MR-Imaging in Osteoarthritis: Current Standard of Practice and Future Outlook
Abstract
1. Introduction
2. Osteoarthritis—A Whole Joint Disease
3. Diagnosing Osteoarthritis
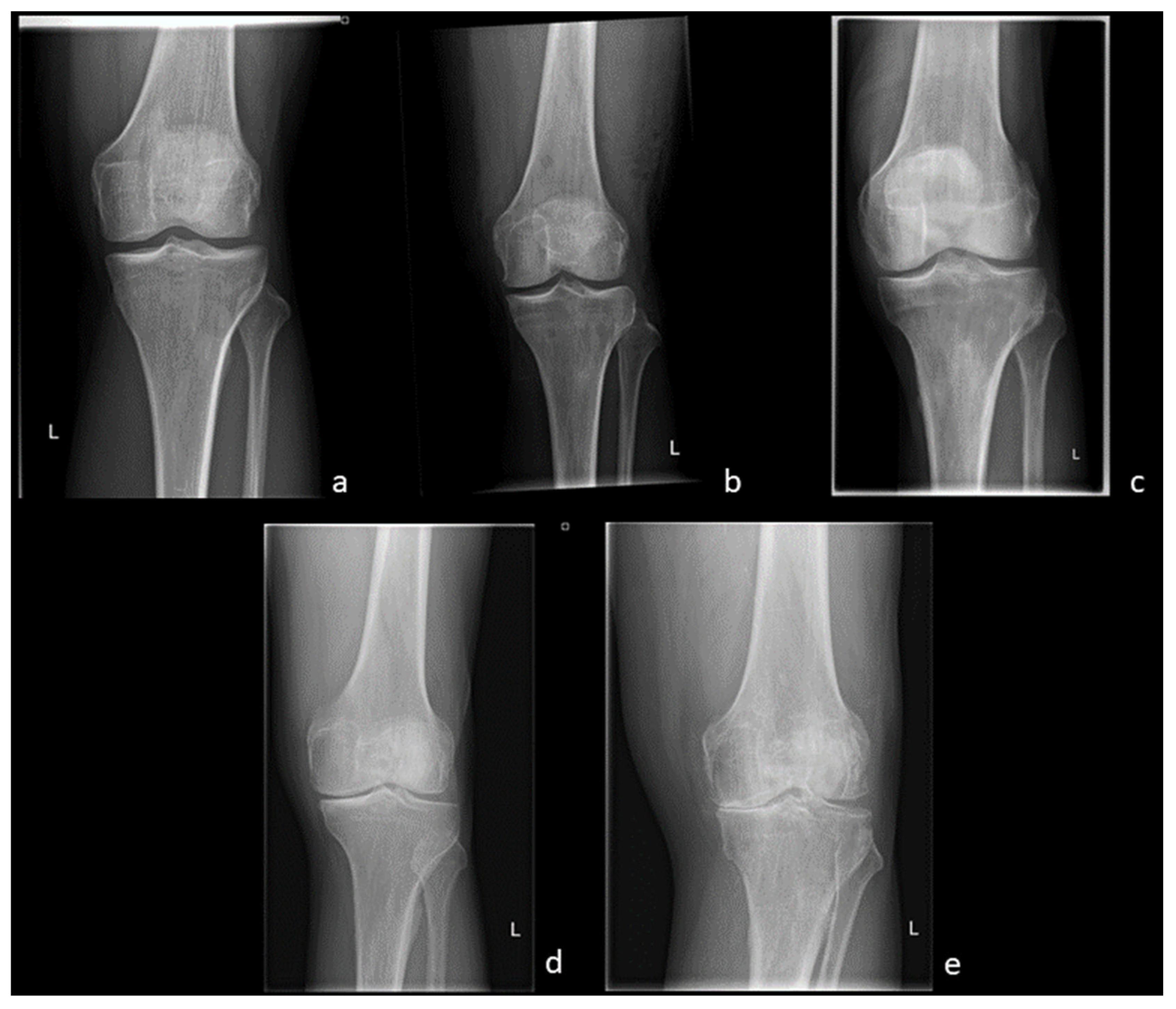
3.1. Radiography
3.2. MRI in Musculoskeletal Imaging
3.3. MR Acquisition Protocols—The Current Standard of Clinical Care
3.4. Fat Suppression
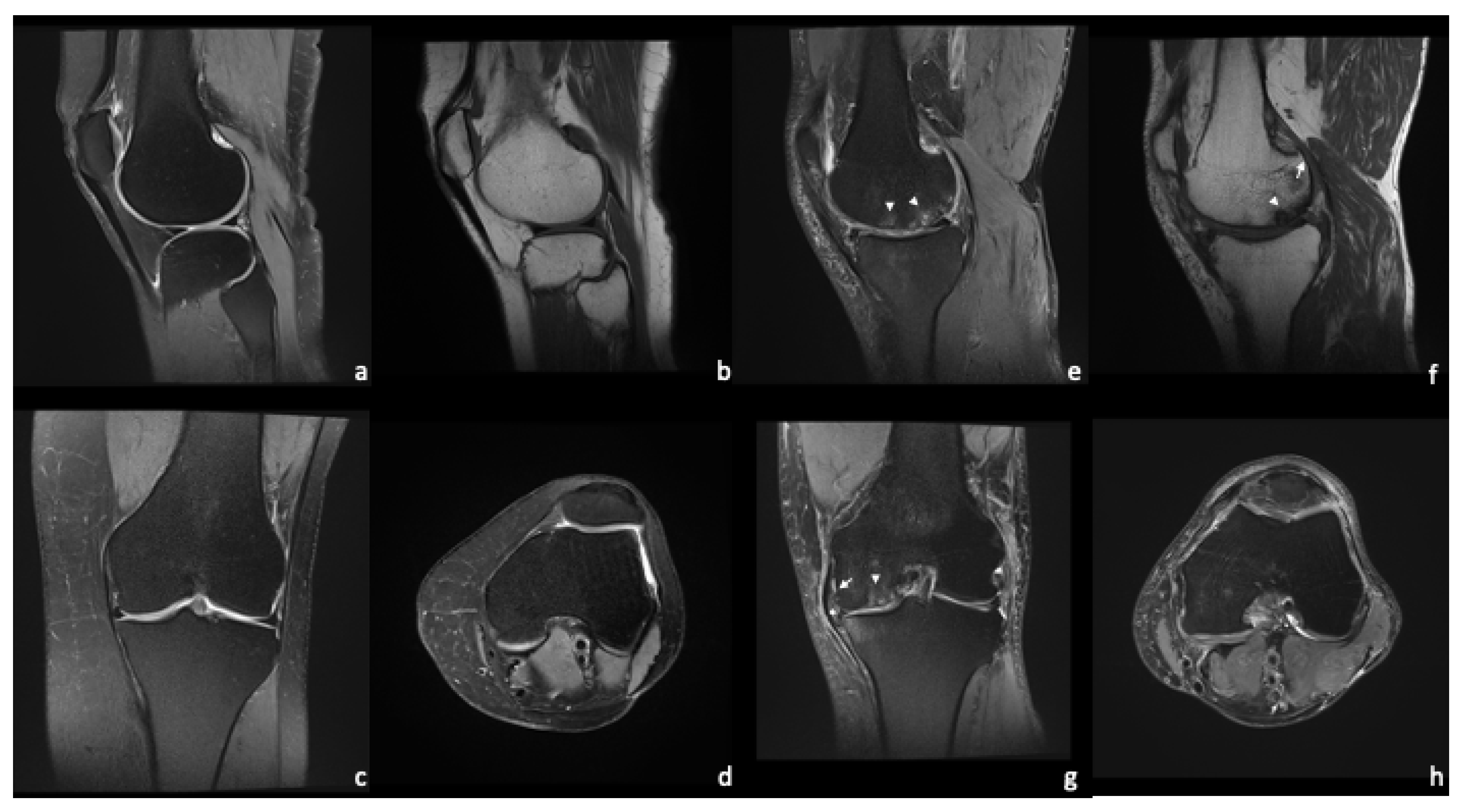
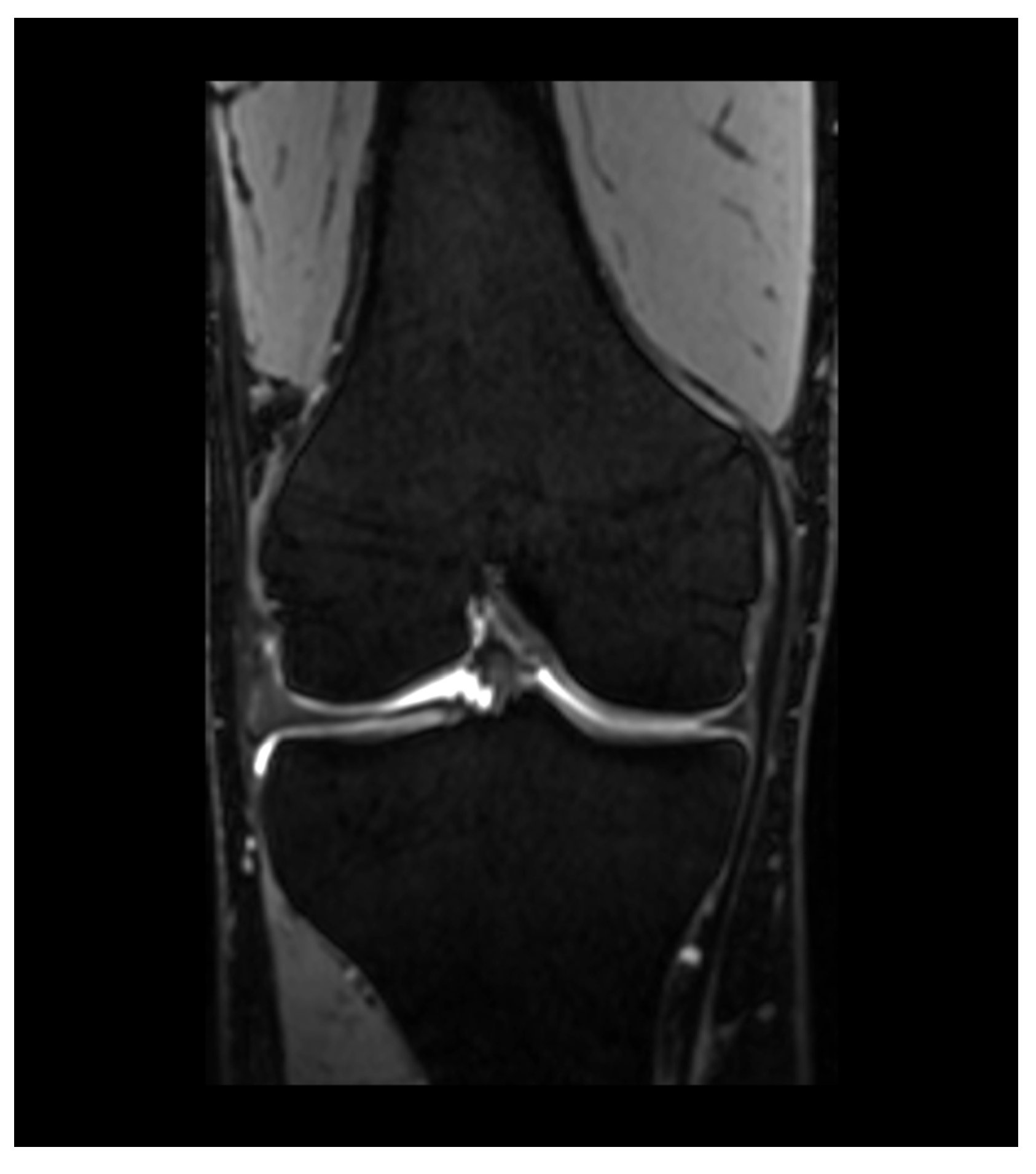
4. Magnetic Resonance Imaging—Common Findings
5. Additional MRI-Techniques
5.1. Three-Dimensional Image Acquisition
5.2. UTE-/ZTE-Imaging
6. Functional Assessment on Real-Time MRI
6.1. Quantitative MRI
6.2. Compositional MRI
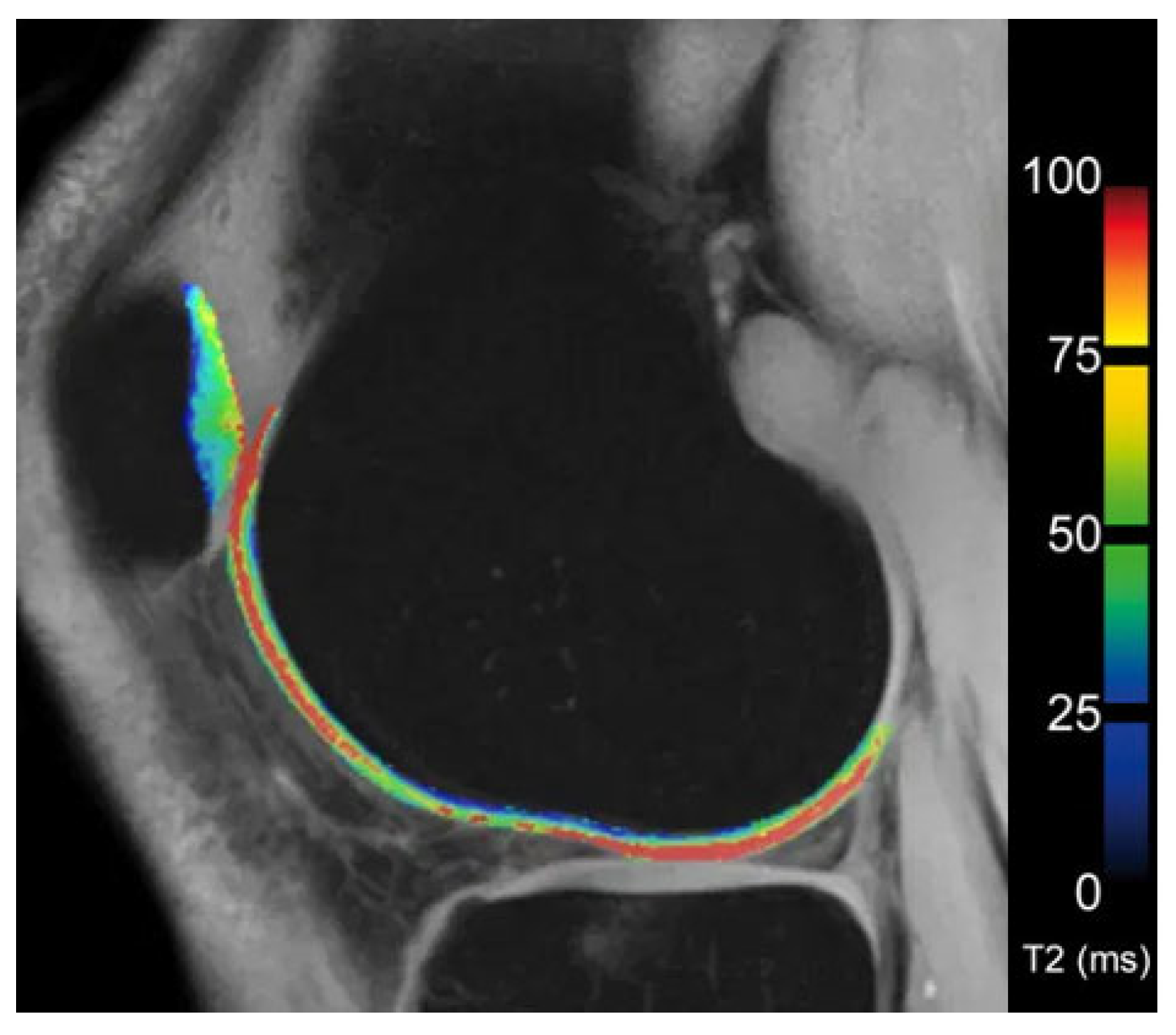
6.3. T2 Mapping
6.4. T1-Rho Mapping
6.5. dGEMRIC
6.6. DWI
6.7. gagCest Imaging
6.8. Sodium Imaging
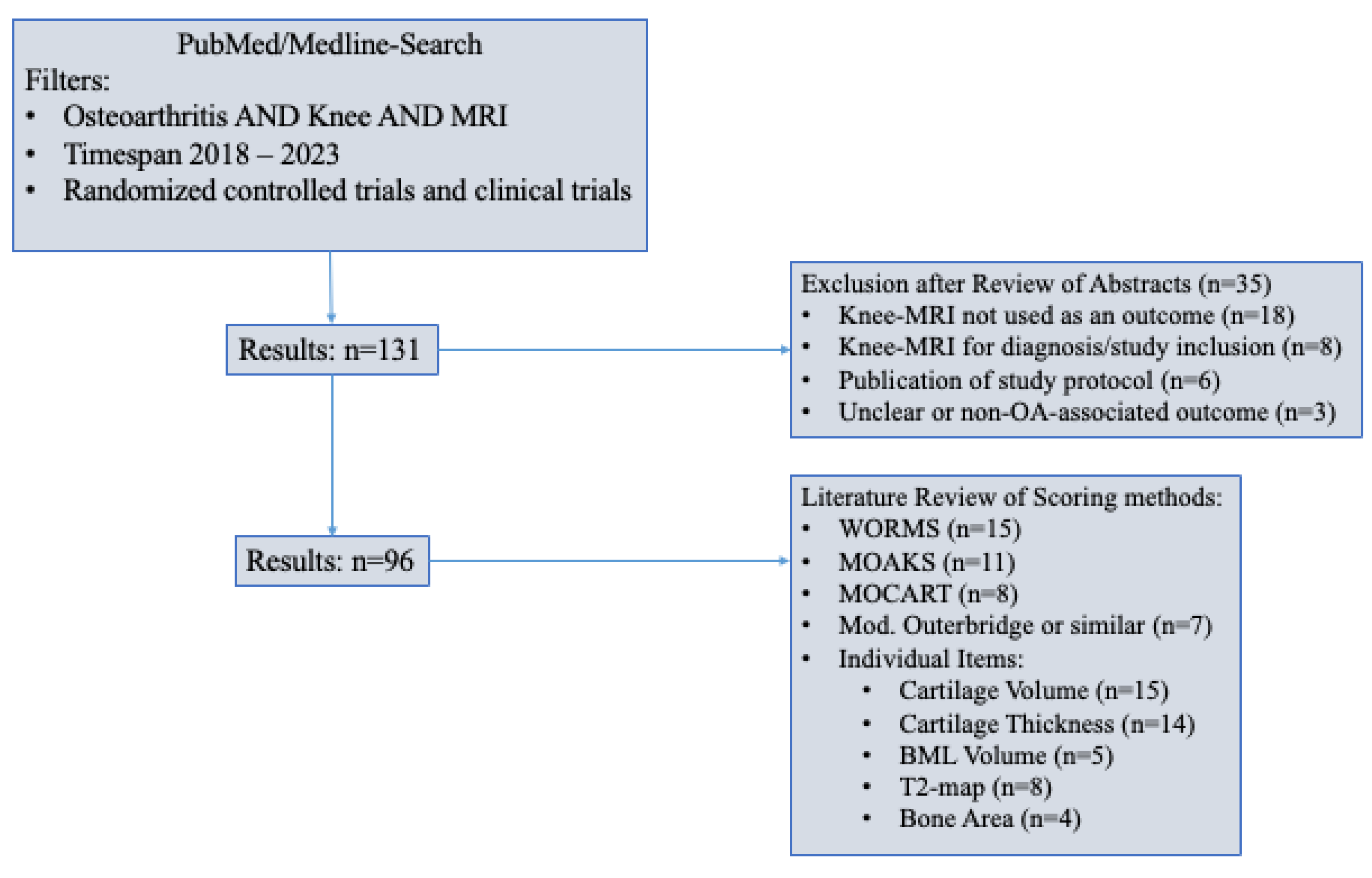
6.9. Semiquantitative Scoring Methods
6.10. Reduction in Acquisition Time
7. Recent Developments—The Advent of Deep Learning
8. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Glyn-Jones, S.; Palmer, A.J.R.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.J.; March, L.; Chew, M. Osteoarthritis in 2020 and beyond: A Lancet Commission. Lancet 2020, 396, 1711–1712. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Nüesch, E.; Dieppe, P.; Reichenbach, S.; Williams, S.; Iff, S.; Jüni, P. All cause and disease specific mortality in patients with knee or hip osteoarthritis: Population based cohort study. BMJ 2011, 342, d1165. [Google Scholar] [CrossRef]
- March, L.M.; Bachmeier, C.J. 10 Economics of osteoarthritis: A global perspective. Baillieres Clin. Rheumatol. 1997, 11, 817–834. [Google Scholar] [CrossRef]
- Hall, M.; van der Esch, M.; Hinman, R.S.; Peat, G.; de Zwart, A.; Quicke, J.G.; Runhaar, J.; Knoop, J.; van der Leeden, M.; de Rooij, M.; et al. How does hip osteoarthritis differ from knee osteoarthritis? Osteoarthr. Cartil. 2021, 30, 32–41. [Google Scholar] [CrossRef]
- Murphy, N.J.; Eyles, J.P.; Hunter, D.J. Hip Osteoarthritis: Etiopathogenesis and Implications for Management. Adv. Ther. 2016, 33, 1921–1946. [Google Scholar] [CrossRef]
- Delpachitra, S.; Dimitroulis, G. Osteoarthritis of the temporomandibular joint: A review of aetiology and pathogenesis. Br. J. Oral Maxillofac. Surg. 2022, 60, 387–396. [Google Scholar] [CrossRef]
