Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: The Next Threat after Viral Hepatitis
Abstract
:1. Introduction
2. Pathogenesis: What Do We Know?
2.1. Genetic and Epigenetic Factors
2.2. Epigenetics
2.3. Non-Coding RNA (ncRNA)
2.4. Role of Gut Microbiome
3. Surveillance and Diagnosis of NAFLD-Related HCC
3.1. Current Difficulties and Challengnes
- A significant burden on susceptible populations with NAFLD [1].
- Only 30% of patients with cirrhosis adhere to surveillance programs, and this ratio decreases among patients with NAFLD-related cirrhosis [40].
- Difficulties and limitations in ultrasound examination in patients with fatty liver disease, with up to 25% of ultrasound examinations being suboptimal [41]. Ultrasound alone has a very low sensitivity for detecting early HCC in cirrhotic patients. Although MRI has made remarkable progress in diagnosing HCC in NAFLD patients, the high cost and low availability still constitute a major limitation to its use in every patient [42].
- Variable natural history of NAFLD progression.
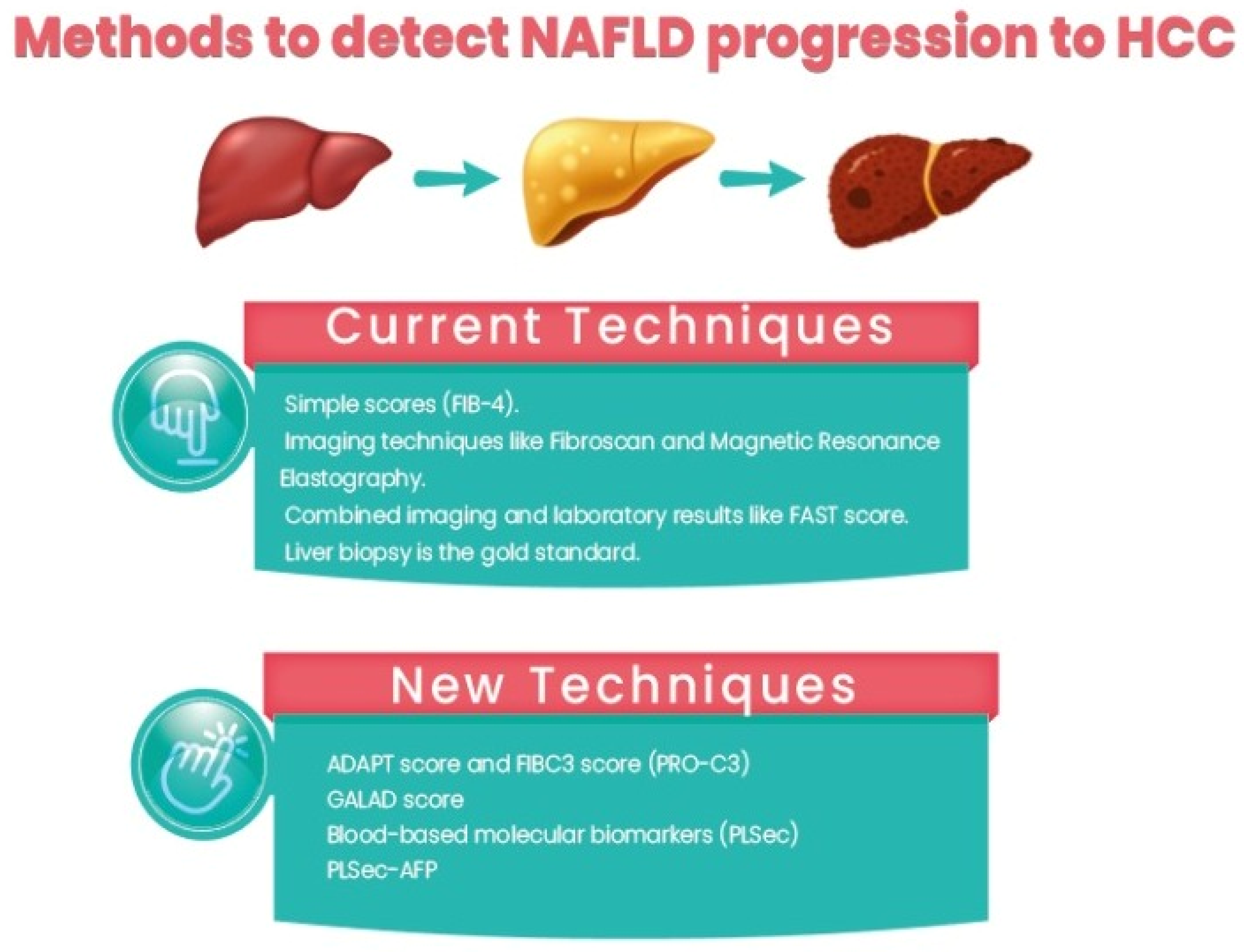
3.2. Current Techniques
- Using simple scores such as the Fibrosis-4 (Fib-4) score, the NAFLD fibrosis score (NFS), the Hepamet fibrosis score (HFS), and the enhanced liver fibrosis (ELF) panel. Among them, Fib-4 is easy and widely used. In a recent study comparing different non-invasive scores, HFS was the best performer for the identification of significant (F0–1 vs. F2–4, AUC = 0.758) and advanced (F0–2 vs. F3–4, AUC = 0.805) fibrosis, while NFS and FIB-4 showed the best performance for detecting histological cirrhosis (range AUCs 0.85–0.88) [50].
- Imaging techniques such as Fibroscan and magnetic resonance elastography.
- Combined imaging and laboratory results such as the FAST score [51].
- Liver biopsy is the gold standard for assessment, but due to its invasiveness, sampling errors, and inconvenience in follow-up, its role in clinical practice is limited.
3.3. What Is New?
3.4. Selection of Treatment Strategy
3.5. Non-Pharmacological Prevention
3.6. Chemoprevention
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| NAFLD | Nonalcoholic fatty liver disease |
| HCC | Hepatocellular carcinoma |
| HCV | Hepatitis C virus |
| HBV | Hepatitis B virus |
| SNPs | Single nucleotide polymorphisms |
| PNPLA3 | Patatin-like phospholipase domain containing 3 |
| TM6SF2 | Transmembrane 6 superfamily member 2 |
| MBOAT7 | Membrane-bound 0-acyltransferase domain containing 7 |
| PDCD-1 | Programmed cell death 1 |
| Tubb2b | Tubulin beta 2B class IIB |
| cfDNA | Circulating cell-free DNA |
| MAPK | Mitogen-activated protein kinase |
| HULC Lnc RNA | Highly upregulated in liver cancer long non-coding RNA |
| MALAT1 Lnc RNA | Metastases associated lung adenocarcinoma transcript 1 long non-coding RNA |
| SIRT1 | Silent information regulator 1 |
| IL8 | Interleukin 8 |
