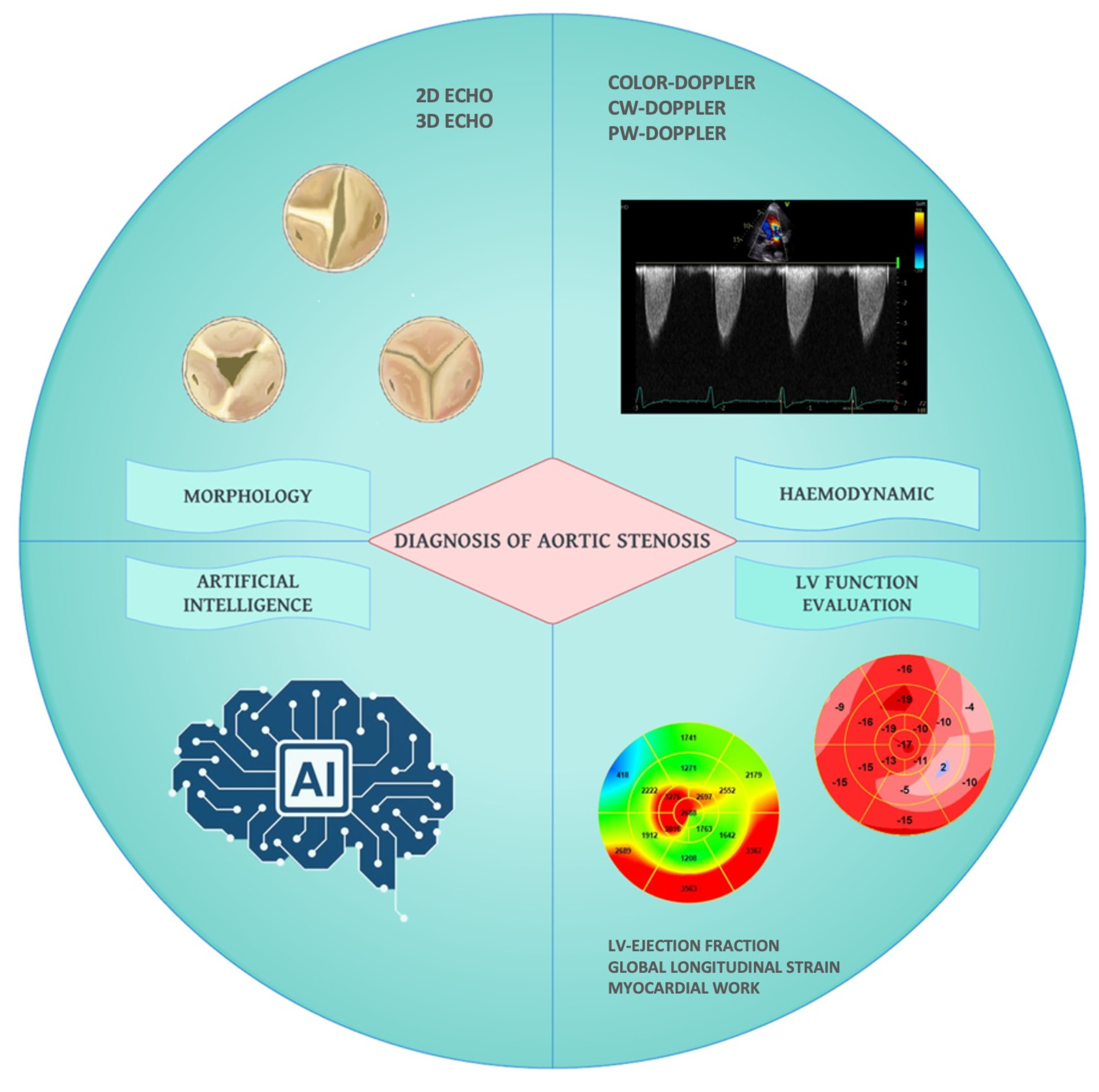
Echocardiographic Evaluation of Aortic Stenosis: A Comprehensive Review
Abstract
1. Introduction
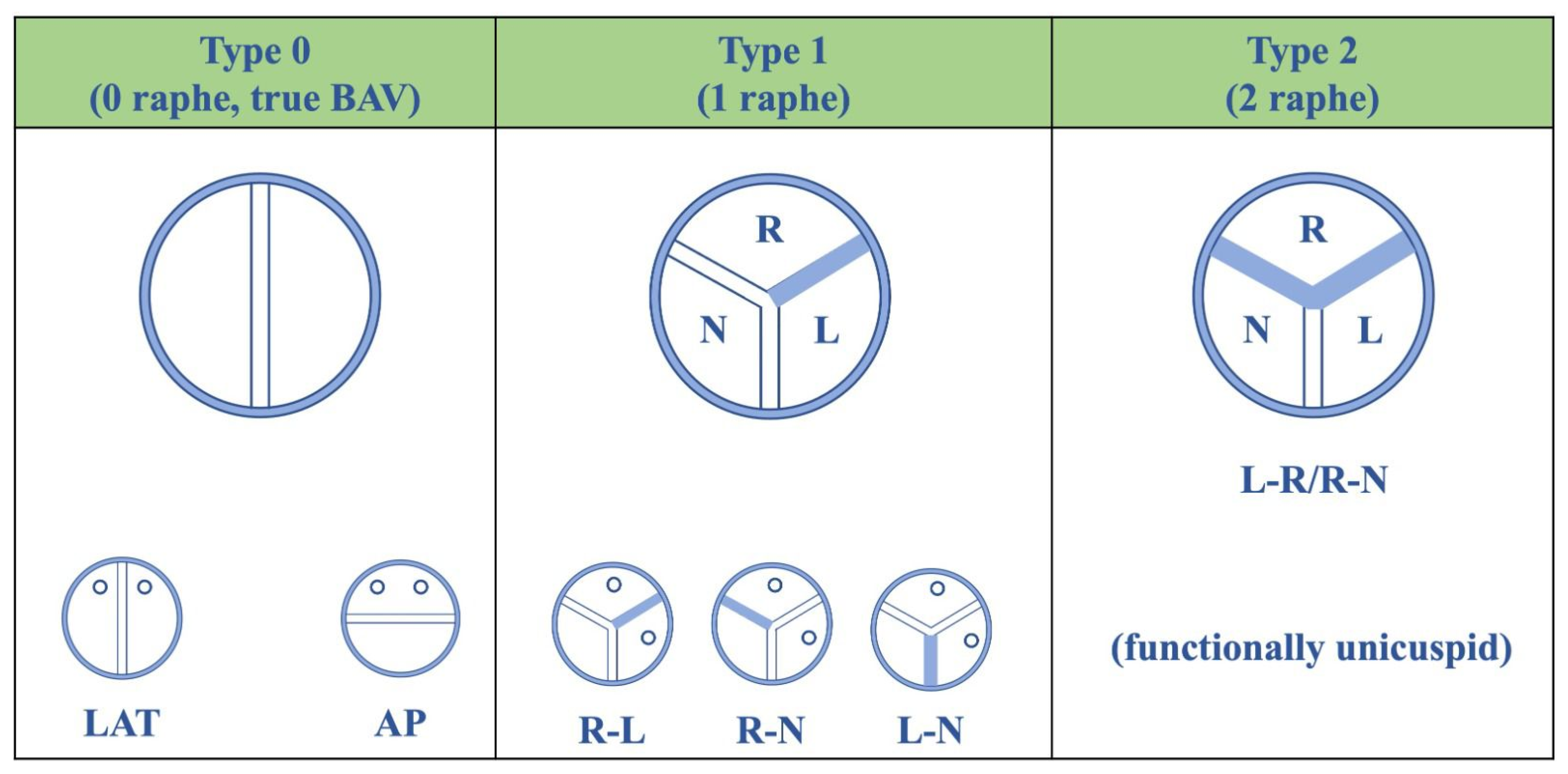
2. Aortic Stenosis Etiology and Echocardiographic Morphologic Assessment
3. Echocardiographic Assessment of AS Severity
3.1. Alternative Parameters Indicative of AS Severity
3.2. Role of 3D Echocardiography in the Evaluation of Aortic Stenosis
4. Discordant Grading of Aortic Stenosis
- a valve area >1.5 cm2, a peak velocity between 2.6 and 2.9 m/s, a mean gradient <20 mmHg define a mild stenosis;
- a valve area between 1.5 and 1 cm2, a peak velocity between 3 and 4 m/s, or a mean gradient between 20 and 40 mmHg define a moderate stenosis;
- a peak velocity ≥ 4 m/s, a mean gradient ≥ 40 mmHg and an AVA ≤ 1 cm2, or an indexed (to body surface area) AVA (AVAi) ≤ 0.6 cm2/m2 are the criteria proposed by current guidelines to identify severe stenosis.
- Classical low-flow low-gradient (cLFLG) AS, characterized by mean aortic transvalvular pressure gradient < 40 mmHg, SVi ≤ 35 mL/m2 and LVEF < 50%, and is detected in 5–10% of the AS cases;
- Paradoxical low-flow low-gradient (pLFLG) AS, characterized by mean aortic transvalvular pressure gradient < 40 mmHg, SVi ≤ 35 mL/m2 and LVEF ≥ 50%. In the vast majority, this type of AS affects women with small and concentric remodeled ventricles, that are responsible for the low flow state. In these patients, even the concomitant presence of mitral regurgitation, mitral stenosis, tricuspid regurgitation, and atrial fibrillation might explain the SV reduction.
- normal-flow low-gradient (NFLG) AS, instead, is defined by mean aortic transvalvular pressure gradient < 40 mmHg, a SVi > 35 mL/m2, and LVEF ≥ 50%. The reduction of the gradient despite a normal SVi can depend on a low transvalvular flow rate, calculated as SVi/LV ejection time. If the latter is prolonged, such as during bradycardia or because of systemic hypertension, the flow rate and consequently, the mean gradient will be lower [44].
4.1. Role of Dobutamine Stress Echocardiography
4.2. Role of Exercise Stress Echocardiography
5. Aortic Stenosis-Related Structural and Functional Changes
6. Artificial Intelligence in Aortic Stenosis Severity Assessment
7. Limitations of Echocardiography in the Assessment of Aortic Stenosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durko, A.P.; Osnabrugge, R.L.; van Mieghem, N.M.; Milojevic, M.; Mylotte, D.; Nkomo, V.T.; Pieter Kappetein, A. Annual Number of Candidates for Transcatheter Aortic Valve Implantation per Country: Current Estimates and Future Projections. Eur. Heart J. 2018, 39, 2635–2642. [Google Scholar] [CrossRef] [PubMed]
- Nicoara, A.; Skubas, N.; Ad, N.; Finley, A.; Hahn, R.T.; Mahmood, F.; Mankad, S.; Nyman, C.B.; Pagani, F.; Porter, T.R.; et al. Guidelines for the Use of Transesophageal Echocardiography to Assist with Surgical Decision-Making in the Operating Room: A Surgery-Based Approach: From the American Society of Echocardiography in Collaboration with the Society of Cardiovascular Anesthesiologists and the Society of Thoracic Surgeons. J. Am. Soc. Echocardiogr. 2020, 33, 692–734. [Google Scholar] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Edvardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F.; Otto, C.M. Recommendations on the Echocardiographic Assessment of Aortic Valve Stenosis: A Focused Update from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2017, 30, 372–392. [Google Scholar] [CrossRef]
- Lindman, B.R.; Clavel, M.A.; Mathieu, P.; Iung, B.; Lancellotti, P.; Otto, C.M.; Pibarot, P. Calcific Aortic Stenosis. Nat. Rev. Dis. Primers 2016, 2, 16006. [Google Scholar] [CrossRef]