- Shorter, E.; Sannicandro, A.J.; Poulet, B.; Goljanek-Whysall, K. Skeletal Muscle Wasting and Its Relationship with Osteoarthritis: A Mini-Review of Mechanisms and Current Interventions. Curr. Rheumatol. Rep. 2019, 21, 40. [Google Scholar] [CrossRef]
- Adams, B.G.; Houston, M.N.P.; Cameron, K.L.P. The Epidemiology of Meniscus Injury. Sports Med. Arthrosc. Rev. 2021, 29, e24–e33. [Google Scholar] [CrossRef]
- Wang, L.-J.; Zeng, N.; Yan, Z.-P.; Li, J.-T.; Ni, G.-X. Post-traumatic osteoarthritis following ACL injury. Arthritis Res. Ther. 2020, 22, 57. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Madrid, F.; Karvonen, R.L.; Teitge, R.A.; Miller, P.R.; Negendank, W.G. MR features of osteoarthritis of the knee. Magn. Reson. Imaging 1994, 12, 703–709. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chan, Y.T.; Yung, P.S.H.; Tuan, R.S.; Jiang, Y. Subchondral Bone Remodeling: A Therapeutic Target for Osteoarthritis. Front. Cell Dev. Biol. 2021, 8, 607764. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Winkler, T.; Schenk, L.S.; Neuerburg, C.; Baumbach, S.F.; Zustin, J.; Lehmann, W.; Schilling, A.F. Developmental Transformation and Reduction of Connective Cavities within the Subchondral Bone. Int. J. Mol. Sci. 2019, 20, 770. [Google Scholar] [CrossRef] [PubMed]
- Taheri, S.; Yoshida, T.; Böker, K.; Foerster, R.; Jochim, L.; Flux, A.; Grosskopf, B.; Hawellek, T.; Lehmann, W.; Schilling, A. Changes of the subchondral bone microchannel network in early osteoarthritis. Osteoarthr. Cartil. 2023, 31, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hu, Y.; Yu, Y.E.; Zhang, X.; Watts, T.; Zhou, B.; Wang, J.; Wang, T.; Zhao, W.; Chiu, K.Y.; et al. Subchondral Trabecular Rod Loss and Plate Thickening in the Development of Osteoarthritis. J. Bone Miner. Res. 2018, 33, 316–327. [Google Scholar] [CrossRef]
- Dennison, E.M. Osteoarthritis: The importance of hormonal status in midlife women. Maturitas 2022, 165, 8–11. [Google Scholar] [CrossRef]
- Zoli, A.; Lizzio, M.M.; Capuano, A.; Massafra, U.; Barini, A.; Ferraccioli, G. Osteoporosis and bone metabolism in postmenopausal women with osteoarthritis of the hand. Menopause 2006, 13, 462–466. [Google Scholar] [CrossRef]
