Telemedicine in the Era of a Pandemic: Usefulness of a Novel Three-Lead ECG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Data Collection
2.3. Data Analysis
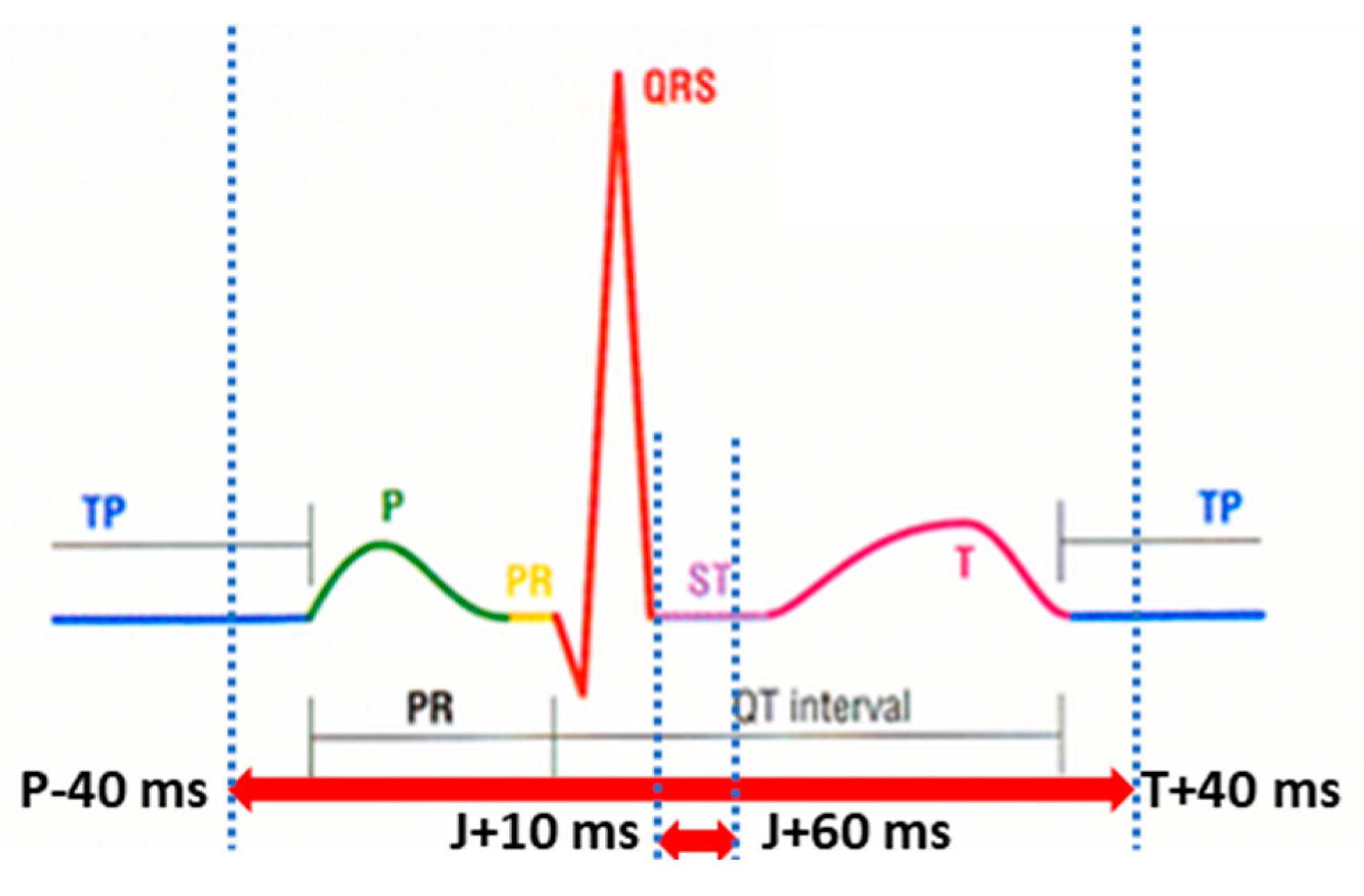
2.4. ECG Signal Parameters Matching Analysis
2.5. Efficiency Analysis
2.6. Statistical Analysis
3. Results
3.1. Demographic and Clinical Characteristics
3.2. ECG Diagnostic Findings
3.3. ECG Clinical Matching
3.4. Diagnostic Accuracy of Clinically Relevant ECG Reconstruction Matching Obtained by the KardioPal Technology
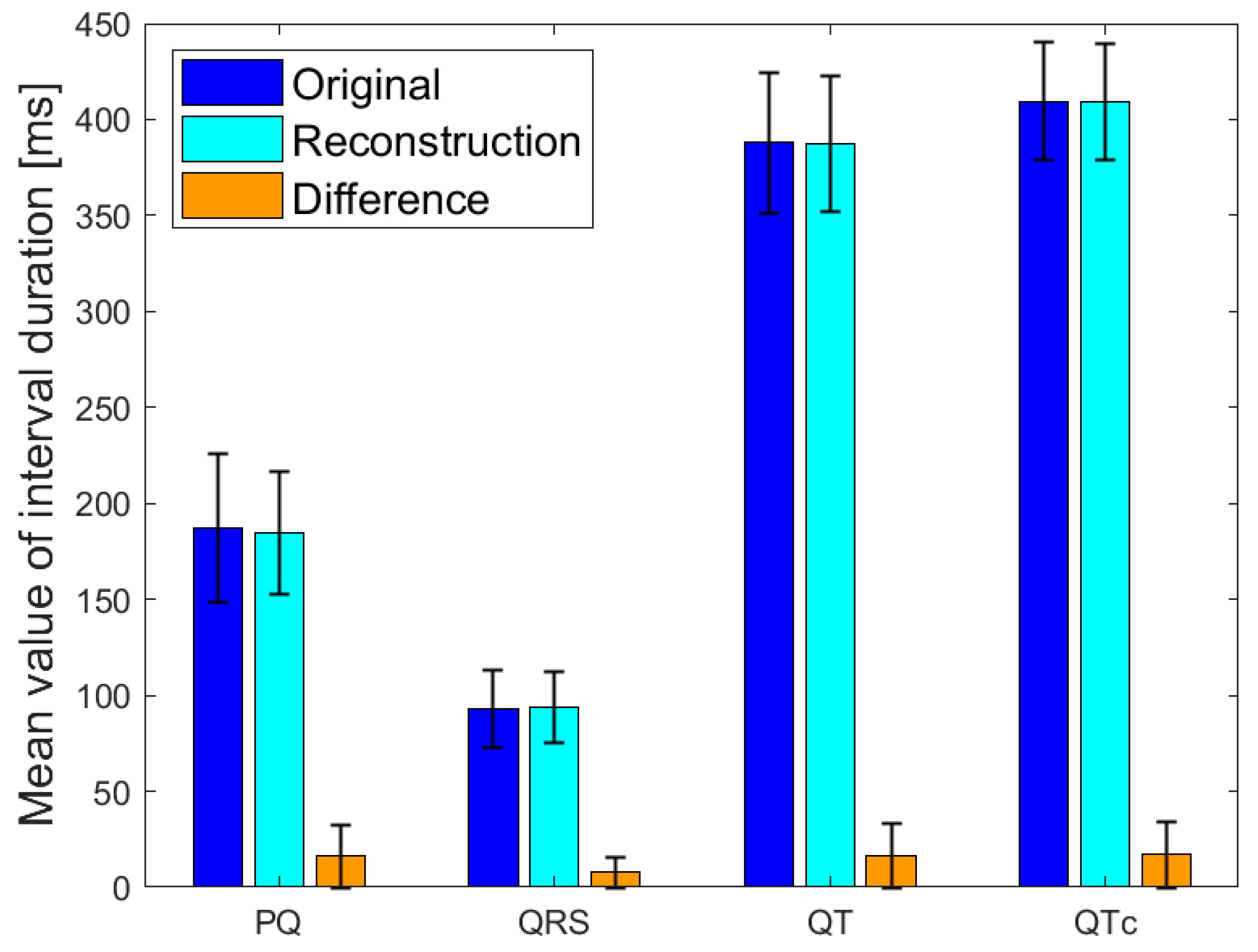
3.5. ECG Signal Parameters Performance Matching
3.6. Efficacy of KardioPal Technology
4. ECG Clinical Matching Analysis: Case Examples
- Inadequate Matching (Figure 6)
- Acceptable Matching (Figure 7)
- Adequate Matching (Figure 8)
5. Discussion
Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hollander, J.E.; Carr, B.G. Virtually Perfect? Telemedicine for COVID-19. N. Engl. J. Med. 2020, 30, 1679–1681. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Freeman, A.; Mullen, B. Telehealth: Rapid Implementation for Your Cardiology Clinic. Am. Colege Cardiol. 2020, 24. [Google Scholar]
- Hong, Z.; Li, N.; Li, D.; Li, J.; Li, B.; Xiong, W.; Lu, L.; Li, W.; Zhou, D. Telemedicine During the COVID-19 Pandemic: Experiences from Western China. J. Med. Internet Res. 2020, 8, e19577. [Google Scholar] [CrossRef]
- Kang, Y.; Chen, T.; Mui, D.; Ferrari, V.; Jagasia, D.; Scherrer-Crosbie, M.; Chen, Y.; Han, Y. Cardiovascular manifestations and treatment considerations in COVID-19. Heart 2020, 106, 1132–1141. [Google Scholar] [CrossRef] [PubMed]
