A Review of Deep Learning Techniques for Lung Cancer Screening and Diagnosis Based on CT Images
Abstract
:1. Introduction
1.1. Deep Learning Methods Overview
1.1.1. Supervised Deep Learning Models
1.1.2. Unsupervised Deep Learning Models
1.1.3. Semi-Supervised Deep Learning Models
1.1.4. Reinforced Deep Learning Models
1.2. Analysis of a CT Image Using DL
1.3. Paper Organization
1.4. Contributions to the Survey
- Giving a thorough analysis of how deep learning methods are used to identify and diagnose lung cancer from CT scans.
- Summarizing the most popular deep learning models for detecting and classifying lung cancer.
- Comparison analysis of the effectiveness of various deep learning models.
- Outlining the shortcomings of the current approaches and recommending potential study areas.
2. Lung Cancer Detection Using Deep Learning Techniques
3. Computer-Assisted Lung Cancer Detection Using CT Images
4. Dataset Discussion
- Lung Image Database (LID): The Lung Image Database is often used to develop and validate computer-aided diagnosis systems for lung cancer based on deep learning algorithms [62].
- LIDC-IDRI: The Lung Image Database Consortium and Image Database Resource Initiative dataset is a widely used dataset for lung cancer research, providing annotated CT images for nodule detection and classification tasks [63].
- NLST: The National Lung Screening Trial dataset is commonly used for low-dose CT imaging research to evaluate the performance of deep learning models in early lung cancer detection [64].
- ImageNet: ImageNet is a vast dataset used for pretraining deep learning models. It has been utilized in transfer learning approaches for lung cancer detection tasks [65].
- Immunotherapy dataset: This dataset has been employed to investigate the relationship between lung cancer and immunotherapy responses using deep learning methods [66].
- PD-L1 expression dataset: The PD-L1 expression dataset has been utilized to explore the use of deep learning in predicting PD-L1 expression levels in lung cancer patients [47].
- Tianchi AI dataset: The Tianchi AI dataset has been used in various studies to develop and evaluate deep learning-based lung cancer detection systems [67].
- CT lung datasets: Different CT lung datasets are used in studies focusing on lung-specific image analysis and nodule detection using deep learning methods [68].
- Cancer Imaging Archive (CIA) dataset: This dataset is commonly used in research to develop and assess deep-learning models for lung cancer diagnosis [69].
- Private datasets: Some studies have utilized private datasets, the sources of which are not publicly disclosed, to evaluate the performance of deep learning techniques [70].
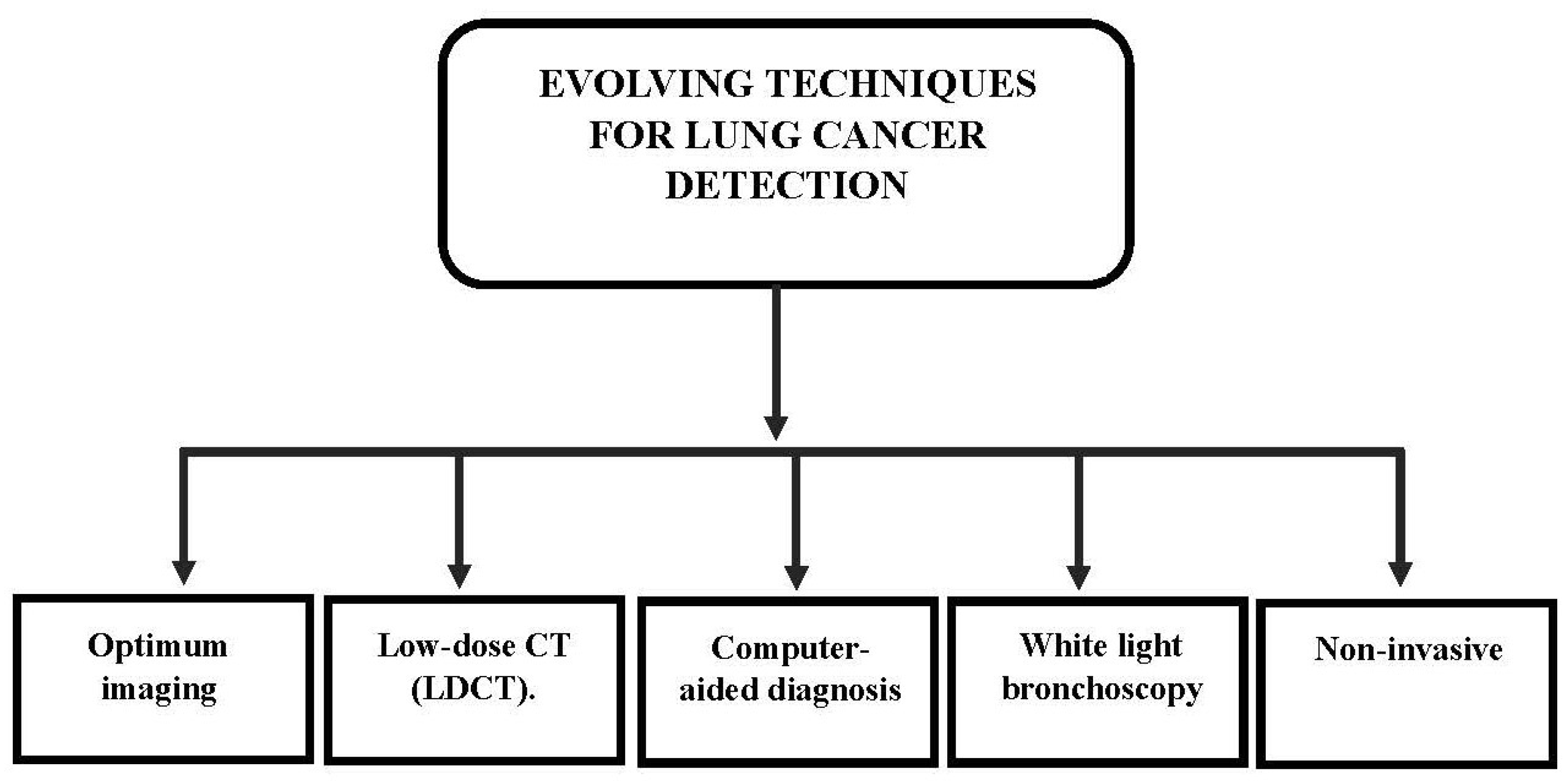
5. Evolving Techniques for Lung Cancer Detection
Research Gaps and Challenges
- Early diagnosis of lung cancer is crucial to improving survival rates, but it remains challenging due to factors like low contrast variation, heterogeneity, and the visual resemblance between benign and malignant nodules in CT images [94].
