CrossRef]
- de Rooij, M.; Hamoen, E.H.; Witjes, J.A.; Barentsz, J.O.; Rovers, M.M. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur. Urol. 2016, 70, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Valentin, B.; Schimmöller, L.; Ullrich, T.; Klingebiel, M.; Demetrescu, D.; Sawicki, L.M.; Lakes, J.; Mally, D.; Quentin, M.; Esposito, I.; et al. Magnetic resonance imaging improves the prediction of tumor staging in localized prostate cancer. Abdom. Radiol. 2021, 46, 2751–2759. [Google Scholar] [CrossRef]
- Lee, H.; Kim, C.K.; Park, B.K.; Sung, H.H.; Han, D.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Jeon, S.S.; Choi, H.Y.; et al. Accuracy of preoperative multiparametric magnetic resonance imaging for prediction of unfavorable pathology in patients with localized prostate cancer undergoing radical prostatectomy. World J. Urol. 2017, 35, 929–934. [Google Scholar] [CrossRef]
- Li, W.; Dong, A.; Hong, G.; Shang, W.; Shen, X. Diagnostic performance of ESUR scoring system for extraprostatic prostate cancer extension: A meta-analysis. Eur. J. Radiol. 2021, 143, 109896. [Google Scholar] [CrossRef] [PubMed]
- Alessi, S.; Maggioni, R.; Luzzago, S.; Colombo, A.; Pricolo, P.; Summers, P.E.; Saia, G.; Manzoni, M.; Renne, G.; Marvaso, G.; et al. Apparent Diffusion Coefficient and Other Preoperative Magnetic Resonance Imaging Features for the Prediction of Positive Surgical Margins in Prostate Cancer Patients Undergoing Radical Prostatectomy. Clin. Genitourin. Cancer 2021, 19, e335–e345. [Google Scholar] [CrossRef] [PubMed]
- Park, M.Y.; Park, K.J.; Kim, M.-H.; Kim, J.K. Preoperative MRI-based estimation of risk for positive resection margin after radical prostatectomy in patients with prostate cancer: Development and validation of a simple scoring system. Eur. Radiol. 2021, 31, 4898–4907. [Google Scholar] [CrossRef]
- Xu, B.; Luo, C.; Zhang, Q.; Jin, J. Preoperative characteristics of the P.R.O.S.T.A.T.E. scores: A novel predictive tool for the risk of positive surgical margin after radical prostatectomy. J. Cancer Res. Clin. Oncol. 2017, 143, 687–692. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M.; QUADAS-2 Group. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Reitsma, J.B.; Glas, A.S.; Rutjes, A.W.; Scholten, R.J.; Bossuyt, P.M.; Zwinderman, A.H. Bivariate analysis of sensitivity and specificity produces informative summary measures in diagnostic reviews. J. Clin. Epidemiol. 2005, 58, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Rutter, C.M.; Gatsonis, C.A. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat. Med. 2001, 20, 2865–2884. [Google Scholar] [CrossRef]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef]
- Brown, J.A.; Rodin, D.M.; Harisinghani, M.; Dahl, D.M. Impact of preoperative endorectal MRI stage classification on neurovascular bundle sparing aggressiveness and the radical prostatectomy positive margin rate. Urol. Oncol. Semin. Orig. Investig. 2009, 27, 174–179. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.Y.; Chi, B.H.; Kim, T.-H.; Lim, B.; Lee, W.; You, D.; Kim, C.-S. Does intraoperative frozen section really predict significant positive surgical margins after robot-assisted laparoscopic prostatectomy? A retrospective study. Asian J. Androl. 2021, 23, 74–79. [Google Scholar] [CrossRef]