- Esmel-Vilomara, R.; Dolader, P.; Sabaté-Rotes, A.; Soriano-Arandes, A.; Gran, F.; Rosés-Noguer, F. QTc interval prolongation in patients infected with SARS-CoV-2 and treated with antiviral drugs. An. Pediatría (Engl. Ed.) 2022, 96, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, S.; Spinelli, F.R.; Agati, L.; Ciardi, M.R.; Garufi, C.; Natalucci, F.; Molteni, E.; Truglia, S.; Riccieri, V.; Priori, R.; et al. Hydroxychloroquine cardiotoxicity: A case-control study comparing patients with COVID-19 and patients with systemic lupus erythematosus. Clin. Exp. Rheumatol. 2022, 40, 890–896. [Google Scholar] [CrossRef]
- Taggar, J.S.; Colema, T.; Lewis, S.; Heneghan, C.; Jones, M. Accuracy of methods for diagnosing atrial fibrillation using 12-lead ECG: A systematic review and meta-analysis. Int. J. Cardiol. 2015, 184, 175–183. [Google Scholar] [CrossRef]
- Haberman, Z.C.; Jahn, R.T.; Bose, R.; Tun, H.; Shinbane, J.S.; Doshi, R.N.; Chang, P.M.; Saxon, L.A. Wireless smartphone ECG enables large-scale screening in diverse populations. J. Cardiovasc. Electrophysiol. 2015, 26, 520–526. [Google Scholar] [CrossRef]
- Steinberg, J.S.; Varma, N.; Cygankiewicz, I.; Aziz, P.; Balsam, P.; Baranchuk, A.; Cantillon, D.J.; Dilaveris, P.; Dubner, S.J.; El-Sherif, N.; et al. 2017 ISHNE-HRS expert consensus statement on ambulatory ECG and external cardiac monitoring/telemetry. Heart Rhythm. 2017, 14, e55–e96. [Google Scholar] [CrossRef] [Green Version]
- Glikson, M.; Nielsen, J.C.; Kronborg, M.B.; Michowitz, Y.; Auricchio, A.; Barbash, I.M.; A Barrabés, J.; Boriani, G.; Braunschweig, F.; Brignole, M.; et al. ESC Scientific Document Group. 2021 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J. 2021, 14, 3427–3520. [Google Scholar] [CrossRef]
- Ahmed, A.; Charate, R.; Pothineni, N.V.K.; Aedma, S.K.; Gopinathannair, R.; Lakkireddy, D. Role of Digital Health During Coronavirus Disease 2019 Pandemic and Future Perspectives. Card. Electrophysiol. Clin. 2022, 14, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Bansal, A.; Joshi, R. Portable out-of-hospital electrocardiography: A review of current technologies. J. Arrhythmia 