- Accurately detecting lung nodules in medical imaging is difficult due to the intricate lung anatomy and the need for labeled samples, which can be time-consuming to acquire [95].
- Deep learning algorithms have shown promise in automatically identifying features in lung nodule CT images, but their performance is often compared to traditional computer-aided diagnosis (CADx) systems that rely on hand-crafted features [96].
- There is limited research on utilizing Convolutional Neural Networks (CNNs) to analyze EBUS images, and distinguishing between benign and potentially cancerous tumors based solely on EBUS images is challenging [97].
- Some studies have focused on predicting mortality risks based on CT scans of NSCLC patients but failed to identify early-stage lung or lobe-related malignant lesions [98].
- Understanding how CNNs predict the malignancy of a specific nodule and the importance of the region within a nodule or contextual information in the CNN’s output remains unclear [99].
- Computer-assisted lung disease diagnosis is essential due to noise signals that degrade the quality of cancer images during the picture capture process [100].
- The diverse appearance of different lung nodules and the scarcity of positive samples in available datasets pose challenges for training Deep Convolutional Neural Networks (DCNNs) [101].
6. Segmentation Process
7. Classification Process
8. Limitations
9. Discussion
10. Conclusions
11. Future Research Direction
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Shah, R.; Sabanathan, S.; Richardson, J.; Mearns, A.; Goulden, C. Results of surgical treatment of stage i and ii lung cancer. J. Cardiovasc. Surg. 1996, 37, 169–172. [Google Scholar]
- Nesbitt, J.C.; Putnam, J.B., Jr.; Walsh, G.L.; Roth, J.A.; Mountain, C.F. Survival in early-stage non-small cell lung cancer. Ann. Thorac. Surg. 1995, 60, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Kuan, K.; Ravaut, M.; Manek, G.; Chen, H.; Lin, J.; Nazir, B.; Chen, C.; Howe, T.C.; Zeng, Z. Deep learning for lung cancer detection: Tackling the kaggle data science bowl 2017 challenge. arXiv 2017, arXiv:1705.09435. [Google Scholar]
- Ciompi, F.; Chung, K.; Gerke, P.K.; Jacobs, C.; Scholten, E.T.; SchaeferProkop, C.; Wille, M.M.W.; Marchianò, A.; Pastorino, U.; van Ginneken, B. Towards automatic pul-monary nodule management in lung cancer screening with deep learning. Sci. Rep. 2017, 7, 46479. [Google Scholar]
- Sun, W.; Tseng, T.-L.B.; Qian, W.; Zhang, J.; Saltzstein, E.C.; Zheng, B.; Lure, F.; Yu, H.; Zhou, S. Using multiscale texture and density features for near-term breast cancer risk analysis. Med. Phys. 2015, 42 Pt 1, 2853–2862. [Google Scholar]
- Hossain, M.S.; Muhammad, G. Cloud-Based Collaborative Media Service Framework for HealthCare. Int. J. Distrib. Sens. Netw. 2014, 10, 858712. [Google Scholar]
- Amin, S.U.; Alsulaiman, M.; Muhammad, G.; Mekhtiche, M.A.; Hossain, M.S. Deep Learning for EEG motor imagery classification based on multi-layer CNNs feature fusion. Future Gener. Comput. Syst. 2019, 101, 542–554. [Google Scholar]
- Jiang, H.; Ma, H.; Qian, W.; Gao, M.; Li, Y. An Automatic Detection System of Lung Nodule Based on Multi-Group Patch-Based Deep Learning Network. IEEE J. Biomed. Health Inform. 2018, 22, 1227–1237. [Google Scholar]
- Skourt, B.A.; El Hassani, A. Lung CT image segmentation using deep neural networks. Procedia Comput. Sci. 2018, 127, 109–113. [Google Scholar] [CrossRef]
- Krishnaiah, V.; Narsimha, G.; Chandra, N.S. Diagnosis of lung cancer prediction system using data mining classification techniques. Int. J. Comput. Sci. Inf. Technol. 2013, 4, 39–45. [Google Scholar]
- Begum, S.; Sarkar, R.; Chakraborty, D.; Maulik, U. Identification of biomarker on biological and gene expression data using fuzzy preference based rough set. J. Intell. Syst. 2021, 30, 130–141. [Google Scholar]
- Razzak, M.I.; Naz, S.; Zaib, A. Deep learning for medical image processing: Overview, challenges and the future. Lect. Notes Comput. Vis. Biomech. 2018, 26, 323–350. [Google Scholar]
- Roy, R.; Chakraborti, T.; Chowdhury, A.S. A deep learning shape driven level set synergism for pulmonary nodule segmentation. Pattern Recognit. Lett. 2019, 123, 31–38. [Google Scholar]
- Brown, M.S.; Mcnitt-Gray, M.F.; Mankovich, N.J.; Goldin, J.G.; Hiller, J.; Wilson, L.S.; Aberie, D. Method for segmenting chest CT image data using an anatomical model: Preliminary results. IEEE Trans. Med. Imaging 1997, 16, 828–839. [Google Scholar]
- Brown, M.S.; Goldin, J.G.; McNitt-Gray, M.F.; Greaser, L.E.; Sapra, A.; Li, K.-T.; Sayre, J.W.; Martin, K.; Aberle, D.R. Knowledge-based segmentation of thoracic computed tomography images for assessment of split lung function. Med. Phys. 2000, 27, 592–598. [Google Scholar]
- Hu, S.; Hoffman, E.A.; Reinhardt, J.M. Automatic lung segmentation for accurate quantitation of volumetric x-ray ct images. IEEE Trans. Med. Imaging 2001, 20, 490–498. [Google Scholar] [CrossRef]
- Leader, J.K.; Zheng, B.; Rogers, R.M.; Sciurba, F.C.; Perez, A.; Chapman, B.E.; Patel, S.; Fuhrman, C.R.; Gur, D. Automated lung segmentation in x-ray computed tomography: Development and evaluation of a heuristic threshold-based scheme 1. Acad. Radiol. 2003, 10, 1224–1236. [Google Scholar]
- Sun, X.; Zhang, H.; Duan, H. 3d computerized segmentation of lung volume with computed tomography. Acad. Radiol. 2006, 13, 670–677. [Google Scholar] [CrossRef]
- Swierczynski, P.; Papie, B.W.; Schnabel, J.A.; Macdonald, C. A level-set approach to joint image segmentation and registration with application to CT lung imaging. Comput. Med. Imaging Graph. 2018, 65, 58–68. [Google Scholar]