| IL13 | Interleukin 13 |
| CCL3 | Chemokine (C-C motif) ligand 3 |
| CCL4 | Chemokine (C-C motif) ligand 4 |
| CCL5 | Chemokine (C-C motif) ligand 5 |
| Fib-4 score | Fibrosis 4 score |
| NFS Score | NAFLD fibrosis score |
| HFS | Hepamet fibrosis score |
| ELF | enhanced liver fibrosis panel |
| FAST | Fibroscan-AST score |
| PRO-C3 | N-terminal type III collagen propeptide |
| ADAPT score | Age, diabetes, PRO-C3, and platelet count |
| PLSec | Prognostic liver signature secretome |
References
- El-Kassas, M.; Cabezas, J.; Coz, P.I.; Zheng, M.H.; Arab, J.P.; Awad, A. Nonalcoholic Fatty Liver Disease: Current Global Burden. Semin. Liver Dis. 2022, 42, 401–412. [Google Scholar] [CrossRef]
- Ong, J.; Alswat, K.; Hamid, S.; El-Kassas, M. Nonalcoholic Fatty Liver Disease in Asia, Africa, and Middle East Region. Clin. Liver Dis. 2023, 27, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Akinyemiju, T.; Abera, S.; Ahmed, M.; Alam, N.; Alemayohu, M.A.; Allen, C.; Al-Raddadi, R.; Alvis-Guzman, N.; Amoako, Y.; Artaman, A.; et al. The Burden of Primary Liver Cancer and underlying etiologies from 1990 to 2015 at the Global, Regional, and National Level: Results from the Global Burden of Disease Study 2015. JAMA Oncol. 2017, 3, 1683–1691. [Google Scholar] [PubMed]
- Benhammou, J.N.; Lin, J.; Aby, E.S.; Markovic, D.; Raman, S.S.; Lu, D.S.; Tong, M.J. Nonalcoholic fatty liver disease-related hepatocellular carcinoma growth rates and their clinical outcomes. Hepatoma Res. 2021, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Piscaglia, F.; Svegliati-Baroni, G.; Barchetti, A.; Pecorelli, A.; Marinelli, S.; Tiribelli, C.; Bellentani, S. HCC-NAFLD Italian Study Group. Clinical patterns of hepatocellular carcinoma in nonalcoholic fatty liver disease: A multicenter prospective study. Hepatology 2016, 63, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.; Stepanova, M.; Ong, J.P.; Eguchi, Y.; Wong, V.W.; Negro, F.; Yilmaz, Y.; Romero-Gomez, M.; George, J.; Ahmed, A.; et al. Nonalcoholic Steatohepatitis Is the Fastest Growing Cause of Hepatocellular Carcinoma in Liver Transplant Candidates. Clin. Gastroenterol. Hepatol. 2019, 17, 748–755.e3. [Google Scholar] [CrossRef] [Green Version]
- Vitale, A.; Svegliati-Baroni, G.; Ortolani, A.; Cucco, M.; Dalla Riva, G.V.; Giannini, E.G.; Piscaglia, F.; Rapaccini, G.; Di Marco, M.; Caturelli, E.; et al. Epidemiological trends and trajectories of MAFLD-associated hepatocellular carcinoma 2002–2033: The ITA.LI.CA database. Gut 2023, 72, 141–152. [Google Scholar] [CrossRef]
- Dyson, J.; Jaques, B.; Chattopadyhay, D.; Lochan, R.; Graham, J.; Das, D.; Aslam, T.; Patanwala, I.; Gaggar, S.; Cole, M.; et al. Hepatocellular cancer: The impact of obesity, type 2 diabetes and a multidisciplinary team. J. Hepatol. 2014, 60, 110–117. [Google Scholar] [CrossRef]