- Wang, A.; Zawadzki, N.; Hedlin, H.; LeBlanc, E.; Budrys, N.; Van Horn, L.; Gass, M.; Westphal, L.; Stefanick, M. Reproductive history and osteoarthritis in the Women’s Health Initiative. Scand. J. Rheumatol. 2021, 50, 58–67. [Google Scholar] [CrossRef]
- Jung, J.H.; Bang, C.H.; Song, G.G.; Kim, C.; Kim, J.-H.; Choi, S.J. Knee osteoarthritis and menopausal hormone therapy in postmenopausal women: A nationwide cross-sectional study. Menopause 2019, 26, 598–602. [Google Scholar] [CrossRef]
- Noehren, B.; Kosmac, K.; Walton, R.; Murach, K.; Lyles, M.; Loeser, R.; Peterson, C.; Messier, S. Alterations in quadriceps muscle cellular and molecular properties in adults with moderate knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 1359–1368. [Google Scholar] [CrossRef]
- Alnahdi, A.H.; Zeni, J.A.; Snyder-Mackler, L. Muscle Impairments in Patients with Knee Osteoarthritis. Sports Health Multidiscip. Approach 2012, 4, 284–292. [Google Scholar] [CrossRef]
- Di Pietro, G.; Scimeca, M.; Iundusi, R.; Celi, M.; Gasbarra, E.; Tarantino, U.; Capuani, S. Differences between muscle from osteoporotic and osteoarthritic subjects: In vitro study by diffusion-tensor MRI and histological findings. Aging Clin. Exp. Res. 2020, 32, 2489–2499. [Google Scholar] [CrossRef]
- Pereira, D.; Peleteiro, B.; Araújo, J.; Branco, J.; Santos, R.A.; Ramos, E. The effect of osteoarthritis definition on prevalence and incidence estimates: A systematic review. Osteoarthr. Cartil. 2011, 19, 1270–1285. [Google Scholar] [CrossRef]
- Guermazi, A.; Niu, J.; Hayashi, D.; Roemer, F.W.; Englund, M.; Neogi, T.; Aliabadi, P.; McLennan, C.E.; Felson, D.T. Prevalence of abnormalities in knees detected by MRI in adults without knee osteoarthritis: Population based observational study (Framingham Osteoarthritis Study). BMJ 2012, 345, e5339. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Demehri, S.; Wirth, W.; Kijowski, R. Imaging in Osteoarthritis. Osteoarthr. Cartil. 2022, 30, 913–934. [Google Scholar] [CrossRef]
