Is Prone Position [18F]FDG PET/CT Useful in Reducing Respiratory Motion Artifacts in Evaluating Hepatic Lesions?
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
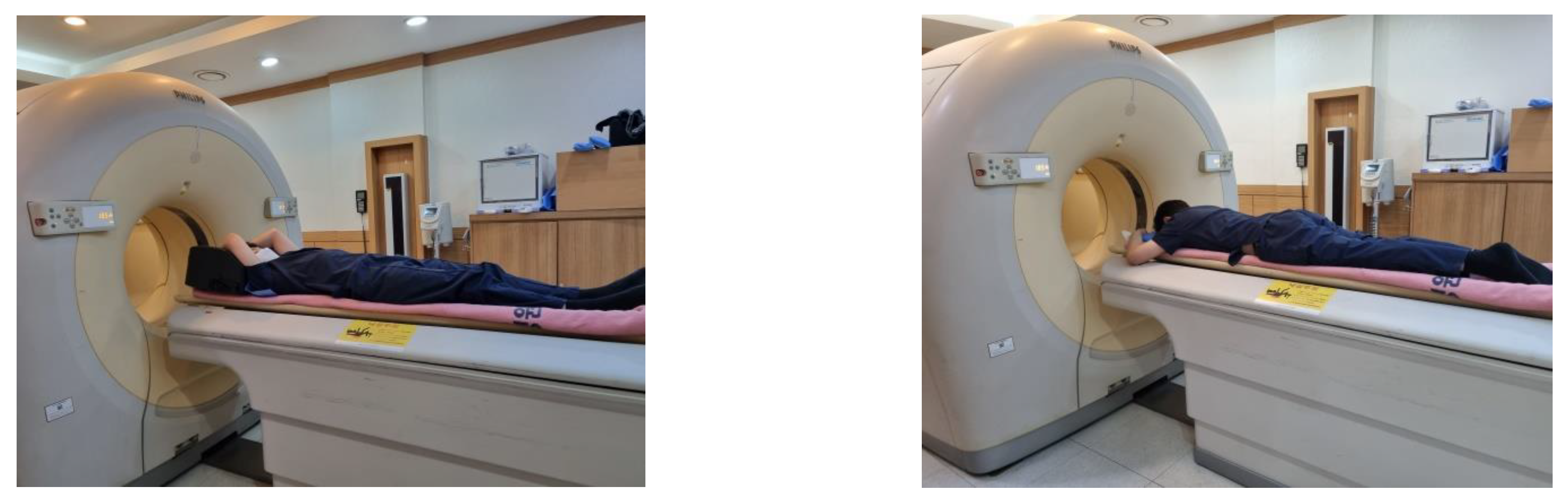
2.2. PET/CT Imaging Protocol
2.3. PET/CT Image Analysis
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moon, S.H.; Cho, Y.S.; Choi, J.Y. KSNM60 in clinical nuclear oncology. Nucl. Med. Mol. Imaging 2021, 55, 210–224. [Google Scholar] [CrossRef]
- Patton, J.A.; Delbeke, D.; Sandler, M.P. Image fusion using an integrated, dual-head coincidence camera with X-ray tube-based attenuation maps. J. Nucl. Med. 2000, 41, 1364–1368. [Google Scholar] [PubMed]
- Hasegawa, B.H.; Iwata, K.; Wong, K.H.; Wu, M.C.; Da Silva, A.J.; Tang, H.R.; Barber, W.C.; Hwang, A.H.; Sakdinawat, A.E. Dual-modality imaging of function and physiology. Acad. Radiol. 2002, 9, 1305–1321. [Google Scholar] [CrossRef] [PubMed]
- Schillaci, O.; Simonetti, G. Fusion imaging in nuclear medicine—Applications of dual-modality systems in oncology. Cancer Biother. Radiopharm. 2004, 19, 1–10. [Google Scholar] [CrossRef]
- Ozaki, K.; Harada, K.; Terayama, N.; Kosaka, N.; Kimura, H.; Gabata, T. FDG-PET/CT imaging findings of hepatic tumors and tumor-like lesions based on molecular background. Jpn. J. Radiol. 2020, 38, 697–718. [Google Scholar] [CrossRef]
- Blechacz, B.; Gores, G.J. Positron emission tomography scan for a hepatic mass. Hepatology 2010, 52, 2186–2191. [Google Scholar] [CrossRef]
- Joshi, U.; Raijmakers, P.G.; Riphagen, I.I.; Teule, G.J.; van Lingen, A.; Hoekstra, O.S. Attenuation-corrected vs. nonattenuation-corrected 2-deoxy-2-[F-18]fluoro-D-glucose-positron emission tomography in oncology: A systematic review. Mol. Imaging Biol. 2007, 9, 99–105. [Google Scholar] [CrossRef][Green Version]
- Kinahan, P.E.; Hasegawa, B.H.; Beyer, T. X-ray-based attenuation correction for positron emission tomography/computed tomography scanners. Semin. Nucl. Med. 2003, 33, 166–179. [Google Scholar] [CrossRef]
- Ay, M.R.; Zaidi, H. Computed tomography-based attenuation correction in neurological positron emission tomography: Evaluation of the effect of the X-ray tube voltage on quantitative analysis. Nucl. Med. Commun. 2006, 27, 339–346. [Google Scholar] [CrossRef]
- Gorospe, L.; Raman, S.; Echeveste, J.; Avril, N.; Herrero, Y.; Herna, S. Whole-body PET/CT: Spectrum of physiological variants, artifacts and interpretative pitfalls in cancer patients. Nucl. Med. Commun. 2005, 26, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Pettinato, C.; Nanni, C.; Farsad, M.; Castellucci, P.; Sarnelli, A.; Civollani, S.; Franchi, R.; Fanti, S.; Marengo, M.; Bergamini, C. Artefacts of PET/CT images. Biomed. Imaging Interv. J. 2006, 2, e60. [Google Scholar] [CrossRef] [PubMed]
- Lemeunier, L.; Maassmoreno, R.; Carrasquillo, J.A.; Dieckmann, W.; Bacharach, S.L. PET/CT imaging: Effect of respiratory motion on apparent myocardial uptake. J. Nucl. Cardiol. 2006, 13, 821–830. [Google Scholar] [CrossRef]