2018, 34, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Lowres, N.; Neubeck, L.; Salkeld, G.; Krass, I.; McLachlan, A.J.; Redfern, J.; Bennett, A.A.; Briffa, T.; Bauman, A.; Martinez, C.; et al. Feasibility and cost-effectiveness of stroke prevention through community screening for atrial fibrillation using iPhone ECG in pharmacies. Thromb. Haemost. 2014, 111, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Le Page, P. Living with the handheld ECG. BMJ Innov. 2015, 1, 46–48. [Google Scholar] [CrossRef]
- Vukčević, V.; Panescu, D.; Bojović, B.; George, S.; Gussak, I.; Giga, V.; Stanković, I. Wireless remote monitoring of myocardial ischemia using reconstructed 12-lead EKGs. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 1, pp. 2215–2220. [Google Scholar] [CrossRef]
- Vukajlović, D.; Bojović, B.; Hadžievski, L.; George, S.; Gussak, I.; Panescu, D. Wireless remote monitoring of atrial fibrillation using reconstructed 12-lead EKGs. In Proceedings of the 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, Buenos Aires, Argentina, 31 August–4 September 2010; Volume 2010, pp. 1113–1118. [Google Scholar] [CrossRef]
- George, S.; Vukčević, V.; Bojović, B.; Gussak, I.; Giga, V.; Stanković, I. Clinical utility of cardiobip in remote detection of 12-lead EKGs in patients with exercise-induced ischemia international academy of cardiology. In Proceedings of the 15th World Congress on Heart Disease, Vancouver, BC, Canada, 24–27 July 2010. [Google Scholar]
- Vukajlović, D.; Bojović, B.; Vukčević, V.; Gussak, I.; Mitrović, U.; Simić, G.; George, S. Atrial fibrillation remote monitoring using a handheld wireless remote monitor, international academy of cardiology. In Proceedings of the 15th World Congress on Heart Disease, Vancouver, BC, Canada, 24–27 July 2010. [Google Scholar]