- Bijlsma, J.W.; Berenbaum, F.; Lafeber, F.P. Osteoarthritis: An update with relevance for clinical practice. Lancet 2011, 377, 2115–2126. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Crema, M.D.; Nevitt, M.; Guermazi, A.; Felson, D.; Wang, K.; Lynch, J.; Marra, M.D.; Torner, J.; Lewis, C.; Roemer, F. Progression of cartilage damage and meniscal pathology over 30 months is associated with an increase in radiographic tibiofemoral joint space narrowing in persons with knee OA—The MOST study. Osteoarthr. Cartil. 2014, 22, 1743–1747. [Google Scholar] [CrossRef][Green Version]
- Kijowski, R.; Blankenbaker, D.G.; Davis, K.W.; Shinki, K.; Kaplan, L.D.; De Smet, A.A. Comparison of 1.5- and 3.0-T MR Imaging for Evaluating the Articular Cartilage of the Knee Joint. Radiology 2009, 250, 839–848. [Google Scholar] [CrossRef]
- Masi, J.N.; Sell, C.A.; Phan, C.; Han, E.; Newitt, D.; Steinbach, L.; Majumdar, S.; Link, T.M. Cartilage MR Imaging at 3.0 versus That at 1.5 T: Preliminary Results in a Porcine Model. Radiology 2005, 236, 140–150. [Google Scholar] [CrossRef] [PubMed]
- Arnold, T.C.; Freeman, C.W.; Litt, B.; Stein, J.M. Low-field MRI: Clinical promise and challenges. J. Magn. Reson. Imaging 2023, 57, 25–44. [Google Scholar] [CrossRef] [PubMed]
- Khodarahmi, I.M.; Keerthivasan, M.B.; Brinkmann, I.M.; Grodzki, D.; Fritz, J. Modern Low-Field MRI of the Musculoskeletal System. Investig. Radiol. 2023, 58, 76–87. [Google Scholar] [CrossRef] [PubMed]
- Sutter, R.; Tresch, F.; Buck, F.M.; Pfirrmann, C.W.A. Is Dedicated Extremity 1.5-T MRI Equivalent to Standard Large-Bore 1.5-T MRI for Foot and Knee Examinations? Am. J. Roentgenol. 2014, 203, 1293–1302. [Google Scholar] [CrossRef]
- Klein, H.-M. Low-Field Magnetic Resonance Imaging. RöFo-Fortschritte Geb. Röntgenstrahlen Bildgeb. Verfahr. 2020, 192, 537–548. [Google Scholar] [CrossRef]
- Lutterbey, G.; Behrends, K.; Falkenhausen, M.V.; Wattjes, M.P.; Morakkabati, N.; Gieseke, J.; Schild, H. Is the body-coil at 3 Tesla feasible for the MRI evaluation of the painful knee? A comparative study. Eur. Radiol. 2007, 17, 503–508. [Google Scholar] [CrossRef]
- Link, T.M. MR Imaging in Osteoarthritis: Hardware, Coils, and Sequences. Radiol. Clin. N. Am. 2009, 47, 617–632. [Google Scholar] [CrossRef]
- Guglielmi, G.; Lennart, J.; Simoni, P.; Mascarenhas, V. Knee. MRI Protocols of the ESSR Arthritis Subcommittee. 2018. Available online: https://www.essr.org/content-essr/uploads/2018/05/Knee.pdf (accessed on 22 February 2023).