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of Valvular Heart Diseases: A Population-Based Study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Sverdlov, A.L.; Ngo, D.T.; Chapman, M.J.; Ali, O.A.; Chirkov, Y.Y.; Horowitz, J.D. Pathogenesis of Aortic Stenosis: Not Just a Matter of Wear and Tear. Am. J. Cardiovasc. Dis. 2011, 1, 185–199. [Google Scholar]
- Roberts, W.C.; Ko, J.M. Frequency by Decades of Unicuspid, Bicuspid, and Tricuspid Aortic Valves in Adults Having Isolated Aortic Valve Replacement for Aortic Stenosis, with or without Associated Aortic Regurgitation. Circulation 2005, 111, 920–925. [Google Scholar] [CrossRef]
- Movahed, M.R.; Hepner, A.D.; Ahmadi-Kashani, M. Echocardiographic Prevalence of Bicuspid Aortic Valve in the Population. Heart Lung Circ. 2006, 15, 297–299. [Google Scholar] [CrossRef]
- Sillesen, A.-S.; Vøgg, O.; Pihl, C.; Raja, A.A.; Sundberg, K.; Vedel, C.; Zingenberg, H.; Jørgensen, F.S.; Vejlstrup, N.; Iversen, K.; et al. Prevalence of Bicuspid Aortic Valve and Associated Aortopathy in Newborns in Copenhagen, Denmark. JAMA 2021, 325, 561–567. [Google Scholar] [CrossRef]
- Remenyi, B.; Elguindy, A.; Smith, S.C.; Yacoub, M.; Holmes, D.R. Valvular Aspects of Rheumatic Heart Disease. Lancet 2016, 387, 1335–1346. [Google Scholar] [CrossRef]
- Novaro, G.M.; Mishra, M.; Griffin, B.P. Incidence and Echocardiographic Features of Congenital Unicuspid Aortic Valve in an Adult Population. J. Heart Valve Dis. 2003, 12, 674–678. [Google Scholar] [PubMed]
- Tsang, M.Y.C.; Abudiab, M.M.; Ammash, N.M.; Naqvi, T.Z.; Edwards, W.D.; Nkomo, V.T.; Pellikka, P.A. Quadricuspid Aortic Valve: Characteristics, Associated Structural Cardiovascular Abnormalities, and Clinical Outcomes. Circulation 2016, 133, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Nemchyna, O.; Soltani, S.; Solowjowa, N.; Schoenrath, F.; Hrytsyna, Y.; Unbehaun, A.; Kempfert, J.; Stein, J.; Knosalla, C.; Hagendorff, A.; et al. Validity of Visual Assessment of Aortic Valve Morphology in Patients with Aortic Stenosis Using Two-Dimensional Echocardiography. Int. J. Cardiovasc. Imaging 2021, 37, 813–823. [Google Scholar] [CrossRef]
- Devabhaktuni, S.R.; Chakfeh, E.; Malik, A.O.; Pengson, J.A.; Rana, J.; Ahsan, C.H. Subvalvular Aortic Stenosis: A Review of Current Literature. Clin. Cardiol. 2018, 41, 131–136. [Google Scholar] [CrossRef]
- Merla, G.; Brunetti-Pierri, N.; Piccolo, P.; Micale, L.; Loviglio, M.N. Supravalvular Aortic Stenosis. Circ. Cardiovasc. Genet. 2012, 5, 692–696. [Google Scholar] [CrossRef]
- Guner, A.; Havan, N.; Gunduz, S.; Akgun, T.; Guvendi, B.; Kahveci, G. Evaluation of the Congenital Supravalvular Aortic Stenosis by Different Imaging Modalities. Echocardiography 2017, 34, 1376–1378. [Google Scholar] [CrossRef] [PubMed]
- Currie, P.J.; Seward, J.B.; Reeder, G.S.; Vlietstra, R.E.; Bresnahan, D.R.; Bresnahan, J.F.; Smith, H.C.; Hagler, D.J.; Tajik, A.J. Continuous-Wave Doppler Echocardiographic Assessment of Severity of Calcific Aortic Stenosis: A Simultaneous Doppler-Catheter Correlative Study in 100 Adult Patients. Circulation 1985, 71, 1162–1169. [Google Scholar] [CrossRef]
- Thaden, J.J.; Nkomo, V.T.; Lee, K.J.; Oh, J.K. Doppler Imaging in Aortic Stenosis: The Importance of the Nonapical Imaging Windows to Determine Severity in a Contemporary Cohort. J. Am. Soc. Echocardiogr. 2015, 28, 780–785. [Google Scholar] [CrossRef]
- Benfari, G.; Gori, A.M.; Rossi, A.; Papesso, B.; Vassanelli, C.; Zito, G.B.; Nistri, S. Feasibility and Relevance of Right Parasternal View for Assessing Severity and Rate of Progression of Aortic Valve Stenosis in Primary Care. Int. J. Cardiol. 2017, 240, 446–451. [Google Scholar] [CrossRef]
- Kang, D.H.; Park, S.J.; Rim, J.H.; Yun, S.C.; Kim, D.H.; Song, J.M.; Choo, S.J.; Park, S.W.; Song, J.K.; Lee, J.W.; et al. Early Surgery versus Conventional Treatment in Asymptomatic Very Severe Aortic Stenosis. Circulation 2010, 121, 1502–1509. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Nishimura, R.A.; Bailey, K.R.; Tajik, A.J. The Natural History of Adults with Asymptomatic, Hemodynamically Significant Aortic Stenosis. J. Am. Coll. Cardiol. 1990, 15, 1012–1017. [Google Scholar] [CrossRef]