- Hadžievski, L.; Bojović, B.; Vukčević, V.; Beličev, P.; Pavlović, S.; Vasiljević-Pokrajčić, Z.; Ostojić, M. A novel mobile transtelephonic system with synthesized 12-lead EKG. IEEE Trans. Inf. Technol. Biomed. 2004, 8, 428–438. [Google Scholar] [CrossRef]
- Rothman, S.A.; Laughlin, J.C.; Seltzer, J.; Walia, J.S.; Baman, R.I.; Siouffi, S.Y.; Sangrigoli, R.M.; Kowey, P.R. The diagnosis of cardiac arrhythmias: A prospective multi-center randomized study comparing mobile cardiac outpatient telemetry versus standard loop event monitoring. J. Cardiovasc. Electrophysiol. 2007, 18, 241–247. [Google Scholar] [CrossRef]
- Spaich, S.; Kern, H.; Zelniker, T.A.; Stiepak, J.; Gabel, M.; Popp, E.; Katus, H.A.; Preusch, M.R. Feasibility of CardioSecur®, a Mobile 4-Electrode/22-Lead ECG Device, in the Prehospital Emergency Setting. Front. Cardiovasc. Med. 2020, 9, 551796. [Google Scholar] [CrossRef]
- Evans, G.F.; Shirk, A.; Muturi, P.; Soliman, E.Z. Feasibility of Using Mobile ECG Recording Technology to Detect Atrial Fibrillation in Low-Resource Settings. Glob. Heart 2017, 12, 285–289. [Google Scholar] [CrossRef]
- García-Niebla, J.; Llontop-García, P.; Valle-Racero, J.I.; Serra-Autonell, G.; Batchvarov, V.N.; de Luna, A.B. Technical Mistakes during the Acquisition of the Electrocardiogram. Ann. Noninvasive Electrocardiol. 2009, 14, 389–403. [Google Scholar] [CrossRef]
- Stroobandt, R.X.; Barold, S.S.; Sinnaeve, A.F. ECG from Basics to Essentials, Step by Step; Wiley Blackwell: Hoboken, NJ, USA, 2016. [Google Scholar]
- Nigolian, A.; Dayal, N.; Nigolian, H.; Stettler, C.; Burri, H. Diagnostic accuracy of multi-lead ECGs obtained using a pocket-sized bipolar handheld event recorder. J. Electrocardiol. 2018, 51, 278–281. [Google Scholar] [CrossRef]
- McCullough, S.A.; Goyal, P.; Krishnan, U.; Choi, J.J.; Safford, M.M.; Okin, P.M. Electrocardiographic Findings in Coronavirus Disease-19, Insights on Mortality and Underlying Myocardial Processes. J. Card. Fail. 2020, 26, 626–632. [Google Scholar] [CrossRef] [PubMed]