- Sudoł-Szopińska, I.; Jurik, A.G.; Eshed, I.; Lennart, J.; Grainger, A.; Østergaard, M.; Klauser, A.; Cotten, A.; Wick, M.C.; Maas, M.; et al. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin. Musculoskelet. Radiol. 2015, 19, 396–411. [Google Scholar] [CrossRef]
- Freeman, D.M.; Bergman, G.; Glover, G. Short TE MR microscopy: Accurate measurement and zonal differentiation of normal hyaline cartilage. Magn. Reson. Med. 1997, 38, 72–81. [Google Scholar] [CrossRef]
- Link, T.M.; Stahl, R.; Woertler, K. Cartilage imaging: Motivation, techniques, current and future significance. Eur. Radiol. 2007, 17, 1135–1146. [Google Scholar] [CrossRef]
- Crema, M.D.; Roemer, F.W.; Marra, M.D.; Burstein, D.; Gold, G.E.; Eckstein, F.; Baum, T.; Mosher, T.J.; Carrino, J.A.; Guermazi, A. Articular Cartilage in the Knee: Current MR Imaging Techniques and Applications in Clinical Practice and Research. RadioGraphics 2011, 31, 37–61. [Google Scholar] [CrossRef]
- Delfaut, E.M.; Beltran, J.; Johnson, G.; Rousseau, J.; Marchandise, X.; Cotten, A. Fat Suppression in MR Imaging: Techniques and Pitfalls. RadioGraphics 1999, 19, 373–382. [Google Scholar] [CrossRef]
- Dixon, W.T. Simple proton spectroscopic imaging. Radiology 1984, 153, 189–194. [Google Scholar] [CrossRef]
- Guerini, H.; Omoumi, P.; Guichoux, F.; Vuillemin, V.; Morvan, G.; Zins, M.; Thevenin, F.; Drape, J.L. Fat Suppression with Dixon Techniques in Musculoskeletal Magnetic Resonance Imaging: A Pictorial Review. Semin. Musculoskelet. Radiol. 2015, 19, 335–347. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Guan, M.; Zhao, W.; Leung, F.-K.; Pan, H.; Cao, X.; Guo, X.; Lu, W. Bone turnover and articular cartilage differences localized to subchondral cysts in knees with advanced osteoarthritis. Osteoarthr. Cartil. 2015, 23, 2174–2183. [Google Scholar] [CrossRef]
- Muratovic, D.; Cicuttini, F.; Wluka, A.; Findlay, D.; Wang, Y.; Otto, S.; Taylor, D.; Humphries, J.; Lee, Y.; Labrinidis, A.; et al. Bone marrow lesions detected by specific combination of MRI sequences are associated with severity of osteochondral degeneration. Arthritis Res. Ther. 2016, 18, 54. [Google Scholar] [CrossRef]
- Peterfy, C.; Guermazi, A.; Zaim, S.; Tirman, P.; Miaux, Y.; White, D.; Kothari, M.; Lu, Y.; Fye, K.; Zhao, S.; et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthr. Cartil. 2004, 12, 177–190. [Google Scholar] [CrossRef]
- Deveza, L.; Melo, L.; Yamato, T.; Mills, K.; Ravi, V.; Hunter, D. Knee osteoarthritis phenotypes and their relevance for outcomes: A systematic review. Osteoarthr. Cartil. 2017, 25, 1926–1941. [Google Scholar] [CrossRef] [PubMed]
- Roemer, F.W.; Kwoh, C.K.; Hayashi, D.; Felson, D.T.; Guermazi, A. The role of radiography and MRI for eligibility assessment in DMOAD trials of knee OA. Nat. Rev. Rheumatol. 2018, 14, 372–380. [Google Scholar] [CrossRef]
- Ryd, L.; Flodström, K.; Manley, M. Patient-Specific Implants for Focal Cartilage Lesions in The Knee: Implant Survivorship Analysis up to Seven Years Post-Implantation. Surg. Technol. Online 2020, 38, 379–386. [Google Scholar] [CrossRef]
- Siepmann, D.B.; McGovern, J.; Brittain, J.H.; Reeder, S.B. High-Resolution 3D Cartilage Imaging with IDEAL–SPGR at 3 T. Am. J. Roentgenol. 2007, 189, 1510–1515. [Google Scholar] [CrossRef] [PubMed]
- Bruder, H.; Fischer, H.; Graumann, R.; Deimling, M. A new steady-state imaging sequence for simultaneous acquisition of two MR images with clearly different contrasts. Magn. Reson. Med. 1988, 7, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Welsch, G.H.; Scheffler, K.; Mamisch, T.C.; Hughes, T.; Millington, S.; Deimling, M.; Trattnig, S. Rapid estimation of cartilage T2 based on double echo at steady state (DESS) with 3 Tesla. Magn. Reson. Med. 2009, 62, 544–549. [Google Scholar] [CrossRef]
- Friedrich, K.M.; Reiter, G.; Kaiser, B.; Mayerhöfer, M.; Deimling, M.; Jellus, V.; Horger, W.; Trattnig, S.; Schweitzer, M.; Salomonowitz, E. High-resolution cartilage imaging of the knee at 3T: Basic evaluation of modern isotropic 3D MR-sequences. Eur. J. Radiol. 2011, 78, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Raithel, E.; Thawait, G.K.; Gilson, W.; Papp, D.F. Six-Fold Acceleration of High-Spatial Resolution 3D SPACE MRI of the Knee Through Incoherent k-Space Undersampling and Iterative Reconstruction—First Experience. Investig. Radiol. 2016, 51, 400–409. [Google Scholar] [CrossRef]
- Van Dyck, P.; Smekens, C.; Roelant, E.; Vyvere, T.V.; Snoeckx, A.; De Smet, E. 3D CAIPIRINHA SPACE versus standard 2D TSE for routine knee MRI: A large-scale interchangeability study. Eur. Radiol. 2022, 32, 6456–6467. [Google Scholar] [CrossRef]
- Roemer, F.W.; Engelke, K.; Li, L.; Laredo, J.-D.; Guermazi, A. MRI underestimates presence and size of knee osteophytes using CT as a reference standard. Osteoarthr. Cartil. 2023, 31, 656–668. [Google Scholar] [CrossRef]
- Cheng, K.Y.; Moazamian, D.; Ma, Y.; Jang, H.; Jerban, S.; Du, J.; Chung, C.B. Clinical application of ultrashort echo time (UTE) and zero echo time (ZTE) magnetic resonance (MR) imaging in the evaluation of osteoarthritis. Skelet. Radiol. 2023, 1–9. [Google Scholar] [CrossRef]
- Aydıngöz, Ü.; Yıldız, A.E.; Ergen, F.B. Zero Echo Time Musculoskeletal MRI: Technique, Optimization, Applications, and Pitfalls. Radiographics 2022, 42, 1398–1414. [Google Scholar] [CrossRef]