- Farag, A.A.; Munim, H.E.A.E.; Graham, J.H.; Farag, A.A. A novel approach for lung nodules segmentation in chest ct using level sets. IEEE Trans. Image Process. 2013, 22, 5202–5213. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Bui, A.A.; Cong, J.; Hsu, W. An automated lung segmentation approach using bidirectional chain codes to improve nodule detection accuracy. Comput. Biol. Med. 2015, 57, 139–149. [Google Scholar]
- Zhang, W.; Wang, X.; Zhang, P.; Chen, J. Global optimal hybrid geometric active contour for automated lung segmentation on CT images. Comput. Biol. Med. 2017, 91, 168–180. [Google Scholar]
- Filho, P.P.R.; Cortez, P.C.; da Silva Barros, A.C.; Albuquerque, V.H.C.; Tavares, J.M.R.S. Novel and powerful 3D adaptive crisp active contour method applied in the segmentation of CT lung images. Med. Image Anal. 2017, 35, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Sun, X.; Guo, X.; Zhang, X.; Yang, X.; Wu, Y.; Zhong, W. Toward an Expert Level of Lung Cancer Detection and Classification Using a Deep Convolutional Neural Network. Oncologist 2019, 24, 1159–1165. [Google Scholar] [PubMed] [Green Version]
- Nasser, I.M.; Naser, A. Lung cancer detection using artificial neural network. Int. J. Eng. Inf. Syst. 2019, 3, 17–23. [Google Scholar]
- Cifci, M.A. SegChaNet: A Novel Model for Lung Cancer Segmentation in CT Scans. Appl. Bionics Biomech. 2022, 2022, 1139587. [Google Scholar] [CrossRef]
- Jakimovski, G.; Davcev, D. Using Double Convolution Neural Network for Lung Cancer Stage Detection. Appl. Sci. 2019, 9, 427. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Wang, J.; Wen, Y.; Lu, H.; Niu, T.; Pan, J.; Qian, D. Pulmonary Nodule Detection in Volumetric Chest CT Scans Using CNNs-Based Nodule-Size-Adaptive Detection and Classification. IEEE Access 2019, 7, 46033–46044. [Google Scholar]
- Wang, C.; Chen, D.; Hao, L.; Liu, X.; Zeng, Y.; Chen, J.; Zhang, G. Pulmonary Image Classification Based on Inception-v3 Transfer Learning Model. IEEE Access 2019, 7, 146533–146541. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, P.; Zhang, P.; Xu, X.; Wu, J.; Chen, W. Dense Convolutional Binary-Tree Networks for Lung Nodule Classification. IEEE Access 2018, 6, 49080–49088. [Google Scholar] [CrossRef]
- Li, L.; Liu, Z.; Huang, H.; Lin, M.; Luo, D. Evaluating the performance of a deep learning-based computer-aided diagnosis (DL-CAD) system for detecting and characterizing lung nodules: Comparison with the performance of double reading by radiologists. Thorac. Cancer 2018, 10, 183–192. [Google Scholar] [PubMed]
- Jin, H.; Li, Z.; Tong, R.; Lin, L. A deep 3D residual CNN for false-positive reduction in pulmonary nodule detection. Med. Phys. 2018, 45, 2097–2107. [Google Scholar]
- Teramoto, A.; Tsukamoto, T.; Kiriyama, Y. Automated classification of lung cancer types from cytological images using deep convolutional neural networks. BioMed Res. Int. 2017, 2017, 4067832. [Google Scholar] [CrossRef] [Green Version]
- Dou, Q.; Chen, H.; Yu, L.; Qin, J.; Heng, P. Multilevel Contextual 3-D CNNs for False Positive Reduction in Pulmonary Nodule Detection. IEEE Trans. Biomed. Eng. 2016, 64, 1558–1567. [Google Scholar]
- Wang, S.; Shi, J.; Ye, Z.; Dong, D.; Yu, D.; Zhou, M.; Liu, Y.; Gevaert, O.; Wang, K.; Zhu, Y.; et al. Predicting EGFR mutation status in lung adenocarcinoma on computed tomography image using deep learning. Eur. Respir. J. 2019, 53, 1800986. [Google Scholar]
- Wang, C.; Shao, J.; Lv, J.; Cao, Y.; Zhu, C.; Li, J.; Shen, W.; Shi, L.; Liu, D.; Li, W. Deep learning for predicting subtype classification and survival of lung adenocarcinoma on computed tomography. Transl. Oncol. 2021, 14, 101141. [Google Scholar] [CrossRef]
- Wu, J.; Qian, T. A survey of pulmonary nodule detection, segmentation and classification in computed tomography with deep learning techniques. J. Med. Artif. Intell. 2019, 2, 1–12. [Google Scholar]
- Ardila, D.; Kiraly, A.P.; Bharadwaj, S.; Choi, B.; Reicher, J.J.; Peng, L.; Tse, D.; Etemadi, M.; Ye, W.; Corrado, G.; et al. End-to-end lung cancer screening with three-dimensional deep learning on low-dose chest computed tomography. Nat. Med. 2019, 25, 954–961. [Google Scholar]
- Shao, J.; Wang, G.; Yi, L.; Wang, C.; Lan, T.; Xu, X.; Guo, J.; Deng, T.; Liu, D.; Chen, B.; et al. Deep learning empowers lung cancer screening based on mobile low-dose computed tomography in resource-constrained sites. Front. Biosci. Landmark 2022, 27, 212. [Google Scholar]
- Wang, C.; Xu, X.; Shao, J.; Zhou, K.; Zhao, K.; He, Y.; Li, J.; Guo, J.; Yi, Z.; Li, W. Deep learning to predict EGFR mutation and PD-L1 expression status in non-small-cell lung cancer on computed tomography images. J. Oncol. 2021, 2021, 5499385. [Google Scholar] [PubMed]
- Li, R.; Xiao, C.; Huang, Y.; Hassan, H.; Huang, B. Deep learning applications in computed tomography images for pulmonary nodule detection and diagnosis: A review. Diagnostics 2022, 12, 298. [Google Scholar]
- Lakshmanaprabu, S.K.; Mohanty, S.N.; Shankar, K.; Arunkumar, N.; Ramirez, G. Optimal deep learning model for classification of lung cancer on CT images. Future Gener. Comput. Syst. 2019, 92, 374–382. [Google Scholar]
- Lee, S.M.; Seo, J.B.; Yun, J.; Cho, Y.H.; Vogel-Claussen, J.; Schiebler, M.L.; Gefter, W.B.; van Beek, E.J.; Goo, J.M.; Lee, K.S.; et al. Deep learning applications in chest radiography and computed tomography. J. Thorac. Imaging 2019, 34, 75–85. [Google Scholar]
- Bhatia, S.; Sinha, Y.; Goel, L. Lung cancer detection: A deep learning approach. In Soft Computing for Problem Solving: SocProS 2017; Springer: Singapore, 2019; Volume 2, pp. 699–705. [Google Scholar]
- Tian, P.; He, B.; Mu, W.; Liu, K.; Liu, L.; Zeng, H.; Liu, Y.; Jiang, L.; Zhou, P.; Huang, Z.; et al. Assessing PD-L1 expression in non-small cell lung cancer and predicting responses to immune checkpoint inhibitors using deep learning on computed tomography images. Theranostics 2021, 11, 2098. [Google Scholar]