- Geh, D.; Anstee, Q.M.; Reeves, H.L. NAFLD-Associated HCC: Progress and Opportunities. J. Hepatocell. Carcinoma 2021, 8, 223–239. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Meroni, M.; Longo, M.; Fargion, S.; Fracanzani, A.L. Genetics, Immunity and Nutrition Boost the Switching from NASH to HCC. Biomedicines 2021, 9, 1524. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Liu, Y.L.; Day, C.P.; Reeves, H.L. Reply to: HCC and liver disease risk in homozygous PNPLA3 p.I148M carriers approach monogenic inheritance. J. Hepatol. 2015, 62, 982–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, C.; Jamialahmadi, O.; Pelusi, S.; Baselli, G.; Dongiovanni, P.; Zanoni, I.; Santoro, L.; Maier, S.; Liguori, A.; Meroni, M.; et al. Non-invasive stratification of hepatocellular carcinoma risk in nonalcoholic fatty liver using polygenic risk scores. J. Hepatol. 2021, 74, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Trépo, E.; Nahon, P.; Bontempi, G.; Valenti, L.; Falleti, E.; Nischalke, H.D.; Hamza, S.; Corradini, S.G.; Burza, M.A.; Guyot, E.; et al. Association between the PNPLA3 (rs738409 C > G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology 2014, 59, 2170–2177. [Google Scholar] [CrossRef] [PubMed]
- Bruschi, F.V.; Claudel, T.; Tardelli, M.; Caligiuri, A.; Stulnig, T.M.; Marra, F.; Trauner, M. The PNPLA3 I148M variant modulates the fibrogenic phenotype of human hepatic stellate cells. Hepatology 2017, 65, 1875–1890. [Google Scholar] [CrossRef]
- Kozlitina, J.; Smagris, E.; Stender, S.; Nordestgaard, B.G.; Zhou, H.H.; Tybjærg-Hansen, A.; Vogt, T.F.; Hobbs, H.H.; Cohen, J.C. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat. Genet. 2014, 46, 352–356. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, E.A.; Yang, R.; Yerges-Armstrong, L.M.; Sreenivasan, U.; McFarland, R.; Leitch, C.C.; Wilson, M.H.; Narina, S.; Gorden, A.; Ryan, K.A.; et al. TM6SF2 rs58542926 impacts lipid processing in liver and small intestine. Hepatology 2017, 65, 1526–1542. [Google Scholar] [CrossRef]
- Longo, M.; Meroni, M.; Paolini, E.; Macchi, C.; Dongiovanni, P. Mitochondrial dynamics and nonalcoholic fatty liver disease (NAFLD): New perspectives for a fairy-tale ending? Metabolism 2021, 117, 154708. [Google Scholar] [CrossRef]
- Longo, M.; Meroni, M.; Paolini, E.; Erconi, V.; Carli, F.; Fortunato, F.; Ronchi, D.; Piciotti, R.; Sabatini, S.; Macchi, C.; et al. TM6SF2/PNPLA3/MBOAT7 Loss-of-Function Genetic Variants Impact on NAFLD Development and Progression Both in Patients and in In Vitro Models. Cell. Mol. Gastroenterol. Hepatol. 2022, 13, 759–788. [Google Scholar] [CrossRef]
- Meroni, M.; Longo, M.; Fracanzani, A.L.; Dongiovanni, P. MBOAT7 down-regulation by genetic and environmental factors predisposes to MAFLD. EBioMedicine 2020, 57, 102866. [Google Scholar] [CrossRef]
- Buch, S.; Stickel, F.; Trépo, E.; Way, M.; Herrmann, A.; Nischalke, H.D.; Brosch, M.; Rosendahl, J.; Berg, T.; Ridinger, M.; et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat. Genet. 2015, 47, 1443–1448. [Google Scholar] [CrossRef]