- Du, J.; Carl, M.; Bae, W.; Statum, S.; Chang, E.; Bydder, G.; Chung, C. Dual inversion recovery ultrashort echo time (DIR-UTE) imaging and quantification of the zone of calcified cartilage (ZCC). Osteoarthr. Cartil. 2012, 21, 77–85. [Google Scholar] [CrossRef]
- Chu, C.R.; Williams, A.A.; West, R.V.; Qian, Y.; Fu, F.H.; Do, B.H.; Bruno, S. Quantitative Magnetic Resonance Imaging UTE-T2* Mapping of Cartilage and Meniscus Healing After Anatomic Anterior Cruciate Ligament Reconstruction. Am. J. Sports Med. 2014, 42, 1847–1856. [Google Scholar] [CrossRef] [PubMed]
- Garetier, M.; Rousset, J.; Makki, K.; Brochard, S.; Rousseau, F.; Ben Salem, D.; Borotikar, B. Assessment and comparison of image quality between two real-time sequences for dynamic MRI of distal joints at 3.0 Tesla. Acta Radiol. 2023, 64, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Garetier, M.; Borotikar, B.; Makki, K.; Brochard, S.; Rousseau, F.; Ben Salem, D. Dynamic MRI for articulating joint evaluation on 1.5 T and 3.0 T scanners: Setup, protocols, and real-time sequences. Insights Imaging 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Bowes, M.A.; Kacena, K.; Alabas, O.A.; Brett, A.D.; Dube, B.; Bodick, N.; Conaghan, P.G. Machine-learning, MRI bone shape and important clinical outcomes in osteoarthritis: Data from the Osteoarthritis Initiative. Ann. Rheum. Dis. 2021, 80, 502–508. [Google Scholar] [CrossRef]
- Kijowski, R.; Fritz, J.; Deniz, C.M. Deep learning applications in osteoarthritis imaging. Skelet. Radiol. 2023, 1–14. [Google Scholar] [CrossRef]
- Desai, A.D.; Caliva, F.; Iriondo, C.; Mortazi, A.; Jambawalikar, S.; Bagci, U.; Perslev, M.; Igel, C.; Dam, E.B.; Gaj, S.; et al. The International Workshop on Osteoarthritis Imaging Knee MRI Segmentation Challenge: A Multi-Institute Evaluation and Analysis Framework on a Standardized Dataset. Radiol. Artif. Intell. 2021, 3, e200078. [Google Scholar] [CrossRef]
- Aigner, T.; McKenna, L. Molecular pathology and pathobiology of osteoarthritic cartilage. Cell. Mol. Life Sci. 2002, 59, 5–18. [Google Scholar] [CrossRef]
- Hohe, J.; Faber, S.; Stammberger, T.; Reiser, M.; Englmeier, K.-H.; Eckstein, F. A technique for 3D in vivo quantification of proton density and magnetization transfer coefficients of knee joint cartilage. Osteoarthr. Cartil. 2000, 8, 426–433. [Google Scholar] [CrossRef][Green Version]
- Lüssea, S.; Claassen, H.; Gehrke, T.; Hassenpflug, J.; Schünke, M.; Heller, M.; Glüer, C.-C. Evaluation of water content by spatially resolved transverse relaxation times of human articular cartilage. Magn. Reson. Imaging 2000, 18, 423–430. [Google Scholar] [CrossRef]
- Dunn, T.C.; Lu, Y.; Jin, H.; Ries, M.D.; Majumdar, S.; Roemer, F.W.; Demehri, S.; Omoumi, P.; Link, T.M.; Kijowski, R.; et al. T2 Relaxation Time of Cartilage at MR Imaging: Comparison with Severity of Knee Osteoarthritis. Radiology 2004, 232, 592–598. [Google Scholar] [CrossRef]
- Koff, M.; Amrami, K.; Kaufman, K. Clinical evaluation of T2 values of patellar cartilage in patients with osteoarthritis. Osteoarthr. Cartil. 2007, 15, 198–204. [Google Scholar] [CrossRef]
- Wang, L.; Regatte, R.R. T1ρMRI of human musculoskeletal system. J. Magn. Reson. Imaging 2015, 41, 586–600. [Google Scholar] [CrossRef]
- Li, X.; Cheng, J.; Lin, K.; Saadat, E.; Bolbos, R.I.; Jobke, B.; Ries, M.D.; Horvai, A.; Link, T.M.; Majumdar, S. Quantitative MRI using T1ρ and T2 in human osteoarthritic cartilage specimens: Correlation with biochemical measurements and histology. Magn. Reson. Imaging 2011, 29, 324–334. [Google Scholar] [CrossRef]
- Sigurdsson, U.; Müller, G.; Siversson, C.; Lammentausta, E.; Svensson, J.; Tiderius, C.-J.; Dahlberg, L.E. Delayed gadolinium-enhanced MRI of meniscus (dGEMRIM) and cartilage (dGEMRIC) in healthy knees and in knees with different stages of meniscus pathology. BMC Musculoskelet. Disord. 2016, 17, 406. [Google Scholar] [CrossRef]
- van Tiel, J.; Kotek, G.; Reijman, M.; Bos, P.K.; Bron, E.E.; Klein, S.; Verhaar, J.A.N.; Krestin, G.P.; Weinans, H.; Oei, E.H.G. Delayed gadolinium-enhanced MRI of the meniscus (dGEMRIM) in patients with knee osteoarthritis: Relation with meniscal degeneration on conventional MRI, reproducibility, and correlation with dGEMRIC. Eur. Radiol. 2014, 24, 2261–2270. [Google Scholar] [CrossRef]
- Hangaard, S.; Gudbergsen, H.; Daugaard, C.L.; Bliddal, H.; Nybing, J.D.; Nieminen, M.T.; Casula, V.; Tiderius, C.-J.; Boesen, M. Delayed gadolinium-enhanced MRI of menisci and cartilage (dGEMRIM/dGEMRIC) in obese patients with knee osteoarthritis: Cross-sectional study of 85 obese patients with intra-articular administered gadolinium contrast. J. Magn. Reson. Imaging 2018, 48, 1700–1706. [Google Scholar] [CrossRef]
- Guermazi, A.; Alizai, H.; Crema, M.D.; Trattnig, S.; Regatte, R.; Roemer, F. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1639–1653. [Google Scholar] [CrossRef]
- Miller, K.L.; Hargreaves, B.A.; Gold, G.E.; Pauly, J.M. Steady-state diffusion-weighted imaging of in vivo knee cartilage. Magn. Reson. Med. 2004, 51, 394–398. [Google Scholar] [CrossRef]
- Mlynárik, V.; Sulzbacher, I.; Bittšanský, M.; Fuiko, R.; Trattnig, S. Investigation of apparent diffusion constant as an indicator of early degenerative disease in articular cartilage. J. Magn. Reson. Imaging 2003, 17, 440–444. [Google Scholar] [CrossRef]