- Hu, D.; Zhang, H.; Li, S.; Wang, Y.; Wu, N.; Lu, X. Automatic extraction of lung cancer staging information from computed tomography reports: Deep learning approach. JMIR Med. Inform. 2021, 9, e27955. [Google Scholar]
- Kozuka, T.; Matsukubo, Y.; Kadoba, T.; Oda, T.; Suzuki, A.; Hyodo, T.; Im, S.; Kaida, H.; Yagyu, Y.; Tsurusaki, M.; et al. Efficiency of a computer-aided diagnosis (CAD) system with deep learning in detection of pulmonary nodules on 1-mm-thick images of computed tomography. Jpn. J. Radiol. 2020, 38, 1052–1061. [Google Scholar]
- Ashraf, S.F.; Yin, K.; Meng, C.X.; Wang, Q.; Wang, Q.; Pu, J.; Dhupar, R. Predicting benign, preinvasive, and invasive lung nodules on computed tomography scans using machine learning. J. Thorac. Cardiovasc. Surg. 2022, 163, 1496–1505. [Google Scholar]
- Subramanian, R.R.; Mourya, R.N.; Reddy, V.P.T.; Reddy, B.N.; Amara, S. Lung Cancer Prediction Using Deep Learning Framework. Int. J. Control. Autom. 2020, 13, 154–160. [Google Scholar]
- Vani, R.; Vaishnnave, M.P.; Premkumar, S.; Sarveshwaran, V.; Rangaraaj, V. Lung cancer disease prediction with CT scan and histopathological images feature analysis using deep learning techniques. Results Eng. 2023, 18, 101111. [Google Scholar]
- Shalini, W.; Vigneshwari, S. A novel hybrid deep learning method for early detection of lung cancer using neural networks. Healthc. Anal. 2023, 3, 100195. [Google Scholar]
- Abunajm, S.; Elsayed, N.; ElSayed, Z.; Ozer, M. Deep Learning Approach for Early-Stage Lung Cancer Detection. arXiv 2023, arXiv:2302.02456. [Google Scholar]
- Avanzo, M.; Stancanello, J.; Pirrone, G.; Sartor, G. Radiomics and deep learning in lung cancer. Strahlenther. Onkol. 2020, 196, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Shan, H.; Homayounieh, F.; Singh, R.; Khera, R.D.; Guo, H.; Su, T.; Wang, G.; Kalra, M.K.; Yan, P. Deep learning predicts cardiovascular disease risks from lung cancer screening low dose computed tomography. Nat. Commun. 2021, 12, 2963. [Google Scholar] [CrossRef] [PubMed]
- Shakeel, P.M.; Burhanuddin, M.A.; Desa, M.I. Lung cancer detection from CT image using improved profuse clustering and deep learning instantaneously trained neural networks. Measurement 2019, 145, 702–712. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, H.; Yoon, S.W.; Won, D.; Srihari, K. Lung nodule diagnosis on 3D computed tomography images using deep convolutional neural networks. Procedia Manuf. 2019, 39, 363–370. [Google Scholar] [CrossRef]
- Zhao, L.; Qian, J.; Tian, F.; Liu, R.; Liu, B.; Zhang, S.; Lu, M. A weighted discriminative extreme learning machine design for lung cancer detection by an electronic nose system. IEEE Trans. Instrum. Meas. 2021, 70, 2509709. [Google Scholar]
- Chen, L.; Liu, K.; Shen, H.; Ye, H.; Liu, H.; Yu, L.; Li, J.; Zhao, K.; Zhu, W. Multimodality Attention-Guided 3-D Detection of Nonsmall Cell Lung Cancer in 18 F-FDG PET/CT Images. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 6, 421–432. [Google Scholar] [CrossRef]
- Naik, A.; Edla, D.R. Lung nodule classification on computed tomography images using deep learning. Wirel. Pers. Commun. 2021, 116, 655–690. [Google Scholar] [CrossRef]
- Atsushi, T.; Fujita, H. Fast lung nodule detection in chest CT images using cylindrical nodule-enhancement filter. Int. J. Comput. Assist. Radiol. Surg. 2013, 8, 193–205. [Google Scholar]
- Armato, S.G.; McLennan, G.; Bidaut, L.; McNitt-Gray, M.F.; Meyer, C.R.; Reeves, A.P.; Zhao, B.; Aberle, D.R.; Henschke, C.I.; Hoffman, E.A.; et al. The Lung Image Database Consortium (LIDC) and Image Database Resource Initiative (IDRI): A completed reference database of lung nodules on CT scans. Med. Phys. 2011, 38, 915–931. [Google Scholar]
- The National Lung Screening Trial Research Team. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N. Engl. J. Med. 2011, 365, 395–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, J.; Dong, W.; Socher, R.; Li, L.J.; Li, K.; Li, F.-F. ImageNet: A large-scale hierarchical image database. In Proceedings of the IEEE Conference on Computer Vision and Pattern Recognition (CVPR), Miami, FL, USA, 20–25 June 2009; pp. 248–255. [Google Scholar]
- Ye, Y.; Zhang, Y.; Yang, N.; Gao, Q.; Ding, X.; Kuang, X.; Bao, R.; Zhang, Z.; Sun, C.; Zhou, B.; et al. Profiling of immune features to predict immunotherapy efficacy. Innovation 2022, 3, 100194. [Google Scholar] [PubMed]
- Hao, T.; Kim, D.R.; Xie, X. Automated pulmonary nodule detection using 3D deep convolutional neural networks. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; IEEE: New York, NJ, USA, 2018. [Google Scholar]
- Zaffino, P.; Marzullo, A.; Moccia, S.; Calimeri, F.; De Momi, E.; Bertucci, B.; Arcuri, P.P.; Spadea, M.F. An open-source COVID-19 ct dataset with automatic lung tissue classification for radiomics. Bioengineering 2021, 8, 26. [Google Scholar] [CrossRef]
- Prior, F.; Smith, K.; Sharma, A.; Kirby, J.; Tarbox, L.; Clark, K.; Bennett, W.; Nolan, T.; Freymann, J. The public cancer radiology imaging collections of The Cancer Imaging Archive. Sci. Data 2017, 4, 170124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.; Yang, Z.; Jiang, S. Automatic lung tumor segmentation from CT images using improved 3D densely connected UNet. Med. Biol. Eng. Comput. 2022, 60, 3311–3323. [Google Scholar] [CrossRef] [PubMed]
- Gindi, A.M.; Al Attiatalla, T.A.; Sami, M.M. A Comparative Study for Comparing Two Feature Extraction Methods and Two Classifiers in Classification of Earlystage Lung Cancer Diagnosis of chest x-ray images. J. Am. Sci. 2014, 10, 13–22. [Google Scholar]
- Suzuki, K.; Kusumoto, M.; Watanabe, S.I.; Tsuchiya, R.; Asamura, H. Radiologic classification of small adenocarcinoma of the lung: Radiologic-pathologic correlation and its prognostic impact. Ann. Thorac. Surg. 2006, 81, 413–419. [Google Scholar] [PubMed]