- Donati, B.; Dongiovanni, P.; Romeo, S.; Meroni, M.; McCain, M.; Miele, L.; Petta, S.; Maier, S.; Rosso, C.; De Luca, L.; et al. MBOAT7 rs641738 variant and hepatocellular carcinoma in non-cirrhotic individuals. Sci. Rep. 2017, 7, 4492. [Google Scholar] [CrossRef] [Green Version]
- Eldafashi, N.; Darlay, R.; Shukla, R.; McCain, M.V.; Watson, R.; Liu, Y.L.; McStraw, N.; Fathy, M.; Fawzy, M.A.; Zaki, M.Y.W.; et al. A PDCD1 Role in the Genetic Predisposition to NAFLD-HCC? Cancers 2021, 13, 1412. [Google Scholar] [CrossRef] [PubMed]
- Dreval, K.; Tryndyak, V.; de Conti, A.; Beland, F.A.; Pogribny, I.P. Gene Expression and DNA Methylation Alterations During Nonalcoholic Steatohepatitis-Associated Liver Carcinogenesis. Front. Genet. 2019, 10, 486. [Google Scholar] [CrossRef]
- Hardy, T.; Zeybel, M.; Day, C.P.; Dipper, C.; Masson, S.; McPherson, S.; Henderson, E.; Tiniakos, D.; White, S.; French, J.; et al. Plasma DNA methylation: A potential biomarker for stratification of liver fibrosis in nonalcoholic fatty liver disease. Gut 2017, 66, 1321–1328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- St Laurent, G.; Wahlestedt, C.; Kapranov, P. The Landscape of long non-coding RNA classification. Trends Genet. 2015, 31, 239–251. [Google Scholar] [CrossRef] [Green Version]
- Coulouarn, C.; Factor, V.M.; Andersen, J.B.; Durkin, M.E.; Thorgeirsson, S.S. Loss of miR-122 expression in liver cancer correlates with suppression of the hepatic phenotype and gain of metastatic properties. Oncogene 2009, 28, 3526–3536. [Google Scholar] [CrossRef] [Green Version]
- Lian, J.; Jing, Y.; Dong, Q.; Huan, L.; Chen, D.; Bao, C.; Wang, Q.; Zhao, F.; Li, J.; Yao, M.; et al. miR-192, a prognostic indicator, targets the SLC39A6/SNAIL pathway to reduce tumor metastasis in human hepatocellular carcinoma. Oncotarget 2016, 7, 2672–2683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afonso, M.B.; Rodrigues, P.M.; Simão, A.L.; Castro, R.E. Circulating microRNAs as Potential Biomarkers in Nonalcoholic Fatty Liver Disease and Hepatocellular Carcinoma. J. Clin. Med. 2016, 5, 30. [Google Scholar] [CrossRef] [Green Version]
- Hou, Z.; Xu, X.; Fu, X.; Tao, S.; Zhou, J.; Liu, S.; Tan, D. HBx-related long non-coding RNA MALAT1 promotes cell metastasis via upregulating LTBP3 in hepatocellular carcinoma. Am. J. Cancer Res. 2017, 7, 845–856. [Google Scholar]
- Li, S.P.; Xu, H.X.; Yu, Y.; He, J.D.; Wang, Z.; Xu, Y.J.; Wang, C.Y.; Zhang, H.M.; Zhang, R.X.; Zhang, J.J.; et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget 2016, 7, 42431–42446. [Google Scholar] [CrossRef] [Green Version]