- Liu, C.; Pierce, L.A., 2nd; Alessio, A.M.; Kinahan, P.E. The impact of respiratory motion on tumor quantification and delineation in static PET/CT imaging. Phys. Med. Biol. 2009, 54, 7345–7362. [Google Scholar] [CrossRef] [PubMed]
- Nehmeh, S.A.; Erdi, Y.E. Respiratory motion in positron emission tomography/computed tomography: A review. Semin. Nucl. Med. 2008, 38, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.M.; Cohade, C.; Nakamoto, Y.; Wahl, R.L. Respiratory motion artifacts on PET emission images obtained using CT attenuation correction on PET-CT. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 603–606. [Google Scholar] [CrossRef]
- Papathanassiou, D.; Becker, S.; Amir, R.; Menéroux, B.; Liehn, J.C. Respiratory motion artefact in the liver dome on FDG PET/CT: Comparison of attenuation correction with CT and a caesium external source. Eur. J. Nucl. Med. Mol. Imaging 2005, 32, 1422–1428. [Google Scholar] [CrossRef]
- Park, S.J.; Ionascu, D.; Killoran, J.; Mamede, M.; Gerbaudo, V.H.; Chin, L.; Berbeco, R. Evaluation of the combined effects of target size, respiratory motion and background activity on 3D and 4D PET/CT images. Phys. Med. Biol. 2008, 53, 3661–3679. [Google Scholar] [CrossRef]
- Song, R.; Tipirneni, A.; Johnson, P.; Loeffler, R.B.; Hillenbrand, C.M. Evaluation of respiratory liver and kidney movements for MRI navigator gating. J. Magn. Reson. Imaging 2011, 33, 143–148. [Google Scholar] [CrossRef]
- Büther, F.; Jones, J.; Seifert, R.; Stegger, L.; Schleyer, P.; Schäfers, M. Clinical evaluation of a data-driven respiratory gating algorithm for whole-body PET with continuous bed motion. J. Nucl. Med. 2020, 61, 1520–1527. [Google Scholar] [CrossRef]
- Pépin, A.; Daouk, J.; Bailly, P.; Hapdey, S.; Meyer, M.E. Management of respiratory motion in PET/computed tomography: The state of the art. Nucl. Med. Commun. 2014, 35, 113–122. [Google Scholar] [CrossRef]
- Walker, M.D.; Morgan, A.J.; Bradley, K.M.; McGowan, D.R. Data-Driven Respiratory Gating Outperforms Device-Based Gating for Clinical 18F-FDG PET/CT. J. Nucl. Med. 2020, 61, 1678–1683. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Son, H.J.; Yun, M.; Moon, J.W.; Kim, Y.N.; Woo, J.Y.; Lee, S.H. Prone position [18F]FDG PET/CT to reduce respiratory motion artefacts in the evaluation of lung nodules. Eur. Radiol. 2021, 31, 4606–4614. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Engelberts, D.; Chen, H.; Li, X.; Katira, B.H.; Otulakowski, G.; Fujino, Y. Prone position minimizes the exacerbation of effort-dependent lung injury: Exploring the mechanism in pigs and evaluating injury in rabbits. Anesthesiology 2022, 136, 779–791. [Google Scholar] [CrossRef]