- Guo, X.; Sun, T.; Wang, H.; Liang, Z. Prediction Models for Malignant Pulmonary Nodules Based-on Texture Features of CT Image. In Theory and Applications of CT Imaging and Analysis; IntechOpen: London, UK, 2011. [Google Scholar] [CrossRef] [Green Version]
- Horeweg, N.; Scholten, E.T.; de Jong, P.A.; van der Aalst, C.M.; Weenink, C.; Lammers, J.-W.J.; Nackaerts, K.; Vliegenthart, R.; ten Haaf, K.; Yousaf-Khan, U.A.; et al. Detection of Lung Cancer through Low-Dose CT Screening (NELSON): A Prespecified Analysis of Screening Test Performance and Interval Cancers. Lancet Oncol. 2014, 15, 1342–1350. [Google Scholar]
- Gartman, E.J.; Jankowich, M.D.; Baptiste, J.; Nici, L. Providence VA lung cancer screening program: Performance: Comparison of local false positive and invasive procedure rates to published trial data. In A98. Clinical Strategies to Improve Lung Cancer Early Detection: Who Is at Risk Here? American Thoracic Society: New York, NY, USA, 2018; p. A2477. [Google Scholar]
- Kuman, V.; Abbas, A.; Fausto, N.; Robbins, S.; Cotran, R. Robbins and Cotran Pathologic Basis of Disease; Elsevier Saunders: Philadelphia, PA, USA, 2005; p. 759. [Google Scholar]
- Travis, W.D. Update on Small Cell Carcinoma and Its Differentiation from Squamous Cell Carcinoma and Other Non-Small Cell Carcinomas. Mod. Pathol. 2012, 25, S18–S30. [Google Scholar]
- Chan, B.A.; Coward, J.I.G. Chemotherapy Advances in Small-Cell Lung Cancer. J. Thorac. Dis. 2013, 5 (Suppl. S5), S565–S578. [Google Scholar]
- Sagawa, M.; Nakayama, T.; Tsukada, H.; Nishii, K.; Baba, T.; Kurita, Y.; Saito, Y.; Kaneko, M.; Sakuma, T.; Suzuki, T.; et al. Te efficacy of lung cancer screening conducted in 1990s: Four case–control studies in Japan. Lung Cancer 2003, 41, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fontana, R.S.; Sanderson, D.R.; Woolner, L.B.; Taylor, W.F.; Miller, W.E.; Muhm, J.R. Lung cancer screening: Te Mayo program. J. Occup. Environ. Med. 1986, 28, 746–750. [Google Scholar] [CrossRef]
- Kubik, A.; Parkin, D.M.; Khlat, M.; Erban, J.; Polak, J.; Adamec, M. Lack of benefit from semi-annual screening for cancer of the lung: Follow-up report of a randomized controlled trial on a population of high-risk males in Czechoslovakia. Int. J. Cancer 1990, 45, 26–33. [Google Scholar] [CrossRef]
- Raghu, V.K.; Zhao, W.; Pu, J.; Leader, J.K.; Wang, R.; Herman, J.; Yuan, J.-M.; Benos, P.V.; Wilson, D.O. Feasibility of lung cancer prediction from low-dose CT scan and smoking factors using causal models. Thorax 2019, 74, 643–649. [Google Scholar] [CrossRef] [Green Version]
- Risse, E.K.; Vooijs, G.P.; van’t Hof, M.A. Relationship between the Cellular Composition of Sputum and the Cytologic Diagnosis of Lung Cancer. Acta Cytol. 1987, 31, 170–176. [Google Scholar] [PubMed]
- MacDougall, B.; Weinerman, B. The Value of Sputum Cytology. J. Gen. Intern. Med. 1992, 7, 11–13. [Google Scholar] [CrossRef]
- Kennedy, T.C.; Hirsch, F.R.; Miller, Y.E.; Prindiville, S.; Murphy, J.R.; Dempsey, E.; Proudfoot, S.; Bunn, P.A.; Franklin, W.A. A Randomized Study of Fluorescence Bronchoscopy versus White-Light Bronchoscopy for Early Detection of Lung Cancer in High Risk Patients. Lung Cancer 2000, 1 (Suppl. S1), 244–245. [Google Scholar]
- Toyoda, Y.; Nakayama, T.; Kusunoki, Y.; Iso, H.; Suzuki, T. Sensitivity and Specificity of Lung Cancer Screening Using Chest Low-Dose Computed Tomography. Br. J. Cancer 2008, 98, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Hinton, G. Deep learning—A technology with the potential to transform health care. JAMA 2018, 320, 1101–1102. [Google Scholar] [CrossRef]
- LeCun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Ueda, D.; Shimazaki, A.; Miki, Y. Technical and clinical overview of deep learning in radiology. Jpn. J. Radiol. 2019, 37, 15–33. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.G.; Park, S.; Hwang, E.J.; Lee, J.H.; Jin, K.-N.; Lim, K.Y.; Vu, T.H.; Sohn, J.H.; Hwang, S.; Goo, J.M.; et al. Development and validation of deep learning–based automatic detection algorithm for malignant pulmonary nodules on chest radiographs. Radiology 2019, 290, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Manser, R.; Irving, L.; Stone, C.; Byrnes, G.; Abramson, M.; Campbell, D. Screening for lung cancer. Cochrane Database Syst. Rev. 2013, CD001991. [Google Scholar] [CrossRef]
- Berlin, L. Radiologic errors, past, present and future. Diagnosis 2014, 1, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, L.H.; Litière, S.; De Vries, E.; Ford, R.; Gwyther, S.; Mandrekar, S.; Shankar, L.; Bogaerts, J.; Chen, A.; Dancey, J.; et al. RECIST 1.1-Update and clarification: From the RECIST committee. Eur. J. Cancer 2016, 62, 132–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwyzer, M.; Ferraro, D.A.; Muehlematter, U.J.; Curioni-Fontecedro, A.; Huellner, M.W.; von Schulthess, G.K.; Kaufmann, P.A.; Burger, I.A.; Messerli, M. Automated detection of lung cancer at ultralow dose PET/CT by deep neural networks—Initial results. Lung Cancer 2018, 126, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Zheng, B.; Qian, W. Automatic feature learning using multichannel ROI based on deep structured algorithms for computerized lung cancer diagnosis. Comput. Biol. Med. 2017, 89, 530–539. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Coroller, T.P.; Grossmann, P.; Zeleznik, R.; Kumar, A.; Bussink, J.; Gillies, R.J.; Mak, R.H.; Aerts, H.J.W.L. Deep learning for lung cancer prognostication: A retrospective multi-cohort radiomics study. PLoS Med. 2018, 15, 1002711. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, L.; Raja, R.; Awasthi, V.; Miri, R.; Sinha, G.R.; Alkinani, M.H.; Polat, K. Detection of lung nodule and cancer using novel Mask-3 FCM and TWEDLNN algorithms. Measurement 2021, 172, 108882. [Google Scholar] [CrossRef]