- Kitai, T.; Kaji, S.; Yamamuro, A.; Tani, T.; Tamita, K.; Kinoshita, M.; Ehara, N.; Kobori, A.; Nasu, M.; Okada, Y.; et al. Clinical Outcomes of Medical Therapy and Timely Operation in Initially Diagnosed Type A Aortic Intramural Hematoma A 20-Year Experience. Circulation 2009, 120, S292–S298. [Google Scholar] [CrossRef] [PubMed]
- Pellikka, P.A.; Sarano, M.E.; Nishimura, R.A.; Malouf, J.F.; Bailey, K.R.; Scott, C.G.; Barnes, M.E.; Tajik, A.J. Outcome of 622 Adults with Asymptomatic, Hemodynamically Significant Aortic Stenosis during Prolonged Follow-Up. Circulation 2005, 111, 3290–3295. [Google Scholar] [CrossRef]
- Nakatsuma, K.; Taniguchi, T.; Morimoto, T.; Shiomi, H.; Ando, K.; Kanamori, N.; Murata, K.; Kitai, T.; Kawase, Y.; Izumi, C.; et al. Prognostic Impact of Peak Aortic Jet Velocity in Conservatively Managed Patients with Severe Aortic Stenosis: An Observation From the CURRENT AS Registry. J. Am. Heart Assoc. 2017, 6, e005524. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur Heart J 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Ring, L.; Shah, B.N.; Bhattacharyya, S.; Harkness, A.; Belham, M.; Oxborough, D.; Pearce, K.; Rana, B.S.; Augustine, D.X.; Robinson, S.; et al. Echocardiographic Assessment of Aortic Stenosis: A Practical Guideline from the British Society of Echocardiography. Echo Res. Pract. 2021, 8, G19–G59. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Taliercio, C.P.; Holmes, D.R.; Reeder, G.S.; Bailey, K.R.; Seward, J.B.; Tajik, A.J. Prediction of the Severity of Aortic Stenosis by Doppler Aortic Valve Area Determination: Prospective Doppler-Catheterization Correlation in 100 Patients. J. Am. Coll. Cardiol. 1988, 11, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- LaBounty, T.M.; Miyasaka, R.; Chetcuti, S.; Grossman, P.M.; Deeb, G.M.; Patel, H.J.; Booher, A.; Patel, S.; Bach, D.S. Annulus Instead of LVOT Diameter Improves Agreement Between Echocardiography Effective Orifice Area and Invasive Aortic Valve Area. JACC Cardiovasc. Imaging 2014, 7, 1065–1066. [Google Scholar] [CrossRef]
- Cotella, J.I.; Miyoshi, T.; Mor-Avi, V.; Addetia, K.; Schreckenberg, M.; Sun, D.; Slivnick, J.A.; Blankenhagen, M.; Hitschrich, N.; Amuthan, V.; et al. Normative Values of the Aortic Valve Area and Doppler Measurements Using Two-Dimensional Transthoracic Echocardiography: Results from the Multicentre World Alliance of Societies of Echocardiography Study. Eur. Heart J. Cardiovasc. Imaging 2023, 24, 415–423. [Google Scholar] [CrossRef]
- Gamaza Chulián, S.; Díaz Retamino, E.; Carmona García, R.; Serrano Muñoz, B.; León Jiménez, J.; González Estriégana, S.; Oneto Otero, J. Prognostic Value of Aortic Valve Area Normalized to Body Size in Native Aortic Stenosis. Rev. Esp. Cardiol. (Engl. Ed.) 2021, 74, 44–50. [Google Scholar] [CrossRef]
- Kitai, T.; Tsutsui, R.S. The Contemporary Role of Echocardiography in the Assessment and Management of Aortic Stenosis. J. Med. Ultrason. 2020, 47, 71–80. [Google Scholar] [CrossRef]
- Gamaza-Chulián, S.; Díaz-Retamino, E.; Camacho-Freire, S.; Ruiz-Fernández, D.; Gutiérrez-Barrios, A.; Oneto-Otero, J. Acceleration Time and Ratio of Acceleration Time to Ejection Time in Aortic Stenosis: New Echocardiographic Diagnostic Parameters. J. Am. Soc. Echocardiogr. 2017, 30, 947–955. [Google Scholar] [CrossRef]
- Lancellotti, P.; Magne, J. Valvuloarterial Impedance in Aortic Stenosis: Look at the Load, but Do Not Forget the Flow. Eur. J. Echocardiogr. 2011, 12, 354–357. [Google Scholar] [CrossRef]