- El-Sherif, N.; Turitto, G. Ambulatory electrocardiographic monitoring between artifacts and misinterpretation, management errors of commission and errors of omission. Ann. Noninvasive Electrocardiol. 2015, 20, 282–289. [Google Scholar] [CrossRef]
- Nelwan, S.; Crater, S.W.; Green, C.L.; Johanson, P.; Dam, T.B.; Meij, S.H.; Simoons, M.L.; Krucoff, M.W. Assessment of derived 12-lead electrocardiograms using general and patient-specific reconstruction strategies at rest and during transient myocardial ischemia. Am. J. Cardiol. 2004, 94, 1529–1533. [Google Scholar] [CrossRef] [PubMed]
- Nelwan, S.; Kors, J.A.; Crater, S.W.; Meij, S.H.; Dam, T.B.; Simoons, M.L.; Krucoff, M.W. Simultaneous comparison of 3 derived 12-lead electrocardiograms with standard electrocardiogram at rest and during percutaneous coronary occlusion. J. Electrocardiol. 2008, 41, 230–237. [Google Scholar] [CrossRef]
- Trobec, R.; Tomasic, I. Synthesis of the 12-lead electrocardiogram from differential leads. IEEE Trans. Inf. Technol. Biomed. 2011, 15, 615–621. [Google Scholar] [CrossRef]
- Tomasic, I.; Trobec, R. Electrocardiographic systems with reduced numbers of leads-synthesis of the 12-Lead ECG. IEEE Rev. Biomed. Eng. 2014, 7, 126–142. [Google Scholar] [CrossRef]
- Sejersten, M.; Pahlm, O.; Pettersson, J.; Clemmensen, P.M.; Rautaharju, F.; Zhou, S.; Maynard, C.; Feldman, C.L.; Wagner, G.S. The relative accuracies of ECG precordial lead waveforms derived from EASI leads and those acquired from paramedic applied standard leads. J. Electrocardiol. 2003, 36, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Sejersten, M.; Pahlm, O.; Pettersson, J.; Zhou, S.; Maynard, C.; Feldman, C.L.; Wagner, G.S. Comparison of EASI-derived 12-lead electrocardiograms versus paramedic-acquired 12-lead electrocardiograms using Mason-Likar limb lead configuration in patients with chest pain. J. Electrocardiol. 2006, 39, 13–21. [Google Scholar] [CrossRef]