- Falagario, U.G.; Jambor, I.; Ratnani, P.; Martini, A.; Treacy, P.-J.; Wajswol, E.; Lantz, A.; Papastefanou, G.; Weil, R.; Phillip, D.; et al. Performance of prostate multiparametric MRI for prediction of prostate cancer extra-prostatic extension according to NCCN risk categories: Implication for surgical planning. Minerva Urol. E Nefrol. Ital. J. Urol. Nephrol. 2020, 72, 746–754. [Google Scholar] [CrossRef]
- Lee, T.; Hoogenes, J.; Wright, I.; Matsumoto, E.D.; Shayegan, B. Utility of preoperative 3 Tesla pelvic phased-array multiparametric magnetic resonance imaging in prediction of extracapsular extension and seminal vesicle invasion of prostate cancer and its impact on surgical margin status: Experience at a Canadian academic tertiary care centre. Can. Urol. Assoc. J. J. L′association Urol. Can. 2017, 11, E174–E178. [Google Scholar] [CrossRef] [Green Version]
- Radtke, J.P.; Hadaschik, B.A.; Wolf, M.B.; Freitag, M.T.; Schwab, C.; Alt, C.; Roth, W.; Duensing, S.; Pahernik, S.A.; Roethke, M.C.; et al. The impact of magnetic resonance imaging on prediction of extraprostatic extension and prostatectomy outcome in patients with low-, intermediate- and high-risk prostate cancer: Try to find a standard. J. Endourol. 2015, 29, 1396–1405. [Google Scholar] [CrossRef]
- Roethke, M.C.; Lichy, M.P.; Kniess, M.; Werner, M.K.; Claussen, C.D.; Stenzl, A.; Schlemmer, H.-P.; Schilling, D. Accuracy of preoperative endorectal MRI in predicting extracapsular extension and influence on neurovascular bundle sparing in radical prostatectomy. World J. Urol. 2013, 31, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Soulié, M.; Aziza, R.; Escourrou, G.; Seguin, P.; Tollon, C.; Molinier, L.; Bachaud, J.-M.; Joffre, F.; Plante, P. Assessment of the risk of positive surgical margins with pelvic phased-array magnetic resonance imaging in patients with clinically localized prostate cancer: A prospective study. Urology 2001, 58, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Tamada, T.; Sone, T.; Kanomata, N.; Miyaji, Y.; Kido, A.; Jo, Y.; Yamamoto, A.; Ito, K. Value of preoperative 3T multiparametric MRI for surgical margin status in patients with prostate cancer. J. Magn. Reson. Imaging JMRI 2016, 44, 584–593. [Google Scholar] [CrossRef]
- Quentin, M.; Schimmöller, L.; Ullrich, T.; Valentin, B.; Demetrescu, D.; Al-Monajjed, R.; Mally, D.; Esposito, I.; Albers, P.; Antoch, G.; et al. Pre-operative magnetic resonance imaging can predict prostate cancer with risk for positive surgical margins. Abdom. Radiol. New York 2022, 47, 2486–2493. [Google Scholar] [CrossRef]
- Zhang, J.Q.; Loughlin, K.R.; Zou, K.H.; Haker, S.; Tempany, C.M.C. Role of Endorectal Coil Magnetic Resonance Imaging in Treatment of Patients with Prostate Cancer and in Determining Radical Prostatectomy Surgical Margin Status: Report of a Single Surgeon’s Practice. Urology 2007, 69, 1134–1137. [Google Scholar] [CrossRef]
- Petralia, G.; Musi, G.; Padhani, A.R.; Summers, P.; Renne, G.; Alessi, S.; Raimondi, S.; Matei, D.V.; Renne, S.L.; Jereczek-Fossa, B.A.; et al. Robot-assisted radical prostatectomy: Multiparametric MR imaging–directed intraoperative frozen-section analysis to reduce the rate of positive surgical margins. Radiology 2015, 274, 434–444. [Google Scholar] [CrossRef] [Green Version]
- Xiang, J.-Y.; Huang, X.-S.; Xu, J.-X.; Huang, R.-H.; Zheng, X.-Z.; Xue, L.-M.; Liu, Y.-L. MRI Extraprostatic Extension Grade: Accuracy and Clinical Incremental Value in the Assessment of Extraprostatic Cancer. BioMed Res. Int. 2022, 2022, 3203965. [Google Scholar] [CrossRef]