- Hachicha, Z.; Dumesnil, J.G.; Pibarot, P. Usefulness of the Valvuloarterial Impedance to Predict Adverse Outcome in Asymptomatic Aortic Stenosis. J. Am. Coll. Cardiol. 2009, 54, 1003–1011. [Google Scholar] [CrossRef] [PubMed]
- Altiok, E.; Koos, R.; Schröder, J.; Brehmer, K.; Hamada, S.; Becker, M.; Mahnken, A.H.; Almalla, M.; Dohmen, G.; Autschbach, R.; et al. Comparison of Two-Dimensional and Three-Dimensional Imaging Techniques for Measurement of Aortic Annulus Diameters before Transcatheter Aortic Valve Implantation. Heart 2011, 97, 1578–1584. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Delgado, V.; van der Kley, F.; Shanks, M.; van de Veire, N.R.L.; Bertini, M.; Nucifora, G.; van Bommel, R.J.; Tops, L.F.; de Weger, A.; et al. Comparison of Aortic Root Dimensions and Geometries Before and After Transcatheter Aortic Valve Implantation by 2- and 3-Dimensional Transesophageal Echocardiography and Multislice Computed Tomography. Circ. Cardiovasc. Imaging 2010, 3, 94–102. [Google Scholar] [CrossRef]
- Dulgheru, R.; Pibarot, P.; Sengupta, P.P.; Piérard, L.A.; Rosenhek, R.; Magne, J.; Donal, E.; Bernard, A.; Fattouch, K.; Cosyns, B.; et al. Multimodality Imaging Strategies for the Assessment of Aortic Stenosis. Circ. Cardiovasc. Imaging 2016, 9, e004352. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.A.; Cowell, S.J.; White, A.C.; Boon, N.A.; Newby, D.E.; Northridge, D.B. Contrast Agent Increases Doppler Velocities and Improves Reproducibility of Aortic Valve Area Measurements in Patients with Aortic Stenosis. J. Am. Soc. Echocardiogr. 2004, 17, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC); European Association for Cardio-Thoracic Surgery (EACTS); Vahanian, A.; Alfieri, O.; Andreotti, F.; Antunes, M.J.; Barón-Esquivias, G.; Baumgartner, H.; Borger, M.A.; Carre, T.P.; et al. Guidelines on the Management of Valvular Heart Disease (Version 2012). Eur. Heart J. 2012, 33, 2451–2496. [Google Scholar]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Guyton, R.A.; O’Gara, P.T.; Ruiz, C.E.; Skubas, N.J.; Sorajja, P.; et al. 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129, 2440–2492. [Google Scholar]
- Minners, J.; Allgeier, M.; Gohlke-Baerwolf, C.; Kienzle, R.-P.; Neumann, F.-J.; Jander, N. Inconsistencies of Echocardiographic Criteria for the Grading of Aortic Valve Stenosis. Eur. Heart J. 2008, 29, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Falk, V.; Bax, J.J.; De Bonis, M.; Hamm, C.; Holm, P.J.; Iung, B.; Lancellotti, P.; Lansac, E.; Rodriguez Muñoz, D.; et al. 2017 ESC/EACTS Guidelines for the Management of Valvular Heart Disease. Eur. Heart J. 2017, 38, 2739–2791. [Google Scholar] [CrossRef] [PubMed]
- Clavel, M.-A.; Magne, J.; Pibarot, P. Low-Gradient Aortic Stenosis. Eur. Heart J. 2016, 37, 2645–2657. [Google Scholar] [CrossRef]
- Kadem, L.; Pibarot, P.; Dumesnil, J.G.; Mouret, F.; Garitey, V.; Durand, L.-G.; Rieu, R. Independent Contribution of Left Ventricular Ejection Time to the Mean Gradient in Aortic Stenosis. J. Heart Valve Dis. 2002, 11, 615–623. [Google Scholar]
- Rusinaru, D.; Bohbot, Y.; Ringle, A.; Maréchaux, S.; Diouf, M.; Tribouilloy, C. Impact of Low Stroke Volume on Mortality in Patients with Severe Aortic Stenosis and Preserved Left Ventricular Ejection Fraction. Eur. Heart J. 2018, 39, 1992–1999. [Google Scholar] [CrossRef] [PubMed]
- Guzzetti, E.; Poulin, A.; Annabi, M.-S.; Zhang, B.; Kalavrouziotis, D.; Couture, C.; Dagenais, F.; Pibarot, P.; Clavel, M.-A. Transvalvular Flow, Sex, and Survival After Valve Replacement Surgery in Patients with Severe Aortic Stenosis. J. Am. Coll. Cardiol. 2020, 75, 1897–1909. [Google Scholar] [CrossRef] [PubMed]
- Stassen, J.; Ewe, S.H.; Singh, G.K.; Butcher, S.C.; Hirasawa, K.; Amanullah, M.R.; Pio, S.M.; Sin, K.Y.K.; Ding, Z.P.; Sia, C.-H.; et al. Prevalence and Prognostic Implications of Discordant Grading and Flow-Gradient Patterns in Moderate Aortic Stenosis. J. Am. Coll. Cardiol. 2022, 80, 666–676. [Google Scholar] [CrossRef]