- Manna, E.M.; Ibraheim, O.A.; Samarkandi, A.H.; Alotaibi, W.M.; Elwatidy, S.M. The effect of prone position on respiratory mechanics during spinal surgery. Middle East J. Anaesthesiol. 2005, 18, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Nam, Y.; Yoon, A.M.; Kim, Y.H.; Yoon, S.H. The effect on respiratory mechanics when using a Jackson surgical table in the prone position during spinal surgery. Korean J. Anesthesiol. 2010, 59, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Şenay, H.; Sıvacı, R.; Kokulu, S.; Koca, B.; Bakı, E.D.; Ela, Y. The effect of pressure-controlled ventilation and volume-controlled ventilation in prone position on pulmonary mechanics and inflammatory markers. Inflammation 2016, 39, 1469–1474. [Google Scholar] [CrossRef]
- Boellaard, R.; Delgado-Bolton, R.; Oyen, W.J.; Giammarile, F.; Tatsch, K.; Eschner, W.; Verzijlbergen, F.J.; Barrington, S.F.; Pike, L.C.; Weber, W.A. FDG PET/CT: EANM Procedure Guidelines for Tumour Imaging, version 2.0. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 328–354. [Google Scholar] [CrossRef]
- van der Vos, C.S.; Grootjans, W.; Osborne, D.R.; Meeuwis, A.P.; Hamill, J.J.; Acuff, S.; de Geus-Oei, L.F.; Visser, E.P. Improving the spatial alignment in PET/CT using amplitude-based respiration-gated PET and respiration-triggered CT. J. Nucl. Med. 2015, 56, 1817–1822. [Google Scholar] [CrossRef]
- D’souza, M.M.; Sharma, R.; Mondal, A.; Jaimini, A.; Tripathi, M.; Saw, S.K.; Singh, D.; Mishra, A.; Tripathi, R.P. Prospective evaluation of CECT and 18F-FDG-PET/CT in detection of hepatic metastases. Nucl. Med. Commun. 2009, 30, 117–125. [Google Scholar] [CrossRef]
- Kim, Y.J.; Yun, M.; Lee, W.J.; Kim, K.S.; Lee, J.D. Usefulness of 18F-FDG PET in intrahepatic cholangiocarcinoma. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 1467–1472. [Google Scholar] [CrossRef]
- Kluge, R.; Schmidt, F.; Caca, K.; Barthel, H.; Hesse, S.; Georgi, P.; Seese, A.; Huster, D.; Berr, F. Positron emission tomography with [(18)F]fluoro-2-deoxy-D-glucose for diagnosis and staging of bile duct cancer. Hepatology 2001, 33, 1029–1035. [Google Scholar] [CrossRef]
- Rachh, S.; Basu, S. PET/CT in patients with liver lesions of different nature. Clin. Transl. Imaging 2014, 2, 139–155. [Google Scholar] [CrossRef]
- Tan, G.J.; Berlangieri, S.U.; Lee, S.T.; Scott, A.M. FDG PET/CT in the liver: Lesions mimicking malignancies. Abdom. Imaging 2014, 39, 187–195. [Google Scholar] [CrossRef]
- Beyer, T.; Antoch, G.; Blodgett, T.; Freudenberg, L.F.; Akhurst, T.; Mueller, S. Dual-modality PET/CT imaging: The effect of respiratory motion on combined image quality in clinical oncology. Eur. J. Nucl. Med. Mol. Imaging 2003, 30, 588–596. [Google Scholar] [CrossRef]
- Vogel, W.V.; Oyen, W.J.; Barentsz, J.O.; Kaanders, J.H.; Corstens, F.H. PET/CT: Panacea, redundancy, or something in between? J. Nucl. Med. 2004, 45, 15S–24S. [Google Scholar]
- Sureshbabu, W.; Mawlawi, O. PET/CT imaging artifacts. J. Nucl. Med. Technol. 2005, 33, 156–161, quiz 163–154. [Google Scholar]