- Schieda, N.; Lim, C.S.; Idris, M.; Lim, R.S.; Morash, C.; Breau, R.H.; Flood, T.A.; McInnes, M.D.F. MRI assessment of pathological stage and surgical margins in anterior prostate cancer (APC) using subjective and quantitative analysis. J. Magn. Reson. Imaging 2017, 45, 1296–1303. [Google Scholar] [CrossRef]
- Bernstein, M.R.; Cangiano, T.; D′Amico, A.; Chittams, J.; Hardy, C.; Whittington, R.D.; Tomaszewski, J.E.; Schnall, M.D.; Wein, A.J.; Malkowicz, S.B. Endorectal coil magnetic resonance imaging and clinicopathologic findings in T1c adenocarcinoma of the prostate. Urol. Oncol. 2000, 5, 104–107. [Google Scholar] [CrossRef]
- Cornud, F.; Hamida, K.; Flam, T.; Hélénon, O.; Chrétien, Y.; Thiounn, N.; Correas, J.M.; Casanova, J.M.; Moreau, J.F. Endorectal color doppler sonography and endorectal MR imaging features of nonpalpable prostate cancer: Correlation with radical prostatectomy findings. AJR Am. J. Roentgenol. 2000, 175, 1161–1168. [Google Scholar] [CrossRef]
- May, F.; Treumann, T.; Dettmar, P.; Hartung, R.; Breul, J. Limited value of endorectal magnetic resonance imaging and transrectal ultrasonography in the staging of clinically localized prostate cancer. BJU Int. 2001, 87, 66–69. [Google Scholar] [CrossRef]
- Park, B.K.; Kim, B.; Kim, C.K.; Lee, H.M.; Kwon, G.Y. Comparison of phased-array 3.0-T and endorectal 1.5-T magnetic resonance imaging in the evaluation of local staging accuracy for prostate cancer. J. Comput. Assist. Tomogr. 2007, 31, 534–538. [Google Scholar] [CrossRef]
- Hegde, J.V.; Mulkern, R.V.; Panych, L.P.; Fennessy, F.M.; Fedorov, A.; Maier, S.E.; Tempany, C.M. Multiparametric MRI of prostate cancer: An update on state-of-the-art techniques and their performance in detecting and localizing prostate cancer. J. Magn. Reson. Imaging JMRI 2013, 37, 1035–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McEvoy, S.H.; Raeside, M.C.; Chaim, J.; Ehdaie, B.; Akin, O. Preoperative Prostate MRI: A Road Map for Surgery. AJR Am. J. Roentgenol. 2018, 211, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Yang, Z.; Qi, L.; Chen, M. Robot-assisted and laparoscopic vs open radical prostatectomy in clinically localized prostate cancer: Perioperative, functional, and oncological outcomes: A Systematic review and meta-analysis. Medicine 2019, 98, e15770. [Google Scholar] [CrossRef] [PubMed]
- Park, B.H.; Jeon, H.G.; Jeong, B.C.; Seo, S.I.; Lee, H.M.; Choi, H.Y.; Jeon, S.S. Influence of magnetic resonance imaging in the decision to preserve or resect neurovascular bundles at robotic assisted laparoscopic radical prostatectomy. J. Urol. 2014, 192, 82–88. [Google Scholar] [CrossRef]
- Sogutdelen, E.; Citamak, B. Is Low-risk Prostate Cancer Really Low-risk? Consider Factors Affecting Biochemical Recurrence and Tumour Upgrade. J. Coll. Physicians Surg. Pak. JCPSP 2023, 33, 193–198. [Google Scholar] [CrossRef]
- Freedland, S.J.; Isaacs, W.B.; Platz, E.A.; Terris, M.K.; Aronson, W.J.; Amling, C.L.; Presti, J.C., Jr.; Kane, C.J. Prostate size and risk of high-grade, advanced prostate cancer and biochemical progression after radical prostatectomy: A search database study. J. Clin. Oncol. 2005, 23, 7546–7554. [Google Scholar] [CrossRef] [PubMed]
- Çaliskan, S.; Kaba, S.L.; Koca, O.; Öztürk, M.I. Does Small Prostate Predict High Grade Prostate Cancer? J. Coll. Physicians Surg. Pak. JCPSP 2017, 27, 97–100. [Google Scholar] [PubMed]
- Epstein, J.I. Update on the Gleason grading system. Ann. Pathol. 2011, 31, S20–S26. [Google Scholar] [CrossRef] [PubMed]