- Nishimura, R.A.; Otto, C.M.; Bonow, R.O.; Carabello, B.A.; Erwin, J.P.; Fleisher, L.A.; Jneid, H.; Mack, M.J.; McLeod, C.J.; O’Gara, P.T.; et al. 2017 AHA/ACC Focused Update of the 2014 AHA/ACC Guideline for the Management of Patients with Valvular Heart Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2017, 70, 252–289. [Google Scholar] [CrossRef]
- Otto, C.M.; Pearlman, A.S.; Kraft, C.D.; Miyake-Hull, C.Y.; Burwash, I.G.; Gardner, C.J. Physiologic Changes with Maximal Exercise in Asymptomatic Valvular Aortic Stenosis Assessed by Doppler Echocardiography. J. Am. Coll. Cardiol. 1992, 20, 1160–1167. [Google Scholar] [CrossRef]
- Blais, C.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Loho, N.; Rader, F.; Baumgartner, H.; Beanlands, R.S.; Chayer, B.; Kadem, L.; et al. Projected Valve Area at Normal Flow Rate Improves the Assessment of Stenosis Severity in Patients with Low-Flow, Low-Gradient Aortic Stenosis: The Multicenter TOPAS (Truly or Pseudo-Severe Aortic Stenosis) Study. Circulation 2006, 113, 711–721. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Burwash, I.G.; Mundigler, G.; Dumesnil, J.G.; Baumgartner, H.; Bergler-Klein, J.; Sénéchal, M.; Mathieu, P.; Couture, C.; Beanlands, R.; et al. Validation of Conventional and Simplified Methods to Calculate Projected Valve Area at Normal Flow Rate in Patients with Low Flow, Low Gradient Aortic Stenosis: The Multicenter TOPAS (True or Pseudo Severe Aortic Stenosis) Study. J. Am. Soc. Echocardiogr. 2010, 23, 380–386. [Google Scholar] [CrossRef]
- Annabi, M.-S.; Touboul, E.; Dahou, A.; Burwash, I.G.; Bergler-Klein, J.; Enriquez-Sarano, M.; Orwat, S.; Baumgartner, H.; Mascherbauer, J.; Mundigler, G.; et al. Dobutamine Stress Echocardiography for Management of Low-Flow, Low-Gradient Aortic Stenosis. J. Am. Coll. Cardiol. 2018, 71, 475–485. [Google Scholar] [CrossRef]
- Clavel, M.-A.; Ennezat, P.V.; Maréchaux, S.; Dumesnil, J.G.; Capoulade, R.; Hachicha, Z.; Mathieu, P.; Bellouin, A.; Bergeron, S.; Meimoun, P.; et al. Stress Echocardiography to Assess Stenosis Severity and Predict Outcome in Patients with Paradoxical Low-Flow, Low-Gradient Aortic Stenosis and Preserved LVEF. JACC Cardiovasc. Imaging 2013, 6, 175–183. [Google Scholar] [CrossRef]
- Monin, J.L.; Monchi, M.; Gest, V.; Duval-Moulin, A.M.; Dubois-Rande, J.L.; Gueret, P. Aortic Stenosis with Severe Left Ventricular Dysfunction and Low Transvalvular Pressure Gradients: Risk Stratification by Low-Dose Dobutamine Echocardiography. J. Am. Coll. Cardiol. 2001, 37, 2101–2107. [Google Scholar] [CrossRef]
- Monin, J.-L.; Quéré, J.-P.; Monchi, M.; Petit, H.; Baleynaud, S.; Chauvel, C.; Pop, C.; Ohlmann, P.; Lelguen, C.; Dehant, P.; et al. Low-Gradient Aortic Stenosis: Operative Risk Stratification and Predictors for Long-Term Outcome: A Multicenter Study Using Dobutamine Stress Hemodynamics. Circulation 2003, 108, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Sankaramangalam, K.; Kandregula, K.; Bullen, J.A.; Kapadia, S.R.; Krishnaswamy, A.; Mick, S.; Rodriguez, L.L.; Grimm, R.A.; Menon, V.; et al. Contemporary Outcomes in Low-Gradient Aortic Stenosis Patients Who Underwent Dobutamine Stress Echocardiography. J. Am. Heart Assoc. 2019, 8, e011168. [Google Scholar] [CrossRef]
- Amato, M.C.; Moffa, P.J.; Werner, K.E.; Ramires, J.A. Treatment Decision in Asymptomatic Aortic Valve Stenosis: Role of Exercise Testing. Heart 2001, 86, 381–386. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The Clinical Use of Stress Echocardiography in Non-Ischaemic Heart Disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef] [PubMed]
- Calin, A.; Calin, A.; Mateescu, A.D.; Mateescu, A.D.; Popescu, A.C.; Popescu, A.C.; Bing, R.; Dweck, M.R.; Popescu, B.A.; Popescu, B.A. Role of Advanced Left Ventricular Imaging in Adults with Aortic Stenosis. Heart 2020, 106, 962–969. [Google Scholar] [CrossRef] [PubMed]