- Malakar, P.; Shilo, A.; Mogilevsky, A.; Stein, I.; Pikarsky, E.; Nevo, Y.; Benyamini, H.; Elgavish, S.; Zong, X.; Prasanth, K.V.; et al. Long Non-coding RNA MALAT1 promotes hepatocellular carcinoma development by SRSF1 upregulation and mTOR activation. Cancer Res. 2017, 77, 1155–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, X.; Guo, H.; Xu, J.; Wang, J. Inhibition of lncRNA HULC improves hepatic fibrosis and hepatocyte apoptosis by inhibiting the MAPK signaling pathway in rats with nonalcoholic fatty liver disease. J. Cell. Physiol. 2019, 234, 18169–18179. [Google Scholar] [CrossRef] [PubMed]
- Sookoian, S.; Flichman, D.; Garaycoechea, M.E.; San Martino, J.; Castaño, G.O.; Pirola, C.J. Metastasis-associated lung adenocarcinoma transcript 1 as a common molecular driver in the pathogenesis of nonalcoholic steatohepatitis and chronic immune-mediated liver damage. Hepatol. Commun. 2018, 2, 654–665. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, X.; Zhou, Q.; Huang, C.; Meng, X.; Xu, F.; Li, J. Silent information regulator 1 (SIRT1) ameliorates liver fibrosis via promoting activated stellate cell apoptosis and reversion. Toxicol. Appl. Pharmacol. 2015, 289, 163–176. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Reeves, H.L.; Kotsiliti, E.; Govaere, O.; Heikenwalder, M. From NASH to HCC: Current concepts and future challenges. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 411–428. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Paroni Sterbini, F.; Petito, V.; et al. Hepatocellular carcinoma is associated with gut microbiota profile and inflammation in nonalcoholic fatty liver disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Said, I.; Ahad, H.; Said, A. Gut microbiome in nonalcoholic fatty liver disease associated hepatocellular carcinoma: Current knowledge and potential for therapeutics. World J. Gastrointest. Oncol. 2022, 14, 947–958. [Google Scholar] [CrossRef] [PubMed]
- Marrero, J.A.; Kulik, L.M.; Sirlin, C.B.; Zhu, A.X.; Finn, R.S.; Abecassis, M.M.; Roberts, L.R.; Heimbach, J.K. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018, 68, 723–750. [Google Scholar] [CrossRef] [Green Version]
- Goossens, N.; Singal, A.G.; King, L.Y.; Andersson, K.L.; Fuchs, B.C.; Besa, C.; Taouli, B.; Chung, R.T.; Hoshida, Y. Cost-Effectiveness of Risk Score-Stratified Hepatocellular Carcinoma Screening in Patients with Cirrhosis. Clin. Transl. Gastroenterol. 2017, 8, e101. [Google Scholar] [CrossRef]
- Wolf, E.; Rich, N.E.; Marrero, J.A.; Parikh, N.D.; Singal, A.G. Use of Hepatocellular Carcinoma Surveillance in Patients with Cirrhosis: A Systematic Review and Meta-Analysis. Hepatology 2021, 73, 713–725. [Google Scholar] [CrossRef]