- Langer, D.L.; van der Kwast, T.H.; Evans, A.J.; Plotkin, A.; Trachtenberg, J.; Wilson, B.C.; Haider, M.A. Prostate tissue composition and MR measurements: Investigating the relationships between ADC, T2, Ktrans, ve, and corresponding histologic features. Radiology 2010, 255, 485–494. [Google Scholar] [CrossRef] [PubMed]
- Grivas, N.; Zachos, I.; Georgiadis, G.; Karavitakis, M.; Tzortzis, V.; Mamoulakis, C. Learning curves in laparoscopic and robot-assisted prostate surgery: A systematic search and review. World J. Urol. 2022, 40, 929–949. [Google Scholar] [CrossRef] [PubMed]

| Author | Year | Country | N | Age (Years), Median (Range) | PSA (ng/mL), Median (Range) | No. of Biopsies with Gleason Score ≥7 | Proportion and Mode of Biopsies | DRE (+) |
|---|---|---|---|---|---|---|---|---|
| Soulie [25] | 2001 | France | 176 | 64.2 * (49–74) | 10.9 * (1.2–39) | NA | 97.2%, TRUS | NA |
| Zhang [28] | 2007 | China | 110 | 62.2 * (NA) | 8.1 * (NA) | NA | All, NA | NA |
| Alessi [12] | 2021 | Italy | 400 | 65 (NA) | 6.3 (NA) | 220 | All, NA | NA |
| Lee, H. [10] | 2017 | South Korea | 1145 | 65 (NA) | 6.1 (NA) | 594 | All, NA | NA |
| Radtke [23] | 2015 | Germany | 132 | 66 (NA) | 8.2 (NA) | NA | All, MRI/TRUS-fusion | 28 |
| Yang [4] | 2016 | China | 296 | 70 (51–80) | 12.6 (2.9–180) | 196 | ALL, TRUS | NA |
| Lee, T. [22] | 2017 | Canada | 48 | 60.9 * (NA) | 9.7 * (NA) | 36 | ALL, TRUS | NA |
| Falagario [21] | 2020 | Italy | 664 | 63 (NA) | 6 (NA) | NA | All, NA | NA |
| Tamada [26] | 2016 | Japan | 56 | 69 (54–84) | 7.26 (4.09–24.5) | 49 | All, NA | 15 |
| Roethke [24] | 2013 | Germany | 385 | 62.7 * (42–77) | 8.9 * (0.4–52.5) | NA | All, NA | NA |
| Brown [19] | 2009 | United States | 62 | 58 * (42–73) | 9.3 * (0.5–30.5) | NA | All, NA | NA |
| Quentin [27] | 2022 | Germany | 179 | NA | NA | NA | All, NA | NA |
| Choi [20] | 2021 | South Korea | 271 | 64.9 * (NA) | 7.9 * (NA) | 78 | All, NA | NA |
| First Author | Study Design | Patient Enrollment | Index | NeuroSAFE Technique | Radical Prostatectomy Technique | Interval between Biopsy and MRI | No. of Surgeons | Reference |
|---|---|---|---|---|---|---|---|---|
| Soulie [25] | Prospective | Consecutive | T3 | NA | RRP | 2 to 9 w | 2 | RP |
| Zhang [28] | Retrospective | Consecutive | T3 | NA | NA | 3 to 10 w | 1 | RP |
| Alessi [12] | Retrospective | Consecutive | ECE score ≥4 | yes | RARP | NA | Multiple | RP |
| Lee, H. [10] | Retrospective | Consecutive | T3 | NA | RARP, LRP, ORP | NA | NA | RP |
| Radtke [23] | Retrospective | Consecutive | ECE score ≥4 | NA | RARP, RRP | NA | 4 | RP |
| Yang [4] | Retrospective | Consecutive | EPE | NA | LRP | NA | 1 | RP |
| Lee, T. [22] | Retrospective | Consecutive | ECE | NA | LRP, RARP, ORP | At least 6 w | 1 | RP |
| Falagario [21] | Retrospective | Consecutive | EPE | Yes | NA | 4 w | Multiple | RP |
| Tamada [26] | Retrospective | Consecutive | ECE | NA | LRP, RRP | NA | NA | RP |
| Roethke [24] | Retrospective | Consecutive | ECE | Yes | RRP | At least 6 w | 1 | RP |
| Brown [19] | Retrospective | Consecutive | T3 | NA | LRP, ORP | At least 8–10 w | 2 | RP |
| Quentin [27] | Retrospective | Consecutive | EPE | NA | RARP | NA | 4 | RP |
| Choi [20] | Retrospective | Consecutive | ECE | Yes | RALP | NA | 1 | RP |
| Author | Field Strength (T) | Blinded | No. of Radiologist | Experience of Radiologist (Years/Numbers) | Endorectal coil | MR Techniques | Vendor |
|---|---|---|---|---|---|---|---|
| Soulie [25] | 1.0 | Yes | Single | NA | No | T1, T2 | Siemens |
| Zhang [28] | 1.5 | Yes | Single | NA | Yes | T1, T2 | GE |