- Davin, L.; Nchimi, A.; Ilardi, F.; Dulgheru, R.; Marchetta, S.; Gach, O.; Marechal, P.; Cimino, S.; Bruyère, P.-J.; Georgiopoulos, A.; et al. Epicardial Adipose Tissue and Myocardial Fibrosis in Aortic Stenosis Relationship with Symptoms and Outcomes: A Study Using Cardiac Magnetic Resonance Imaging. JACC Cardiovasc. Imaging 2019, 12, 213–214. [Google Scholar] [CrossRef]
- Ito, S.; Miranda, W.R.; Oh, J.K. Assessment of Aortic Stenosis Beyond the Aortic Valve Area. Struct. Heart 2019, 3, 268–279. [Google Scholar] [CrossRef]
- Asami, M.; Stortecky, S.; Praz, F.; Lanz, J.; Räber, L.; Franzone, A.; Piccolo, R.; Siontis, G.C.M.; Heg, D.; Valgimigli, M.; et al. Prognostic Value of Right Ventricular Dysfunction on Clinical Outcomes After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Imaging 2019, 12, 577–587. [Google Scholar] [CrossRef]
- Eleid, M.F.; Padang, R.; Pislaru, S.V.; Greason, K.L.; Crestanello, J.; Nkomo, V.T.; Pellikka, P.A.; Jentzer, J.C.; Gulati, R.; Sandhu, G.S.; et al. Effect of Transcatheter Aortic Valve Replacement on Right Ventricular–Pulmonary Artery Coupling. JACC Cardiovasc. Interv. 2019, 12, 2145–2154. [Google Scholar] [CrossRef] [PubMed]
- Hutter, A.; Bleiziffer, S.; Richter, V.; Opitz, A.; Hettich, I.; Mazzitelli, D.; Ruge, H.; Lange, R. Transcatheter Aortic Valve Implantation in Patients with Concomitant Mitral and Tricuspid Regurgitation. Ann. Thorac. Surg. 2013, 95, 77–84. [Google Scholar] [CrossRef]
- Barbanti, M.; Binder, R.K.; Dvir, D.; Tan, J.; Freeman, M.; Thompson, C.R.; Cheung, A.; Wood, D.A.; Leipsic, J.; Webb, J.G. Prevalence and Impact of Preoperative Moderate/Severe Tricuspid Regurgitation on Patients Undergoing Transcatheter Aortic Valve Replacement. Catheter. Cardiovasc. Interv. 2015, 85, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Galli, E.; Guirette, Y.; Feneon, D.; Daudin, M.; Fournet, M.; Leguerrier, A.; Flecher, E.; Mabo, P.; Donal, E. Prevalence and Prognostic Value of Right Ventricular Dysfunction in Severe Aortic Stenosis. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Généreux, P.; Pibarot, P.; Redfors, B.; Mack, M.J.; Makkar, R.R.; Jaber, W.A.; Svensson, L.G.; Kapadia, S.; Tuzcu, E.M.; Thourani, V.H.; et al. Staging Classification of Aortic Stenosis Based on the Extent of Cardiac Damage. Eur. Heart J. 2017, 38, 3351–3358. [Google Scholar] [CrossRef]
- Avvedimento, M.; Franzone, A.; Leone, A.; Piccolo, R.; Castiello, D.S.; Ilardi, F.; Mariani, A.; Esposito, R.; Iapicca, C.; Angellotti, D.; et al. Extent of Cardiac Damage and Mortality in Patients Undergoing Transcatheter Aortic Valve Implantation. J. Clin. Med. 2021, 10, 4563. [Google Scholar] [CrossRef]
- Wang, Z. Echocardiographic Assessment in Patients with Aortic Stenosis and LV Dysfunction: Is It Time to Add Strain? Cardiovasc. Revasc Med. 2020, 21, 986–988. [Google Scholar] [CrossRef]
- Ng, A.C.T.; Prihadi, E.A.; Antoni, M.L.; Bertini, M.; Ewe, S.H.; Marsan, N.A.; Leung, D.Y.; Delgado, V.; Bax, J.J. Left Ventricular Global Longitudinal Strain Is Predictive of All-Cause Mortality Independent of Aortic Stenosis Severity and Ejection Fraction. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 859–867. [Google Scholar] [CrossRef]
- Fries, B.; Liu, D.; Gaudron, P.; Hu, K.; Nordbeck, P.; Ertl, G.; Weidemann, F.; Herrmann, S. Role of Global Longitudinal Strain in the Prediction of Outcome in Patients with Severe Aortic Valve Stenosis. Am. J. Cardiol. 2017, 120, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Kusunose, K.; Goodman, A.; Parikh, R.; Barr, T.; Agarwal, S.; Popovic, Z.B.; Grimm, R.A.; Griffin, B.P.; Desai, M.Y. Incremental Prognostic Value of Left Ventricular Global Longitudinal Strain in Patients with Aortic Stenosis and Preserved Ejection Fraction. Circ. Cardiovasc. Imaging 2014, 7, 938–945. [Google Scholar] [CrossRef]