- Schreck, D.M.; Fishberg, R.D. Derivation of the 12-lead electrocardiogram and 3-lead vectorcardiogram. Am. J. Emerg. Med. 2013, 31, 1183–1190. [Google Scholar] [CrossRef]
- Chantad, D.; Krittayaphong, R.; Komoltri, C. Derived 12-lead electrocardiogram in the assessment of ST-segment deviation and cardiac rhythm. J. Electrocardiol. 2006, 39, 7–12. [Google Scholar] [CrossRef]
- Lancia, L.; Toccaceli, A.; Petrucci, C.; Romano, S.; Penco, M. Continuous ECG Monitoring in Patients with Acute Coronary Syndrome or Heart Failure: EASI Versus Gold Standard. Clin. Nurs. Res. 2018, 27, 433–449. [Google Scholar] [CrossRef] [PubMed]
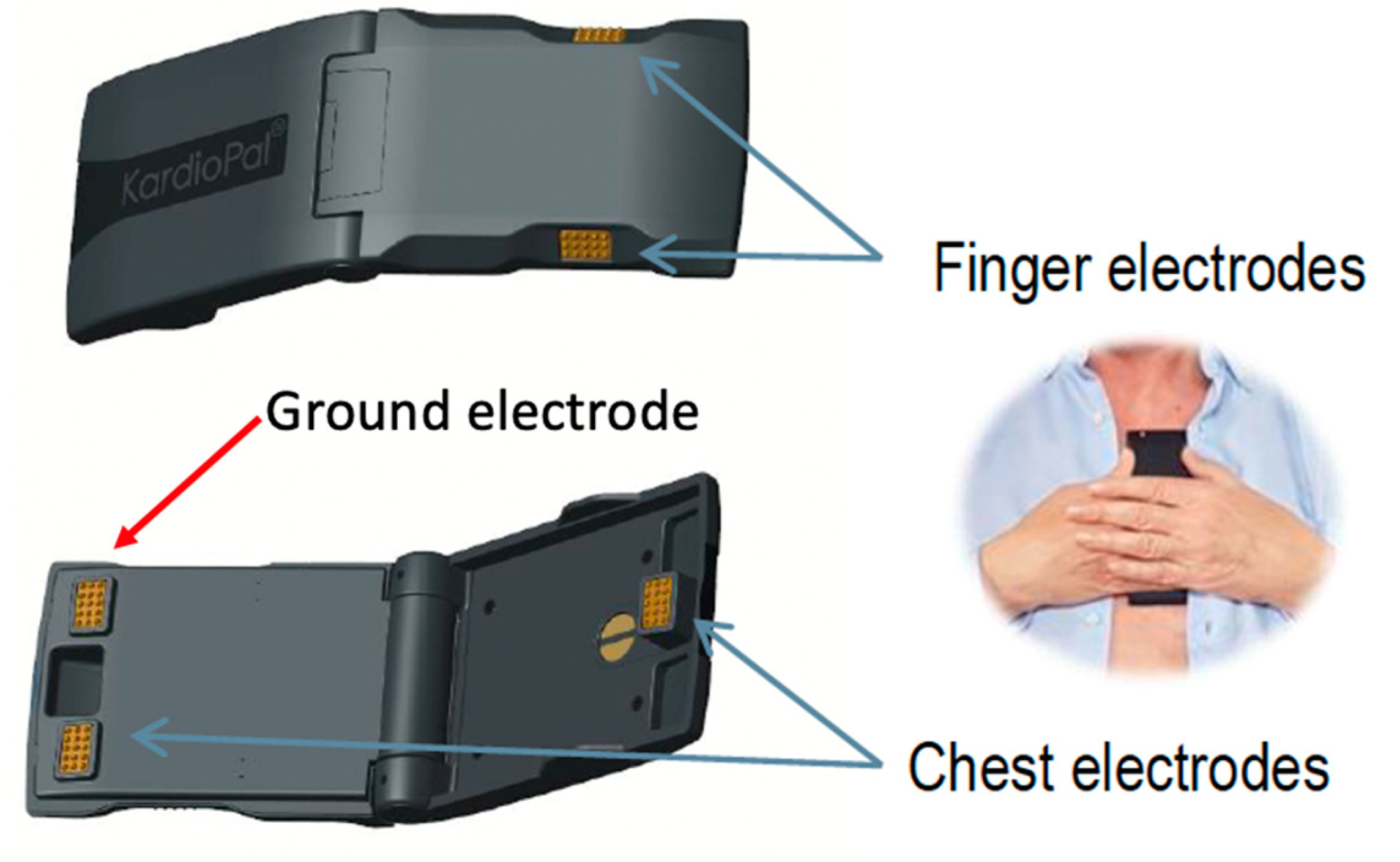





| Adequate | 3 Points (Pass) | No significant differences in signal shapes or voltages |
| Acceptable | 2 Points (Pass) | Noticeable differences in signal shapes or voltages, but these differences do not affect the appropriate clinical decision-making (no need for additional diagnostic procedures or different therapeutic approach). |
| Inadequate | 1 Point (Fail) | Significant differences in signal shapes or voltages that affect the appropriate clinical decision-making |
| Age | 57.0 ± 14.3 |
| >65 years | 25 (24.5%) |
| BMI | 27.4 ± 3.8 |
| >30 | 24 (23.5%) |
| Gender (Male) | 62 (60.8%) |
| Risk factors: | |
| Hypertension | 67 (65.6%) |
| Diabetes mellitus | 12 (11.7%) |
| Dyslipidemia | 38 (37.2%) |
| Heredity | 47 (46.1%) |
| Smoking habit | 77 (75.5%) |
| Previous myocardial infarction | 16 (15.8%) |
| Therapy: | |
| Beta-blocker | 59 (57.8%) |
| ACE inhibitor | 47 (46.1%) |
| Diuretic | 29 (28.4%) |
| Antiplatelet drug | 47 (46.1%) |
| Anticoagulant drug | 19 (18.6%) |
| Nitroglycerin | 7 (6.8%) |
| Statins | 36 (35.2%) |