| Alessi [12] | 1.5 | NA | Three | 10 y, 8 y, 6 y/>500 | No | T2, DWI, DCE | Siemens |
| Lee, H. [10] | 1.5–3.0 | Yes | Two | NA | No | T2, DWI, ADC | Philips |
| Radtke [23] | 3.0 | Yes | Multiple | 7 y, >10 y | No | T2, DWI, DCE | Siemens |
| Yang [4] | NA | NA | NA | NA | NA | NA | NA |
| Lee, T. [22] | 3.0 | Yes | Single | >10 y | No | T2, DWI, DCE | Philips |
| Falagario [21] | 3.0 | NA | Single | NA | No | T2, DWI, DCE | Siemens |
| Tamada [26] | 3.0 | Yes | Two | 7 y, 16 y | No | T1, T2, DWI, DCE | Toshiba |
| Roethke [24] | 1.5 | Yes | Two | 4–14 y/300–1000 | Yes | T2 | Siemens |
| Brown [19] | 1.5 | No | Multiple | 1–20 y | Yes | T1, T2 | GE |
| Quentin [27] | 3.0 | Yes | Two | >10 y | No | T1, T2, ADC, DCE | Siemens |
| Choi [20] | 3.0 | NA | NA | NA | NA | NA | NA |
| Variable | No. of Studies | Category | Sensitivity | Specificity | LRT Chi-Square | P (Joint Model) | ||
|---|---|---|---|---|---|---|---|---|
| Pooled Value (95% CI) | p | Pooled Value (95% CI) | p | |||||
| Study design | 1 | Prospective | 0.44 (0.14–0.74) | 0.7 | 0.79 (0.63–0.95) | 0.42 | 0.03 | 0.86 |
| 12 | Retrospective | 0.40 (0.31–0.48) | 0.74 (0.69–0.80) | |||||
| Area | 8 | Non-Asian | 0.41 (0.30–0.52) | 0.34 | 0.74 (0.67–0.80) | <0.01 | 0.40 | 0.82 |
| 5 | Asian | 0.39 (0.26–0.51) | 0.77 (0.69–0.84) | |||||
| Prevalence of biopsy Gleason score (≥7) | 5 | >50% | 0.48 (0.38–0.57) | 0.02 | 0.65 (0.58–0.73) | <0.001 | 106.04 | <0.01 |
| 1 | <50% | 0.28 (0.13–0.42) | 0.85 (0.76–0.94) | |||||
| Robotic-assisted | 6 | Yes | 0.44 (0.33–0.56) | 0.36 | 0.72 (0.64–0.81) | 0.01 | 29.21 | <0.01 |
| 5 | No | 0.40 (0.27–0.53) | 0.76 (0.68–0.85) | |||||
| Laparoscopic | 9 | Yes | 0.43 (0.33–0.52) | 0.67 | 0.72 (0.65–0.78) | <0.001 | 31.24 | <0.01 |
| 2 | No | 0.42 (0.22–0.62) | 0.82 (0.74–0.91) | |||||
| Field strength | 7 | 3.0 T | 0.42 (0.29–0.54) | 0.28 | 0.75 (0.68–0.82) | <0.01 | 17.07 | <0.01 |
| 5 | 1.5 T or 1 T | 0.36 (0.22–0.50) | 0.77 (0.69–0.85) | |||||
| Functional technology | 7 | Yes | 0.47 (0.36–0.58) | 0.04 | 0.71 (0.65–0.77) | <0.001 | 42.2 | <0.01 |
| 4 | No | 0.29 (0.15–0.43) | 0.80 (0.74–0.87) | |||||
| Endorectal coil | 3 | Yes | 0.24 (0.10–0.39) | 0.38 | 0.81 (0.73–0.89) | 0.08 | 43.09 | <0.01 |
| 8 | No | 0.47 (0.37–0.57) | 0.72 (0.66–0.78) | |||||
| No. of radiologists | 7 | Multiple | 0.48 (0.39–0.57) | 0.02 | 0.75 (0.69–0.82) | 0.02 | 37.3 | <0.01 |
| 4 | Single | 0.30 (0.18–0.42) | 0.73 (0.64–0.82) | |||||
| No. of cases | 8 | ≥150 | 0.44 (0.35–0.53) | 0.04 | 0.76 (0.70–0.82) | 0.03 | 3.36 | 0.19 |
| 5 | <50 | 0.31 (0.18–0.44) | 0.72 (0.63–0.82) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, Y.; Zhu, M.; Tian, M.; Liu, L.; Yin, L. The Diagnostic Performance of Tumor Stage on MRI for Predicting Prostate Cancer-Positive Surgical Margins: A Systematic Review and Meta-Analysis. Diagnostics 2023, 13, 2497. https://doi.org/10.3390/diagnostics13152497
Wang Y, Wu Y, Zhu M, Tian M, Liu L, Yin L. The Diagnostic Performance of Tumor Stage on MRI for Predicting Prostate Cancer-Positive Surgical Margins: A Systematic Review and Meta-Analysis. Diagnostics. 2023; 13(15):2497. https://doi.org/10.3390/diagnostics13152497
Chicago/Turabian StyleWang, Yu, Ying Wu, Meilin Zhu, Maoheng Tian, Li Liu, and Longlin Yin. 2023. "The Diagnostic Performance of Tumor Stage on MRI for Predicting Prostate Cancer-Positive Surgical Margins: A Systematic Review and Meta-Analysis" Diagnostics 13, no. 15: 2497. https://doi.org/10.3390/diagnostics13152497