- Dahl, J.S.; Videbæk, L.; Poulsen, M.K.; Rudbæk, T.R.; Pellikka, P.A.; Maller, J.E. Global Strain in Severe Aortic Valve Stenosis Relation to Clinical Outcome after Aortic Valve Replacement. Circ. Cardiovasc. Imaging 2012, 5, 613–620. [Google Scholar] [CrossRef]
- Ilardi, F.; Marchetta, S.; Martinez, C.; Sprynger, M.; Ancion, A.; Manganaro, R.; Sugimoto, T.; Tsugu, T.; Postolache, A.; Piette, C.; et al. Impact of Aortic Stenosis on Layer-Specific Longitudinal Strain: Relationship with Symptoms and Outcome. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Magne, J.; Cosyns, B.; Popescu, B.A.; Carstensen, H.G.; Dahl, J.; Desai, M.Y.; Kearney, L.; Lancellotti, P.; Marwick, T.H.; Sato, K.; et al. Distribution and Prognostic Significance of Left Ventricular Global Longitudinal Strain in Asymptomatic Significant Aortic Stenosis: An Individual Participant Data Meta-Analysis. JACC Cardiovasc. Imaging 2019, 12, 84–92. [Google Scholar] [CrossRef]
- Thellier, N.; Altes, A.; Appert, L.; Binda, C.; Leman, B.; Marsou, W.; Debry, N.; Joly, C.; Ennezat, P.-V.; Tribouilloy, C.; et al. Prognostic Importance of Left Ventricular Global Longitudinal Strain in Patients with Severe Aortic Stenosis and Preserved Ejection Fraction. J. Am. Soc. Echocardiogr. 2020, 33, 1454–1464. [Google Scholar] [CrossRef]
- Dahou, A.; Clavel, M.-A.; Capoulade, R.; Bartko, P.E.; Magne, J.; Mundigler, G.; Bergler-Klein, J.; Burwash, I.; Mascherbauer, J.; Ribeiro, H.B.; et al. Right Ventricular Longitudinal Strain for Risk Stratification in Low-Flow, Low-Gradient Aortic Stenosis with Low Ejection Fraction. Heart 2016, 102, 548–554. [Google Scholar] [CrossRef] [PubMed]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A Novel Clinical Method for Quantification of Regional Left Ventricular Pressure-Strain Loop Area: A Non-Invasive Index of Myocardial Work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Ilardi, F.; D’Andrea, A.; D’Ascenzi, F.; Bandera, F.; Benfari, G.; Esposito, R.; Malagoli, A.; Mandoli, G.E.; Santoro, C.; Russo, V.; et al. Myocardial Work by Echocardiography: Principles and Applications in Clinical Practice. J. Clin. Med. 2021, 10, 4521. [Google Scholar] [CrossRef]
- Jain, R.; Bajwa, T.; Roemer, S.; Huisheree, H.; Allaqaband, S.Q.; Kroboth, S.; Perez Moreno, A.C.; Tajik, A.J.; Khandheria, B.K. Myocardial Work Assessment in Severe Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 715–721. [Google Scholar] [CrossRef]
- Fortuni, F.; Butcher, S.C.; van der Kley, F.; Lustosa, R.P.; Karalis, I.; de Weger, A.; Priori, S.G.; van der Bijl, P.; Bax, J.J.; Delgado, V.; et al. Left Ventricular Myocardial Work in Patients with Severe Aortic Stenosis. J. Am. Soc. Echocardiogr. 2021, 34, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, F.; Postolache, A.; Dulgheru, R.; Trung, M.L.N.; de Marneffe, N.; Sugimoto, T.; Go, Y.Y.; Oury, C.; Esposito, G.; Lancellotti, P. Prognostic Value of Non-Invasive Global Myocardial Work in Asymptomatic Aortic Stenosis. J. Clin. Med. 2022, 11, 1555. [Google Scholar] [CrossRef] [PubMed]
- Playford, D.; Bordin, E.; Talbot, L.; Mohamad, R.; Anderson, B.; Strange, G. Analysis of Aortic Stenosis Using Artificial Intelligence. Heart Lung Circ. 2018, 27, S216. [Google Scholar] [CrossRef]
- Kebed, K.; Sun, D.; Addetia, K.; Mor-Avi, V.; Markuzon, N.; Lang, R.M. Measurement Errors in Serial Echocardiographic Assessments of Aortic Valve Stenosis Severity. Int. J. Cardiovasc. Imaging 2020, 36, 471. [Google Scholar] [CrossRef] [PubMed]