- Castera, L.; Friedrich-Rust, M.; Loomba, R. Non-invasive Assessment of Liver Disease in Patients with Nonalcoholic Fatty Liver Disease. Gastroenterology 2019, 156, 1264–1281.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tzartzeva, K.; Obi, J.; Rich, N.E.; Parikh, N.D.; Marrero, J.A.; Yopp, A.; Waljee, A.K.; Singal, A.G. Surveillance Imaging and Alpha Fetoprotein for Early Detection of Hepatocellular Carcinoma in Patients with Cirrhosis: A Meta-analysis. Gastroenterology 2018, 154, 1706–1718.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seif El Dahan, K.; Daher, D.; Singal, A.G. Hepatocellular carcinoma surveillance in patients with non-alcoholic fatty liver disease. Clin. Mol. Hepatol. 2023, 29, S207–S219. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, W.; Joo, S.K.; Kim, J.H.; Harrison, S.A.; Younossi, Z.M.; Ahmed, A. Predictors of nonalcoholic steatohepatitis and significant fibrosis in non-obese nonalcoholic fatty liver disease. Liver Int. 2019, 39, 332–341. [Google Scholar] [CrossRef]
- Younes, R.; Caviglia, G.P.; Govaere, O.; Rosso, C.; Armandi, A.; Sanavia, T.; Pennisi, G.; Liguori, A.; Francione, P.; Gallego-Durán, R.; et al. Long-term outcomes and predictive ability of non-invasive scoring systems in patients with nonalcoholic fatty liver disease. J. Hepatol. 2021, 75, 786–794. [Google Scholar] [CrossRef]
- Park, H.J.; Jang, H.Y.; Kim, S.Y.; Lee, S.J.; Won, H.J.; Byun, J.H.; Choi, S.H.; Lee, S.S.; An, J.; Lim, Y.S. Non-enhanced magnetic resonance imaging as a surveillance tool for hepatocellular carcinoma: Comparison with ultrasound. J. Hepatol. 2020, 72, 718–724. [Google Scholar] [CrossRef]
- Renzulli, M.; Golfieri, R. Bologna Liver Oncology Group (BLOG). Proposal of a new diagnostic algorithm for hepatocellular carcinoma based on the Japanese guidelines but adapted to the Western world for patients under surveillance for chronic liver disease. J. Gastroenterol. Hepatol. 2016, 31, 69–80. [Google Scholar] [CrossRef] [Green Version]
- Jang, W.; Song, J.S. Non-Invasive Imaging Methods to Evaluate Non-Alcoholic Fatty Liver Disease with Fat Quantification: A Review. Diagnostics 2023, 13, 1852. [Google Scholar] [CrossRef]
- Pons, M.; Augustin, S.; Scheiner, B.; Guillaume, M.; Rosselli, M.; Rodrigues, S.G.; Stefanescu, H.; Ma, M.M.; Mandorfer, M.; Mergeay-Fabre, M.; et al. Noninvasive Diagnosis of Portal Hypertension in Patients with Compensated Advanced Chronic Liver Disease. Am. J. Gastroenterol. 2021, 116, 723–732. [Google Scholar] [CrossRef]
- Guerra-Ruiz, A.R.; Casals, G.; Iruzubieta, P.; Lalana, M.; Leis, A.; López, R.M.; Crespo, J.; Morales-Ruiz, M. Biochemical assessment of metabolic associated fatty liver disease. Adv. Lab. Med. 2021, 2, 199–219. [Google Scholar] [CrossRef]
- Newsome, P.N.; Sasso, M.; Deeks, J.J.; Paredes, A.; Boursier, J.; Chan, W.K.; Yilmaz, Y.; Czernichow, S.; Zheng, M.H.; Wong, V.W.; et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with nonalcoholic steatohepatitis with significant activity and fibrosis: A prospective derivation and global validation study. Lancet Gastroenterol. Hepatol. 2020, 5, 362–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniels, S.J.; Leeming, D.J.; Eslam, M.; Hashem, A.M.; Nielsen, M.J.; Krag, A.; Karsdal, M.A.; Grove, J.I.; Neil Guha, I.; Kawaguchi, T.; et al. ADAPT: An algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology 2019, 69, 1075–1086. [Google Scholar] [CrossRef] [PubMed]