- Quirbach, S.; Trattnig, S.; Marlovits, S.; Zimmermann, V.; Domayer, S.; Dorotka, R.; Mamisch, T.C.; Bohndorf, K.; Welsch, G.H. Initial results of in vivo high-resolution morphological and biochemical cartilage imaging of patients after matrix-associated autologous chondrocyte transplantation (MACT) of the ankle. Skelet. Radiol. 2009, 38, 751–760. [Google Scholar] [CrossRef]
- Kogan, F.; Hariharan, H.; Reddy, R. Chemical Exchange Saturation Transfer (CEST) Imaging: Description of Technique and Potential Clinical Applications. Curr. Radiol. Rep. 2013, 1, 102–114. [Google Scholar] [CrossRef] [PubMed]
- Soellner, S.; Welsch, G.; Gelse, K.; Goldmann, A.; Kleyer, A.; Schett, G.; Pachowsky, M. gagCEST imaging at 3 T MRI in patients with articular cartilage lesions of the knee and intraoperative validation. Osteoarthr. Cartil. 2021, 29, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.F.; Ma, Y.; Jang, H.; Jerban, S.; Tang, Q.; Searleman, A.C.; Meyer, R.S.; Du, J.; Chang, E.Y. AcidoCEST-UTE MRI Reveals an Acidic Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 2022, 23, 4466. [Google Scholar] [CrossRef] [PubMed]
- Madelin, G.; Lee, J.-S.; Regatte, R.R.; Jerschow, A. Sodium MRI: Methods and applications. Prog. Nucl. Magn. Reson. Spectrosc. 2014, 79, 14–47. [Google Scholar] [CrossRef]
- Kamp, B.; Frenken, M.; Henke, J.M.; Abrar, D.B.; Nagel, A.M.; Gast, L.V.; Oeltzschner, G.; Wilms, L.M.; Nebelung, S.; Antoch, G.; et al. Quantification of Sodium Relaxation Times and Concentrations as Surrogates of Proteoglycan Content of Patellar CARTILAGE at 3T MRI. Diagnostics 2021, 11, 2301. [Google Scholar] [CrossRef]
- Outerbridge, R.E. The etiology of chondromalacia patellae. J. Bone Jt. Surg. 1961, 43, 752–757. [Google Scholar] [CrossRef]
- Jungius, K.-P.; Schmid, M.R.; Zanetti, M.; Hodler, J.; Koch, P.; Pfirrmann, C.W.A. Cartilaginous Defects of the Femorotibial Joint: Accuracy of Coronal Short Inversion Time Inversion-Recovery MR Sequence. Radiology 2006, 240, 482–488. [Google Scholar] [CrossRef]
- Schreiner, M.M.; Raudner, M.; Marlovits, S.; Bohndorf, K.; Weber, M.; Zalaudek, M.; Röhrich, S.; Szomolanyi, P.; Filardo, G.; Windhager, R.; et al. The MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) 2.0 Knee Score and Atlas. Cartilage 2021, 13, 571S–587S. [Google Scholar] [CrossRef]
- Kornaat, P.R.; Ceulemans, R.Y.T.; Kroon, H.M.; Riyazi, N.; Kloppenburg, M.; Carter, W.O.; Woodworth, T.G.; Bloem, J.L. MRI assessment of knee osteoarthritis: Knee Osteoarthritis Scoring System (KOSS)—Inter-observer and intra-observer reproducibility of a compartment-based scoring system. Skelet. Radiol. 2005, 34, 95–102. [Google Scholar] [CrossRef]
- Hunter, D.; Conaghan, P.; Peterfy, C.; Bloch, D.; Guermazi, A.; Woodworth, T.; Stevens, R.; Genant, H. Responsiveness, effect size, and smallest detectable difference of Magnetic Resonance Imaging in knee osteoarthritis. Osteoarthr. Cartil. 2006, 14, 112–115. [Google Scholar] [CrossRef]
- Hunter, D.J.; Lo, G.H.; Gale, D.; Grainger, A.J.; Guermazi, A.; Conaghan, P.G. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston–Leeds Osteoarthritis Knee Score). Ann. Rheum. Dis. 2008, 67, 206–211. [Google Scholar] [CrossRef]
- Felson, D.; Lynch, J.; Guermazi, A.; Roemer, F.; Niu, J.; McAlindon, T.; Nevitt, M. Comparison of BLOKS and WORMS scoring systems part II. Longitudinal assessment of knee MRIs for osteoarthritis and suggested approach based on their performance: Data from the Osteoarthritis Initiative. Osteoarthr. Cartil. 2010, 18, 1402–1407. [Google Scholar] [CrossRef]
- Hunter, D.; Guermazi, A.; Lo, G.; Grainger, A.; Conaghan, P.; Boudreau, R.; Roemer, F. Evolution of semi-quantitative whole joint assessment of knee OA: MOAKS (MRI Osteoarthritis Knee Score). Osteoarthr. Cartil. 2011, 19, 990–1002. [Google Scholar] [CrossRef]
- Roemer, F.W.; Guermazi, A.; Zhang, Y.; Yang, M.; Hunter, D.J.; Crema, M.D.; Bohndorf, K. Hoffa’s Fat Pad: Evaluation on Unenhanced MR Images as a Measure of Patellofemoral Synovitis in Osteoarthritis. Am. J. Roentgenol. 2009, 192, 1696–1700. [Google Scholar] [CrossRef]
- Guermazi, A.; Roemer, F.W.; Hayashi, D.; Crema, M.D.; Niu, J.; Zhang, Y.; Marra, M.D.; Katur, A.; Lynch, J.A.; El-Khoury, G.Y.; et al. Assessment of synovitis with contrast-enhanced MRI using a whole-joint semiquantitative scoring system in people with, or at high risk of, knee osteoarthritis: The MOST study. Ann. Rheum. Dis. 2011, 70, 805–811. [Google Scholar] [CrossRef]
- Roemer, F.; Collins, J.; Kwoh, C.; Hannon, M.; Neogi, T.; Felson, D.; Hunter, D.; Lynch, J.; Guermazi, A. MRI-based screening for structural definition of eligibility in clinical DMOAD trials: Rapid OsteoArthritis MRI Eligibility Score (ROAMES). Osteoarthr. Cartil. 2020, 28, 71–81. [Google Scholar] [CrossRef]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar] [CrossRef]
- Hutchinson, M.; Raff, U. Fast MRI data acquisition using multiple detectors. Magn. Reson. Med. 1988, 6, 87–91. [Google Scholar] [CrossRef]
- Glockner, J.F.; Hu, H.H.; Stanley, D.W.; Angelos, L.; King, K. Parallel MR Imaging: A User’s Guide. RadioGraphics 2005, 25, 1279–1297. [Google Scholar] [CrossRef]
- Barth, M.; Breuer, F.; Koopmans, P.J.; Norris, D.G.; Poser, B.A. Simultaneous multislice (SMS) imaging techniques. Magn. Reson. Med. 2016, 75, 63–81. [Google Scholar] [CrossRef]
