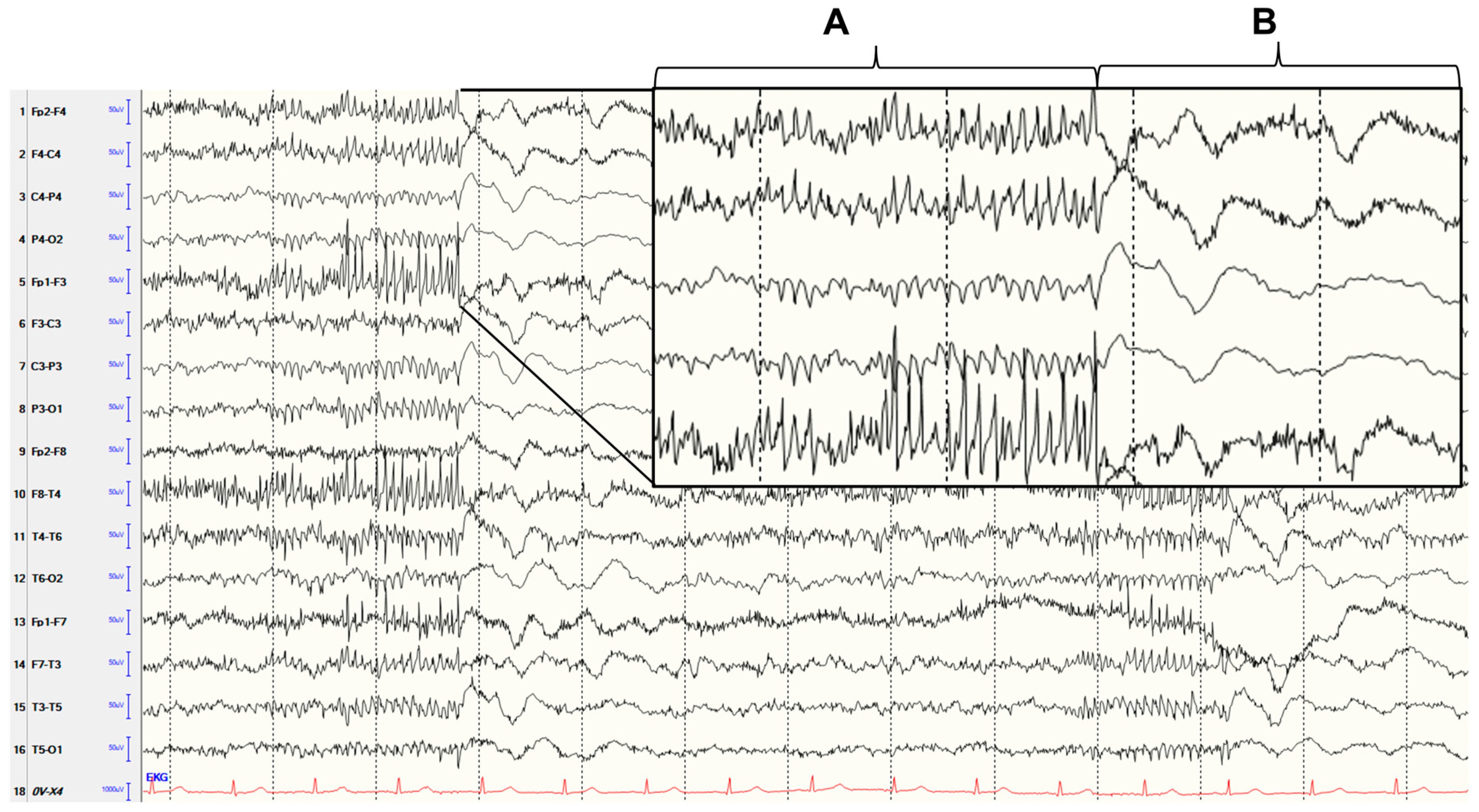
Generalized Polyspike Pattern in EEG Due to Aseptic Meningoencephalitis
Abstract
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maturu, M.V.S.; Datla, A.V.; Maturu, P.; Talla, V.B.; Dalai, S. Unilateral Autoimmune Encephalitis: A Case Report on a Rare Manifestation of Myelin Oligodendrocyte Glycoprotein Antibody Disease. Cureus 2023, 15, e34994. [Google Scholar] [CrossRef] [PubMed]
- Daida, K.; Nishioka, K.; Takanashi, M.; Kobayashi, M.; Yoshikawa, K.; Kusunoki, S.; Yokoyama, K.; Hattori, N. New-onset refractory status epilepticus involving the limbic system, spinal cord, and peripheral nerves. Intern. Med. 2020, 59, 267–270. [Google Scholar] [CrossRef] [PubMed]
- Arya, R.; Anand, V.; Chansoria, M. Hashimoto encephalopathy presenting as progressive myoclonus epilepsy syndrome. Eur. J. Paediatr. Neurol. 2013, 17, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.D.; Solomon, T. Seizures and encephalitis: Clinical features, management, and potential pathophysiologic mechanisms. Epilepsia 2012, 53, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Sellner, J.; Trinka, E. Seizures and epilepsy in herpes simplex virus encephalitis: Current concepts and future directions of pathogenesis and management. J. Neurol. 2012, 259, 2019–2030. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Meng, Y.; Najjar, A.; Lado, F.; Najjar, S. Autoimmune Encephalitis: A Physician’s Guide to the Clinical Spectrum Diagnosis and Management. Brain Sci. 2022, 12, 1130. [Google Scholar] [CrossRef] [PubMed]
- Pollak, L.; Klein, C.; Schiffer, J.; Flechter, S.; Rabey, J.M. Electroencephalographic abnormalities in aseptic meningitis and noninfectious headache. A comparative study. Headache 2001, 41, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.S.; Cross, J.H.; French, J.A.; Higurashi, N.; Hirsch, E.; Jansen, F.E.; Lagae, L.; Moshé, S.L.; Peltola, J.; Roulet Perez, E.; et al. Operational classification of seizure types by the International League Against Epilepsy: Position Paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017, 58, 522–530. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hobert, M.A.; Dargvainiene, J.; Margraf, N.G. Generalized Polyspike Pattern in EEG Due to Aseptic Meningoencephalitis. Diagnostics 2023, 13, 2569. https://doi.org/10.3390/diagnostics13152569
Hobert MA, Dargvainiene J, Margraf NG. Generalized Polyspike Pattern in EEG Due to Aseptic Meningoencephalitis. Diagnostics. 2023; 13(15):2569. https://doi.org/10.3390/diagnostics13152569
Chicago/Turabian StyleHobert, Markus A., Justina Dargvainiene, and Nils G. Margraf. 2023. "Generalized Polyspike Pattern in EEG Due to Aseptic Meningoencephalitis" Diagnostics 13, no. 15: 2569. https://doi.org/10.3390/diagnostics13152569
APA StyleHobert, M. A., Dargvainiene, J., & Margraf, N. G. (2023). Generalized Polyspike Pattern in EEG Due to Aseptic Meningoencephalitis. Diagnostics, 13(15), 2569. https://doi.org/10.3390/diagnostics13152569