- Wu, B.; Zhou, Z.; Wang, J.; Wang, Y. Joint learning for pulmonary nodule segmentation, attributes and malignancy prediction. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; pp. 1109–1113. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Cao, H.; Song, E.; Ma, G.; Xu, X.; Jin, R.; Liu, C.; Hung, C.-C. Multi-model ensemble learning architecture based on 3D CNN for lung nodule malignancy suspiciousness classification. J. Digit. Imaging 2020, 33, 1242–1256. [Google Scholar] [CrossRef]
- Li, J.; Tao, Y.; Cai, T. Predicting lung cancers using epidemiological data: A generative-discriminative framework. IEEE CAA J. Autom. Sin. 2021, 8, 1067–1078. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, J.; Xia, Y. Semi-supervised adversarial model for benign–malignant lung nodule classification on chest CT. Med. Image Anal. 2019, 57, 237–248. [Google Scholar] [CrossRef]
- Abdani, S.R.; Zulkifley, M.A.; Shahrimin, M.I.; Zulkifley, N.H. Computer-Assisted Pterygium Screening System: A Review. Diagnostics 2022, 12, 639. [Google Scholar] [CrossRef] [PubMed]
- Zulkifley, M.A.; Moubark, A.M.; Saputro, A.H.; Abdani, S.R. Automated Apple Recognition System Using Semantic Segmentation Networks with Group and Shuffle Operators. Agriculture 2022, 12, 756. [Google Scholar] [CrossRef]
- Stofa, M.M.; Zulkifley, M.A.; Zainuri, M.A.A.M. Skin Lesions Classification and Segmentation: A Review. Int. J. Adv. Comput. Sci. Appl. 2021, 12, 532–541. [Google Scholar] [CrossRef]
- Stofa, M.M.; Zulkifley, M.A.; Zainuri, M.A.A.M.; Ibrahim, A.A. U-Net with Atrous Spatial Pyramid Pooling for Skin Lesion Segmentation. In Proceedings of the 6th International Conference on Electrical, Control and Computer Engineering: InECCE2021, Kuantan, Malaysia, 23 August 2021; Springer: Singapore, 2022; pp. 1025–1033. [Google Scholar]
- Xu, M.; Qi, S.; Yue, Y.; Teng, Y.; Xu, L.; Yao, Y.; Qian, W. Segmentation of lung parenchyma in CT images using CNN trained with the clustering algorithm generated dataset. Biomed. Eng. Online 2019, 2, 18. [Google Scholar]
- Liu, C.; Pang, M. Automatic lung segmentation based on image decomposition and wavelet transform. Biomed. Signal Process. Control. 2020, 61, 102032. [Google Scholar]
- Khanna, A.; Londhe, N.D.; Gupta, S.; Semwal, A. A deep residual u-net convolutional neural network for automated lung segmentation in computed tomography images. Biocybern. Biomed. Eng. 2020, 40, 1314–1327. [Google Scholar]
- Comelli, A.; Coronnello, C.; Dahiya, N.; Benfante, V.; Palmucci, S.; Basile, A.; Vancheri, C.; Russo, G.; Yezzi, A.; Stefano, A. Lung segmentation on high-resolution computerized tomography images using deep learning: A preliminary step for radiomics studies. J. Imaging 2020, 6, 125. [Google Scholar]
- Hu, Q.; Souza, L.F.D.F.; Holanda, G.B.; Alves, S.S.; Silva, F.H.D.S.; Han, T.; Filho, P.P.R. An effective approach for ct lung segmentation using mask region-based convolutional neural networks. Artif. Intell. Med. 2020, 103, 101792. [Google Scholar] [PubMed]
- Setio, A.A.A.; Ciompi, F.; Litjens, G.; Gerke, P.; Jacobs, C.; Van Riel, S.J.; Wille, M.M.W.; Naqibullah, M.; S’anchez, C.I.; van Ginneken, B. Pulmonary nodule detection in CT images: False positive reduction using multiview convolutional networks. IEEE Trans. Med. Imaging 2016, 35, 1160–1169. [Google Scholar]
- Negahdar, M.; Beymer, D.; Syeda-Mahmood, T.F. Automated volumetric lung segmentation of thoracic CT images using fully convolutional neural network. Med. Imaging 2018, 10575, 105751J. [Google Scholar]
- Liu, K.; Kang, G. Multiview convolutional neural networks for lung nodule classification. Int. J. Imaging Syst. Technol. 2017, 27, 12–22. [Google Scholar] [CrossRef] [Green Version]
- da Silva, G.; da Silva, N. Lung nodules diagnosis based on evolutionary convolutional neural network. Multimed. Tools Appl. 2017, 76, 19039–19055. [Google Scholar]
- Dey, R.; Lu, Z.; Hong, Y. Diagnostic classification of lung nodules using 3d neural networks. In Proceedings of the 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), Washington, DC, USA, 4–7 April 2018; pp. 774–778. [Google Scholar]
- Kirienko, M.; Sollini, M.; Silvestri, G.; Mognetti, S.; Voulaz, E.; Antunovic, L.; Rossi, A.; Antiga, L.; Chiti, A. Convolutional neural networks promising in lung cancer t-parameter assessment on baseline FDG-PET/CT. Contrast Media Mol. Imaging 2018, 2018, 1382309. [Google Scholar]




| Imaging Method | Applications | Pros | Cons |
|---|---|---|---|
| X-ray | Rib fracture detection, pneumonia detection, and lung cancer screening | Quick, affordable, and broadly accessible | Limited sensitivity and specificity, which could miss lung cancer in its early stages. |
| CT (computed tomography) | Lung cancer screening, lung disease diagnosis, lung cancer extent assessment, and pulmonary embolism detection | Detects tiny or early-stage lung malignancies with high resolution and sensitivity and is helpful for examining lung nodules. | High radiation dose, potential need for contrast agent, and high cost |
| Ultrasound | Identifying pleural effusions, directing thoracentesis, and assessing diaphragm performance | Non-invasive, radiation-free, and usable at the bedside | Operator-dependent, limited capacity to scan lung parenchyma; gas or bone obstructions possible. |
| MRI (magnetic resonance imaging) | Evaluation of the invasion of lung cancer, diagnosis of pulmonary embolism, and evaluation of lung function | Good soft tissue contrast, little radiation exposure, and the ability to assess lung function | Long scan times, little availability, high cost, and potential need for contrast agents |
| PET-CT (positron emission tomography—computed tomography) | Assessing lung cancer, staging lung cancer, observing treatment results, and spotting recurrences of cancer | High sensitivity for detecting cancer, early cancer detection capability, and anatomical and functional information provided | False positives caused by inflammation or infection, high radiation dose, price, and possible requirement for fasting before the scan |