- Thalappillil, R.; Datta, P.; Datta, S.; Zhan, Y.; Wells, S.; Mahmood, F.; Cobey, F.C. Artificial Intelligence for the Measurement of the Aortic Valve Annulus. J. Cardiothorac. Vasc. Anesth. 2020, 34, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Wessler, B.S.; Huang, Z.; Long, G.M.; Pacifici, S.; Prashar, N.; Karmiy, S.; Sandler, R.A.; Sokol, J.Z.; Sokol, D.B.; Dehn, M.M.; et al. Automated Detection of Aortic Stenosis Using Machine Learning. J. Am. Soc. Echocardiogr. 2023, 36, 411–420. [Google Scholar] [CrossRef]
- Dai, W.; Nazzari, H.; Namasivayam, M.; Hung, J.; Stultz, C.M. Identifying Aortic Stenosis with a Single Parasternal Long-Axis Video Using Deep Learning. J. Am. Soc. Echocardiogr. 2023, 36, 116–118. [Google Scholar] [CrossRef]
- Sánchez-Puente, A.; Dorado-Díaz, P.I.; Sampedro-Gómez, J.; Bermejo, J.; Martinez-Legazpi, P.; Fernández-Avilés, F.; Sánchez-González, J.; Pérez Del Villar, C.; Vicente-Palacios, V.; Sanchez, P.L. Machine Learning to Optimize the Echocardiographic Follow-Up of Aortic Stenosis. JACC Cardiovasc. Imaging 2023, 16, 733–744. [Google Scholar] [CrossRef]
- Lachmann, M.; Rippen, E.; Schuster, T.; Xhepa, E.; von Scheidt, M.; Pellegrini, C.; Trenkwalder, T.; Rheude, T.; Stundl, A.; Thalmann, R.; et al. Subphenotyping of Patients with Aortic Stenosis by Unsupervised Agglomerative Clustering of Echocardiographic and Hemodynamic Data. JACC Cardiovasc. Interv. 2021, 14, 2127–2140. [Google Scholar] [CrossRef]
- Sengupta, P.P.; Shrestha, S.; Kagiyama, N.; Hamirani, Y.; Kulkarni, H.; Yanamala, N.; Bing, R.; Chin, C.W.L.; Pawade, T.A.; Messika-Zeitoun, D.; et al. A Machine-Learning Framework to Identify Distinct Phenotypes of Aortic Stenosis Severity. JACC Cardiovasc. Imaging 2021, 14, 1707–1720. [Google Scholar] [CrossRef]
- Pawade, T.; Sheth, T.; Guzzetti, E.; Dweck, M.R.; Clavel, M.-A. Why and How to Measure Aortic Valve Calcification in Patients with Aortic Stenosis. JACC Cardiovasc. Imaging 2019, 12, 1835–1848. [Google Scholar] [CrossRef] [PubMed]


| Units | Formula/Method | Severe AS Cutoff | |
|---|---|---|---|
| AS jet velocity | m/s | Direct measure | >4.0 |
| Mean pressure gradient | mmHg | ΔP = Σ4v2/N | >40 |
| EOA | cm2 | Continuity equationAVA = (CSALVOT ∗ VTILVOT)/VTIAV | <1.0 |
| Indexed EOA | cm2/m2 | EOA normalized by BSA | <0.6 |
| Dimensionless index | VR = VLVOT/VAV | <0.25 | |
| Energy loss index | cm2/m2 | Indexed EOA accounting for ascending aorta ELI = [(AVA ∗ AoA)/AoA−AVA]/BSA | <0.5–0.6 |
| Valvuloarterial impedence | mmHg/mL/m2 | ZVA = (sBP + ∆Pnet)/SVi | 4.5–5 |
| Projected valve area at normal flow | cm2 | Estimated EOA at normal flowAVAproj = AVArest + VC ∗ (250−Qrest) | <1.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzo, R.; Ilardi, F.; Nappa, D.; Mariani, A.; Angellotti, D.; Immobile Molaro, M.; Sgherzi, G.; Castiello, D.S.; Simonetti, F.; Santoro, C.; et al. Echocardiographic Evaluation of Aortic Stenosis: A Comprehensive Review. Diagnostics 2023, 13, 2527. https://doi.org/10.3390/diagnostics13152527
Manzo R, Ilardi F, Nappa D, Mariani A, Angellotti D, Immobile Molaro M, Sgherzi G, Castiello DS, Simonetti F, Santoro C, et al. Echocardiographic Evaluation of Aortic Stenosis: A Comprehensive Review. Diagnostics. 2023; 13(15):2527. https://doi.org/10.3390/diagnostics13152527
Chicago/Turabian StyleManzo, Rachele, Federica Ilardi, Dalila Nappa, Andrea Mariani, Domenico Angellotti, Maddalena Immobile Molaro, Giulia Sgherzi, Domenico Simone Castiello, Fiorenzo Simonetti, Ciro Santoro, and et al. 2023. "Echocardiographic Evaluation of Aortic Stenosis: A Comprehensive Review" Diagnostics 13, no. 15: 2527. https://doi.org/10.3390/diagnostics13152527
APA StyleManzo, R., Ilardi, F., Nappa, D., Mariani, A., Angellotti, D., Immobile Molaro, M., Sgherzi, G., Castiello, D. S., Simonetti, F., Santoro, C., Canonico, M. E., Avvedimento, M., Piccolo, R., Franzone, A., & Esposito, G. (2023). Echocardiographic Evaluation of Aortic Stenosis: A Comprehensive Review. Diagnostics, 13(15), 2527. https://doi.org/10.3390/diagnostics13152527