- Farid, K.; Poullias, X.; Alifano, M.; Regnard, J.F.; Servois, V.; Caillat-Vigneron, N.; Petras, S. Respiratory-gated imaging in metabolic evaluation of small solitary pulmonary nodules: 18F-FDG PET/CT and correlation with histology. Nucl. Med. Commun. 2015, 36, 722–727. [Google Scholar] [CrossRef]
- Guerra, L.; De Ponti, E.; Elisei, F.; Bettinardi, V.; Landoni, C.; Picchio, M.; Gilardi, M.C.; Versari, A.; Fioroni, F.; Dziuk, M.; et al. Respiratory gated PET/CT in a European multicentre retrospective study: Added diagnostic value in detection and characterization of lung lesions. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1381–1390. [Google Scholar] [CrossRef]
- Van Der Gucht, A.; Serrano, B.; Hugonnet, F.; Paulmier, B.; Garnier, N.; Faraggi, M. Impact of a new respiratory amplitude-based gating technique in evaluation of upper abdominal PET lesions. Eur. J. Radiol. 2014, 83, 509–515. [Google Scholar] [CrossRef]
- Otani, Y.; Fukuda, I.; Tsukamoto, N.; Kumazaki, Y.; Sekine, H.; Imabayashi, E.; Kawaguchi, O.; Nose, T.; Teshima, T.; Dokiya, T. A comparison of the respiratory signals acquired by different respiratory monitoring systems used in respiratory gated radiotherapy. Med. Phys. 2010, 37, 6178–6186. [Google Scholar] [CrossRef]
- Boucher, L.; Rodrigue, S.; Lecomte, R.; Bénard, F. Respiratory gating for 3-dimensional PET of the thorax: Feasibility and initial results. J. Nucl. Med. 2004, 45, 214–219. [Google Scholar]
- Zhang, T.; Keller, H.; O’Brien, M.J.; Mackie, T.R.; Paliwal, B. Application of the spirometer in respiratory gated radiotherapy. Med. Phys. 2003, 30, 3165–3171. [Google Scholar] [CrossRef]
- Daouk, J.; Fin, L.; Bailly, P.; Meyer, M.E. Respiratory-gated positron emission tomography and breath-hold computed tomography coupling to reduce the influence of respiratory motion: Methodology and feasibility. Acta Radiol. 2009, 50, 144–155. [Google Scholar] [CrossRef]
- Nehmeh, S.A.; Erdi, Y.E.; Ling, C.C.; Rosenzweig, K.E.; Schoder, H.; Larson, S.M.; Macapinlac, H.A.; Squire, O.D.; Humm, J.L. Effect of respiratory gating on quantifying PET images of lung cancer. J. Nucl. Med. 2002, 43, 876–881. [Google Scholar]
- Shin, K.M.; Choi, J.; Chae, K.J.; Jin, G.Y.; Eskandari, A.; Hoffman, E.A.; Hall, C.; Castro, M.; Lee, C.H. Quantitative CT-based image registration metrics provide different ventilation and lung motion patterns in prone and supine positions in healthy subjects. Respir. Res. 2020, 21, 254. [Google Scholar] [CrossRef]
- Frood, R.; McDermott, G.; Scarsbrook, A. Respiratory-gated PET/CT for pulmonary lesion characterisation-promises and problems. Br. J. Radiol. 2018, 91, 20170640. [Google Scholar] [CrossRef]




| Patient Characteristics | n = 20 |
|---|---|
| Age at diagnosis, year (mean ± SD) | 67.0 ± 9.7 |
| Weight, kg (mean ± SD) | 62.5 ± 9.0 |
| Sex | |
| Male, n (%) | 14 (70%) |
| Female, n (%) | 6 (30%) |
| Reason for [18F]FDG PET/CT | |
| Diagnosis and initial staging, n (%) | 15 (75%) |