| Reference | Year | Application | Method Used and Dataset | Pros | Cons |
|---|---|---|---|---|---|
| [21] | 2018 | CT Lung image | Level-set approach for joint image segmentation and registration and CT image dataset | The technique allows for the simultaneous processing of these tasks by combining image segmentation and registration into an identical framework | The technique is only directly applicable to CT lung imaging, excluding additional imaging modalities or anatomical locations |
| [43] | 2018 | Segmenting a CT image of the lung | Deep neural networks and the Lung Image Database | Increases efficiency and accuracy | A small sample size |
| [32] | 2018 | Identification of lung nodules | Dense Convolutional Binary-Tree Networks and LIDC-IDRI | Achieved great accuracy in classifying lung nodules. | Restricted by the lack of readily accessible, large training datasets |
| [33] | 2018 | DL-CAD, or deep learning-based computer-aided diagnosis, is a method for identifying and classifying lung nodules | Deep Learning-based Computer-Aided Diagnosis System | Achieved great accuracy in lung nodule detection and characterization and showed promise for enhancing radiologists’ performance | It is constrained by the availability of huge training datasets, and it might not work well on images with poor contrast or strange morphology |
| [34] | 2018 | Reduced likelihood of false-positive lung nodule identification | Deep 3D Residual CNN and CT data | Reduced false positives in the detection of lung nodules with excellent accuracy | Depending on the quality of the input photographs, the algorithm may not function properly on images with poor contrast or strange morphology |
| [26] | 2019 | Identification and classification of lung cancer | Deep Convolutional Neural Networks (CNNs), en-source data sets, and multicenter data sets have been used. | Displayed expert-level proficiency in the spotting and sizing of lung cancer | Requires massive datasets for training and may struggle with images that have poor contrast or a strange shape |
| [37] | 2019 | Lung adenocarcinoma | Deep learning on CT images and imageNet | Predicting the status of the EGFR mutation | A small sample size |
| [44] | 2019 | CT imaging for the identification of lung cancer | Improved profuse clustering and deep learning using instantaneously trained neural networks and CT image datasets | Increased precision and fewer false positives | Large datasets and specialized knowledge are required |
| [27] | 2019 | Artificial neural network-based lung cancer detection | Artificial Neural Network (ANN) and our ANN established, trained, and validated using a data set, whose title is “survey lung cancer” | Classification of benign and malignant nodules with good accuracy | Limited by the poor interpretability of the model and the caliber of the input images |
| [57] | 2019 | CT imaging for the identification of lung cancer | Improved profuse clustering and deep learning instantaneously trained neural networks and the lung CT images are collected from the cancer imaging archive (CIA) dataset | Increased precision and fewer false positives | Large datasets and specialized knowledge are required |
| [58] | 2019 | 3D CT images used to diagnose a lung nodule | Deep convolutional neural networks and LIDC-IDRI | Improved diagnostic precision and decreased inter-observer variability | Large datasets and specialized knowledge are required |
| [40] | 2019 | Screening for lung cancer | Three-dimensional deep learning on low-dose CT and NLST datasets | High efficiency and accuracy | Huge volumes of training data are necessary |
| [29] | 2019 | Finding the stage of lung cancer | Double Convolutional Neural Network (CNN) And CT images from the initial dataset so that the training of the CDNN could be focused | Achieved great accuracy in identifying the stage of lung cancer | Depending on the quality of the input photographs, the algorithm may not function properly on images with poor contrast or strange morphology |
| [30] | 2019 | Classification and identification of pulmonary nodules | CNN-based nodule-size-adaptive detection and classification and the Tianchi AI dataset | The detection and classification of pulmonary nodules were carried out with great accuracy | Depending on the quality of the input photographs, the algorithm may not function properly on images with poor contrast or strange morphology |
| [39] | 2019 | Identification, segmentation, and classification of pulmonary nodules | Deep learning on CT images and NLST | Increases efficiency and accuracy | A small sample size |
| [31] | 2019 | Image categorization for the lungs | Inception-v3 Transfer Learning Model and ImageNet dataset | Good classification accuracy for pulmonary images. | Restricted by the lack of readily accessible, large training datasets. |
| [37] | 2019 | Lung adenocarcinoma | Deep learning on CT images and ImageNet datasets | Predicting the status of the EGFR mutation | A small sample size |
| [49] | 2020 | Finding pulmonary nodules | Computer-aided diagnosis (CAD) system with deep learning and CT image dataset | Extremely sensitive and specific | Large volumes of training data are required |
| [51] | 2020 | Lung cancer prediction | Deep learning framework and eCT image dataset | Early, non-intrusive detection | Limited information and possible false positives |
| [55] | 2020 | Lung cancer | Radiomics and deep learning | Improved prognosis and diagnosis accuracy | Large datasets and specialized knowledge are required |
| [38] | 2021 | Lung adenocarcinoma | Deep learning on CT images | Predicting survival and subtype classification | A small sample size |
| [61] | 2021 | Classification of lung nodules | Deep learning on CT images | High accuracy and efficiency | A small sample size |