- Boyle, M.; Tiniakos, D.; Schattenberg, J.M.; Ratziu, V.; Bugianessi, E.; Petta, S.; Oliveira, C.P.; Govaere, O.; Younes, R.; McPherson, S.; et al. performance of the PRO-C3 collagen neo-epitope biomarker in nonalcoholic fatty liver disease. JHEP Rep. 2019, 1, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Bechmann, L.P.; Sowa, J.P.; Sydor, S.; Dechêne, A.; Pflanz, K.; Bedreli, S.; Schotten, C.; Geier, A.; Berg, T.; et al. GALAD score detects early hepatocellular carcinoma in an international cohort of patients with nonalcoholic steatohepatitis. Clin. Gastroenterol. Hepatol. 2020, 18, 728–735.e724. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, N.; Kobayashi, M.; Fobar, A.J.; Hoshida, A.; Marquez, C.A.; Koneru, B.; Panda, G.; Taguri, M.; Qian, T.; Raman, I.; et al. A blood-based prognostic liver secretome signature and long-term hepatocellular carcinoma risk in advanced liver fibrosis. Med 2021, 2, 836–850.e10. [Google Scholar] [CrossRef]
- Schotten, C.; Bechmann, L.P.; Manka, P.; Theysohn, J.; Dechêne, A.; El Fouly, A.; Barbato, F.; Neumann, U.; Radünz, S.; Sydor, S.; et al. NAFLD-Associated Comorbidities in Advanced Stage HCC Do Not Alter the Safety and Efficacy of Yttrium-90 Radioembolization. Liver Cancer 2019, 8, 491–504. [Google Scholar] [CrossRef]
- Kogiso, T.; Tokushige, K. The Current View of Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma. Cancers 2021, 13, 516. [Google Scholar] [CrossRef]
- Viganò, L.; Kluger, M.D.; Laurent, A.; Tayar, C.; Merle, J.C.; Lauzet, J.Y.; Andreoletti, M.; Cherqui, D. Liver resection in obese patients: Results of a case-control study. HPB 2011, 13, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Pfister, D.; Núñez, N.G.; Pinyol, R.; Govaere, O.; Pinter, M.; Szydlowska, M.; Gupta, R.; Qiu, M.; Deczkowska, A.; Weiner, A.; et al. NASH limits anti-tumour surveillance in immunotherapy-treated HCC. Nature 2021, 592, 450–456. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef]
- Luu, H.N.; Behari, J.; Goh, G.B.; Wang, R.; Jin, A.; Thomas, C.E.; Clemente, J.C.; Odegaard, A.O.; Koh, W.P.; Yuan, J.M. Composite Score of Healthy Lifestyle Factors and Risk of Hepatocellular Carcinoma: Findings from a Prospective Cohort Study. Cancer Epidemiol. Biomark. Prev. 2021, 30, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Costante, F.; Airola, C.; Santopaolo, F.; Gasbarrini, A.; Pompili, M.; Ponziani, F.R. Immunotherapy for nonalcoholic fatty liver disease-related hepatocellular carcinoma: Lights and shadows. World J. Gastrointest. Oncol. 2022, 14, 1622–1636. [Google Scholar] [CrossRef] [PubMed]