| Atrial Fibrillation | 14 (13.7%) |
| First-degree AV block | 3 (2.9%) |
| Inverted T wave | 6 (7.8%) |
| LBBB | 4 (3.9%) |
| RBBB | 4 (3.9%) |
| IVCD | 3 (2.9%) |
| LVH | 3 (2.9%) |
| Pacemaker | 1 (1.0%) |
| Q wave | 6 (5.9%) |
| Sinus Tachycardia | 1 (1.0%) |
| WPW | 1 (1.0%) |
| VPC | 1 (1.0%) |
| ST segment elevation | 4 (3.9%) |
| ST segment depression | 9 (9.8%) |
| Normal findings | 48 (47.1%) |
| Sensitivity | 96.30% (87.25–99.55%) |
| Specificity | 95.83% (85.75–99.49%) |
| Prevalence of pathological findings | 52.94% |
| Positive predictive value | 95.83% (85.50–98.90%) |
| Negative predictive value | 96.30% (86.99–99.02%) |
| Diagnostic accuracy | 96.08% (90.26–98.92%) |
| QRS (S) | QRS (K) | T (S) | T (K) | P (S) | P (K) | |
|---|---|---|---|---|---|---|
| Axes (degrees) | 19.35 | 10.87 | 26.63 | 21.20 | 38.84 | 33.41 |
| Standard deviation (degrees) | 36.89 | 36.61 | 62.18 | 65.05 | 44.62 | 48.82 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babic, M.D.; Veljkovic, S.; Lakcevic, J.; Babic, R.; Ostojic, M.; Petrovic, M.; Boljevic, D.; Tomic, S.; Bojic, M.; Nikolic, A. Telemedicine in the Era of a Pandemic: Usefulness of a Novel Three-Lead ECG. Diagnostics 2023, 13, 2525. https://doi.org/10.3390/diagnostics13152525
Babic MD, Veljkovic S, Lakcevic J, Babic R, Ostojic M, Petrovic M, Boljevic D, Tomic S, Bojic M, Nikolic A. Telemedicine in the Era of a Pandemic: Usefulness of a Novel Three-Lead ECG. Diagnostics. 2023; 13(15):2525. https://doi.org/10.3390/diagnostics13152525
Chicago/Turabian StyleBabic, Milos D., Stefan Veljkovic, Jovana Lakcevic, Rade Babic, Miodrag Ostojic, Masa Petrovic, Darko Boljevic, Stanko Tomic, Milovan Bojic, and Aleksandra Nikolic. 2023. "Telemedicine in the Era of a Pandemic: Usefulness of a Novel Three-Lead ECG" Diagnostics 13, no. 15: 2525. https://doi.org/10.3390/diagnostics13152525