| [42] | 2021 | Non-small-cell lung cancer | Deep learning on CT images and immunotherapy datasets | PD-L1 expression and EGFR mutation prediction | A small sample size |
| [47] | 2021 | Evaluation and forecasting of PD-L1 expression | Deep learning on CT images and the PD-L1 expression dataset | Enhanced precision and non-invasive | Large volumes of training data are required |
| [48] | 2021 | Extraction of information on lung cancer staging | Deep learning approach and CT dataset | Automated and saving time | Limited information and possible mistakes |
| [56] | 2021 | Prediction of cardiovascular disease risk from CT of lung cancer | Deep learning and NLST and MGH datasets | Enhanced early detection and risk assessment | Restricted by computing power and labeled data accessibility |
| [59] | 2021 | Using an electronic nasal device and identify lung cancer | Weighted discriminative extreme learning machine design and lung cancer datasets and public datasets | Invasive-free detection technique | Only able to identify lung cancer in its first stages |
| [60] | 2021 | PET/CT imaging for non-small cell lung cancer detection | Multimodality attention-guided 3-D detection using deep learning and a private dataset | Improved diagnostic precision and fewer false positives | Large datasets and specialized knowledge are required |
| [50] | 2022 | Prediction of benign, preinvasive, and invasive lung nodules | Machine learning and CT dataset | Enhanced accuracy and early detection potential | Large training data requirements and the risk for false positives |
| [41] | 2022 | Screening for lung cancer | Deep learning on mobile low-dose CT and CT image datasets | Improved access in areas with limited resources | A small sample size |
| [43] | 2022 | Identification and detection of pulmonary nodules | Deep learning on CT image and CT lung datasets | Increases efficiency and accuracy | A small sample size |
| [28] | 2022 | CT imaging for the identification of lung cancer | Improved Profuse Clustering and Deep Learning Instantaneously Trained Neural Networks and images of CT scans | More accuracy compared to conventional clustering-based approaches and shorter training times | Compared to other deep learning models, it requires more parameters and more training time |
| [52] | 2023 | Prediction with CT scan and histopathological images | Six different deep learning algorithms like Convolutional Neural Network (CNN), CNN Gradient Descent (CNN GD), VGG-16, VGG-19, Inception V3, and Resnet-50. | Using the CNN GD provides the ability to learn from training data over time and its efficient cost function within gradient descent, which continuously assesses accuracy during parameter updates | The lack of integration with fuzzy genetic optimization techniques, which could potentially enhance the methodology’s performance and effectiveness |
| [53] | 2023 | A novel method called Cancer Cell Detection uses Hybrid Neural Network (CCDCHNN) to extract features from the CT scan images using deep neural networks | The approach in this research suggests a sophisticated 3DCNN with an RNN algorithm for classifying cancerous lung nodules. The system makes use of the LUNA 16 database. | The proposed improved model provides single 3D-CNN and RNN classifications with high selectivity, sensitivity, and accuracy | Enhanced efficiency by integrating big-data analytics and cascaded classifiers, which are not currently utilized in the proposed approach |
| [54] | 2023 | The proposed model used several convolutional layers to perform the detection task from CT scan imaging | The study developed a CNN-based model with 99.45% accuracy for early lung cancer prediction using CT scan images. The dataset used was the IQ-OTH/NCCD-lung cancer dataset from Kaggle | The proposed CNN-based model achieved a high accuracy rate of 99.45% for early lung cancer prediction and successfully reduced false positives | The major gap in this work is the limited number of epochs used during training |
| S. No | Method | Accuracy (%) |
|---|---|---|
| 1 | Lung CT image segmentation using deep neural networks [11]. | 95% |
| 2 | Biological and gene expression data using a fuzzy preference-based rough set [13]. | 96.19% |
| 3 | Lung cancer detection using an artificial neural network [27]. | 96.67% |
| 4 | SegChaNet: A novel model for lung cancer segmentation in CT scans [28]. | 98.48% |
| 5 | Optimal deep learning model for classification of lung cancer on CT images [44]. | 94.56% |
| 6 | Lung cancer prediction using a deep learning framework [51]. | 99.52% |
| 7 | An effective approach for CT lung segmentation using mask region-based convolutional neural networks [110]. | 97.68% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanoon, M.A.; Zulkifley, M.A.; Mohd Zainuri, M.A.A.; Abdani, S.R. A Review of Deep Learning Techniques for Lung Cancer Screening and Diagnosis Based on CT Images. Diagnostics 2023, 13, 2617. https://doi.org/10.3390/diagnostics13162617
Thanoon MA, Zulkifley MA, Mohd Zainuri MAA, Abdani SR. A Review of Deep Learning Techniques for Lung Cancer Screening and Diagnosis Based on CT Images. Diagnostics. 2023; 13(16):2617. https://doi.org/10.3390/diagnostics13162617
Chicago/Turabian StyleThanoon, Mohammad A., Mohd Asyraf Zulkifley, Muhammad Ammirrul Atiqi Mohd Zainuri, and Siti Raihanah Abdani. 2023. "A Review of Deep Learning Techniques for Lung Cancer Screening and Diagnosis Based on CT Images" Diagnostics 13, no. 16: 2617. https://doi.org/10.3390/diagnostics13162617
APA StyleThanoon, M. A., Zulkifley, M. A., Mohd Zainuri, M. A. A., & Abdani, S. R. (2023). A Review of Deep Learning Techniques for Lung Cancer Screening and Diagnosis Based on CT Images. Diagnostics, 13(16), 2617. https://doi.org/10.3390/diagnostics13162617