| Recurrence, n (%) | 5 (25%) |
| Number of measured hepatic lesions | |
| 1 | 12 (60%) |
| 2 | 5 (25%) |
| 4 | 2 (10%) |
| 5 | 1 (5%) |
| Lesion Characteristics | n = 35 |
|---|---|
| Size, mm (mean ± SD) | 13.0 ± 5.8 |
| Diagnosis of hepatic lesions | |
| Hepatic metastasis, n (%) | 30 (86%) |
| Pancreas cancer, n (%) | 8 (23%) |
| Breast cancer, n (%) | 6 (17%) |
| Gastric cancer, n (%) | 6 (17%) |
| Urothelial cancer, n (%) | 4 (11%) |
| Colorectal cancer, n (%) | 3 (9%) |
| Lung cancer, n (%) | 1 (3%) |
| Common bile duct cancer, n (%) | 1 (3%) |
| Subglottic cancer, n (%) | 1 (3%) |
| Intrahepatic cholangiocarcinoma, n (%) | 3 (9%) |
| Hepatic abscess, n (%) | 2 (6%) |
| Confirmation of hepatic lesions | |
| Pathological, n (%) | 6 (17%) |
| Clinical, n (%) | 29 (83%) |
| Location | |
| I, n (%) | 0 (0%) |
| II, n (%) | 6 (17%) |
| III, n (%) | 2 (6%) |
| IV, n (%) | 8 (23%) |
| V, n (%) | 3 (9%) |
| VI, n (%) | 7 (20%) |
| VII, n (%) | 3 (9%) |
| VIII, n (%) | 6 (17%) |
| Distance from the diaphragm, mm (mean ± SD) | 32.2 ± 25.1 |
| Parameters | Supine | Prone | p-Value |
|---|---|---|---|
| SUVmax, (mean ± SD) | 4.41 ± 2.05 | 4.23 ± 1.83 | 0.240 |
| MTV, cm3 (mean ± SD) | 5.83 ± 6.69 | 5.95 ± 6.24 | 0.672 |
| Location | |||
| Anterior, n (%) | 17 (49%) | 23 (66%) | 0.005 * |
| Middle, n (%) | 10 (29%) | 8 (23%) | |
| Posterior, n (%) | 8 (23%) | 4 (11%) |
| Reasons | Change of SUVmax (%) | n (%) | |
|---|---|---|---|
| Median | Range | ||
| [18F]FDG uptake outside the liver on CT in sPET/CT | 15% | [7% to 71%] | 4 (11%) |
| More blurring in sPET/CT | 11% | [−3% to 32%] | 6 (17%) |
| Unremarkable | 1% | [−8% to 18%] | 12 (34%) |
| More blurring in pPET/CT | −19% | [−30% to −8%] | 12 (34%) |
| [18F]FDG uptake outside the liver on CT in pPET/CT | −30% | [−30% to −30%] | 1 (3%) |
| Total patients | −4% | [−30% to 71%] | 35 (100%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.W.; Son, H.J.; Woo, J.Y.; Lee, S.H. Is Prone Position [18F]FDG PET/CT Useful in Reducing Respiratory Motion Artifacts in Evaluating Hepatic Lesions? Diagnostics 2023, 13, 2539. https://doi.org/10.3390/diagnostics13152539
Lee CW, Son HJ, Woo JY, Lee SH. Is Prone Position [18F]FDG PET/CT Useful in Reducing Respiratory Motion Artifacts in Evaluating Hepatic Lesions? Diagnostics. 2023; 13(15):2539. https://doi.org/10.3390/diagnostics13152539
Chicago/Turabian StyleLee, Chung Won, Hye Joo Son, Ji Young Woo, and Suk Hyun Lee. 2023. "Is Prone Position [18F]FDG PET/CT Useful in Reducing Respiratory Motion Artifacts in Evaluating Hepatic Lesions?" Diagnostics 13, no. 15: 2539. https://doi.org/10.3390/diagnostics13152539
APA StyleLee, C. W., Son, H. J., Woo, J. Y., & Lee, S. H. (2023). Is Prone Position [18F]FDG PET/CT Useful in Reducing Respiratory Motion Artifacts in Evaluating Hepatic Lesions? Diagnostics, 13(15), 2539. https://doi.org/10.3390/diagnostics13152539