- Geethanath, S.; Reddy, R.; Konar, A.S.; Imam, S.; Sundaresan, R.; Ramesh Babu, D.R.; Venkatesan, R. Compressed Sensing MRI: A Review. Crit. Rev. Biomed. Eng. 2013, 41, 183–204. [Google Scholar] [CrossRef] [PubMed]
- Akai, H.; Yasaka, K.; Sugawara, H.; Tajima, T.; Kamitani, M.; Furuta, T.; Akahane, M.; Yoshioka, N.; Ohtomo, K.; Abe, O.; et al. Acceleration of knee magnetic resonance imaging using a combination of compressed sensing and commercially available deep learning reconstruction: A preliminary study. BMC Med. Imaging 2023, 23, 5. [Google Scholar] [CrossRef] [PubMed]
- Müller-Franzes, G.; Nolte, T.; Ciba, M.; Schock, J.; Khader, F.; Prescher, A.; Wilms, L.M.; Kuhl, C.; Nebelung, S.; Truhn, D. Fast, Accurate, and Robust T2 Mapping of Articular Cartilage by Neural Networks. Diagnostics 2022, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Knoll, F.; Hammernik, K.; Zhang, C.; Moeller, S.; Pock, T.; Sodickson, D.K.; Akcakaya, M. Deep-Learning Methods for Parallel Magnetic Resonance Imaging Reconstruction: A Survey of the Current Approaches, Trends, and Issues. IEEE Signal Process. Mag. 2020, 37, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Hammernik, K.; Klatzer, T.; Kobler, E.; Recht, M.P.; Sodickson, D.K.; Pock, T.; Knoll, F. Learning a variational network for reconstruction of accelerated MRI data. Magn. Reson. Med. 2018, 79, 3055–3071. [Google Scholar] [CrossRef]
- Roemer, F.W. The Role of Speed and Possible Implications. Radiology 2023, 307, e222872. [Google Scholar] [CrossRef]
- Johnson, P.M.; Lin, D.J.; Zbontar, J.; Zitnick, C.L.; Sriram, A.; Muckley, M.; Babb, J.S.; Kline, M.; Ciavarra, G.; Alaia, E.; et al. Deep Learning Reconstruction Enables Prospectively Accelerated Clinical Knee MRI. Radiology 2023, 307, e220425. [Google Scholar] [CrossRef]
- Kulseng, C.P.S.; Nainamalai, V.; Grøvik, E.; Geitung, J.-T.; Årøen, A.; Gjesdal, K.-I. Automatic segmentation of human knee anatomy by a convolutional neural network applying a 3D MRI protocol. BMC Musculoskelet. Disord. 2023, 24, 41. [Google Scholar] [CrossRef]
- Almajalid, R.; Zhang, M.; Shan, J. Fully Automatic Knee Bone Detection and Segmentation on Three-Dimensional MRI. Diagnostics 2022, 12, 123. [Google Scholar] [CrossRef]
- Yeoh, P.S.Q.; Lai, K.W.; Goh, S.L.; Hasikin, K.; Hum, Y.C.; Tee, Y.K.; Dhanalakshmi, S. Emergence of Deep Learning in Knee Osteoarthritis Diagnosis. Comput. Intell. Neurosci. 2021, 2021, 4931437. [Google Scholar] [CrossRef]
- Siouras, A.; Moustakidis, S.; Giannakidis, A.; Chalatsis, G.; Liampas, I.; Vlychou, M.; Hantes, M.; Tasoulis, S.; Tsaopoulos, D. Knee Injury Detection Using Deep Learning on MRI Studies: A Systematic Review. Diagnostics 2022, 12, 537. [Google Scholar] [CrossRef]
| Sequence | FOV | Slice Thickness | TR | TE | Matrix |
|---|---|---|---|---|---|
| Sag PD FS | 160 | 3 | 3570 | 39 | 288 × 384 |
| Cor PD FS | 160 | 3 | 3570 | 39 | 288 × 384 |
| Ax PD FS | 160 | 3 | 3570 | 39 | 288 × 384 |
| Cor/Sag T1 | 180 | 3 | 470 | 13 | 358 × 512 |
| Optional CE T1 FS | 180 | 3 | 470 | 13 | 358 × 512 |
| Scoring Method | Intrarater Kappa | Interrater Kappa | Features Assessed | Number of Compartments Assessed |
|---|---|---|---|---|
| MOCART | 0.57–0.87 | 0.57–1.0 | volume fill of cartilage defect, integration into adjacent cartilage, surface, structure signal intensity, bony defect (overgrowth, subchondral changes) | - |
| WORMS | 0.61–0.99 (ICC) | cartilage, BML, subarticular cysts, subarticular bone attrition, osteophytes, meniscal integrity, anterior and posterior cruciate ligament integrity, medial and lateral collateral ligament integrity, synovitis, loose bodies, and periarticular cysts/bursae | 15 | |
| BLOKS | 0.51–0.79 | BML, cartilage, osteophytes, synovitis effusion, meniscal abnormalities, ligaments, periarticular features | 9 | |
| KOSS | 0.56–0.91 | 0.63–0.91 | cartilaginous lesions, osteophytes, subchondral cysts, bone marrow edema, meniscal abnormalities, effusion, synovitis, and Baker’s cyst | 9 |
| MOAKS | 0.42–1.0 | 0.36–1.0 | BML, cartilage, synovitis, osteophytes, effusion, menisci, ligaments, periarticular features | 14 |
| ROAMES | 0.92–1.0 | 0.85–1.0 | cartilage, BML, osteophytes, menisci, inflammation (Hoffa-synivitis, effusion) | 3 |
| Synovitis score | 0.67–1.0 | 0.67–0.92 | synovial thickness | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehmig, J.; Engel, G.; Lotz, J.; Lehmann, W.; Taheri, S.; Schilling, A.F.; Seif Amir Hosseini, A.; Panahi, B. MR-Imaging in Osteoarthritis: Current Standard of Practice and Future Outlook. Diagnostics 2023, 13, 2586. https://doi.org/10.3390/diagnostics13152586
Ehmig J, Engel G, Lotz J, Lehmann W, Taheri S, Schilling AF, Seif Amir Hosseini A, Panahi B. MR-Imaging in Osteoarthritis: Current Standard of Practice and Future Outlook. Diagnostics. 2023; 13(15):2586. https://doi.org/10.3390/diagnostics13152586
Chicago/Turabian StyleEhmig, Jonathan, Günther Engel, Joachim Lotz, Wolfgang Lehmann, Shahed Taheri, Arndt F. Schilling, Ali Seif Amir Hosseini, and Babak Panahi. 2023. "MR-Imaging in Osteoarthritis: Current Standard of Practice and Future Outlook" Diagnostics 13, no. 15: 2586. https://doi.org/10.3390/diagnostics13152586
APA StyleEhmig, J., Engel, G., Lotz, J., Lehmann, W., Taheri, S., Schilling, A. F., Seif Amir Hosseini, A., & Panahi, B. (2023). MR-Imaging in Osteoarthritis: Current Standard of Practice and Future Outlook. Diagnostics, 13(15), 2586. https://doi.org/10.3390/diagnostics13152586