- Lazo, M.; Solga, S.F.; Horska, A.; Bonekamp, S.; Diehl, A.M.; Brancati, F.L.; Wagenknecht, L.E.; Pi-Sunyer, F.X.; Kahn, S.E.; Clark, J.M. Fatty Liver Subgroup of the Look AHEAD Research Group. Effect of a 12-month intensive lifestyle intervention on hepatic steatosis in adults with type 2 diabetes. Diabetes Care 2010, 33, 2156–2163. [Google Scholar] [CrossRef] [Green Version]
- Vilar-Gomez, E.; Martinez-Perez, Y.; Calzadilla-Bertot, L.; Torres-Gonzalez, A.; Gra-Oramas, B.; Gonzalez-Fabian, L.; Friedman, S.L.; Diago, M.; Romero-Gomez, M. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015, 149, 367–378.e5. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, S.E.; Schlesinger, S.; Aleksandrova, K.; Jochem, C.; Jenab, M.; Gunter, M.J.; Overvad, K.; Tjønneland, A.; Boutron-Ruault, M.C.; Carbonnel, F.; et al. Association between physical activity and risk of hepatobiliary cancers: A multinational cohort study. J. Hepatol. 2019, 70, 885–892. [Google Scholar] [CrossRef] [PubMed]
- Yaskolka Meir, A.; Rinott, E.; Tsaban, G.; Zelicha, H.; Kaplan, A.; Rosen, P.; Shelef, I.; Youngster, I.; Shalev, A.; Blüher, M.; et al. effect of green-Mediterranean diet on intrahepatic fat: The DIRECT PLUS randomised controlled trial. Gut 2021, 70, 2085–2095. [Google Scholar] [CrossRef]
- George, E.S.; Sood, S.; Broughton, A.; Cogan, G.; Hickey, M.; Chan, W.S.; Sudan, S.; Nicoll, A.J. The Association between Diet and Hepatocellular Carcinoma: A Systematic Review. Nutrients 2021, 13, 172. [Google Scholar] [CrossRef]
- Godos, J.; Micek, A.; Marranzano, M.; Salomone, F.; Rio, D.D.; Ray, S. Coffee Consumption and Risk of Biliary Tract Cancers and Liver Cancer: A Dose-Response Meta-Analysis of Prospective Cohort Studies. Nutrients 2017, 9, 950. [Google Scholar] [CrossRef] [Green Version]
- Hayat, U.; Siddiqui, A.A.; Okut, H.; Afroz, S.; Tasleem, S.; Haris, A. The effect of coffee consumption on the nonalcoholic fatty liver disease and liver fibrosis: A meta-analysis of 11 epidemiological studies. Ann. Hepatol. 2021, 20, 100254. [Google Scholar] [CrossRef]
- Kennedy, O.J.; Roderick, P.; Buchanan, R.; Fallowfield, J.A.; Hayes, P.C.; Parkes, J. coffee, including caffeinated and decaffeinated coffee, and the risk of hepatocellular carcinoma: A systematic review and dose-response meta-analysis. BMJ Open 2017, 7, e013739. [Google Scholar] [CrossRef]
- Simon, T.G.; Ma, Y.; Ludvigsson, J.F.; Chong, D.Q.; Giovannucci, E.L.; Fuchs, C.S.; Meyerhardt, J.A.; Corey, K.E.; Chung, R.T.; Zhang, X.; et al. Association Between Aspirin Use and Risk of Hepatocellular Carcinoma. JAMA Oncol. 2018, 4, 1683–1690, Erratum in JAMA Oncol. 2019, 5, 579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simon, T.G.; Duberg, A.S.; Aleman, S.; Chung, R.T.; Chan, A.T.; Ludvigsson, J.F. Association of Aspirin with Hepatocellular Carcinoma and Liver-Related Mortality. N. Engl. J. Med. 2020, 382, 1018–1028. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, P.P.; Singh, A.G.; Murad, M.H.; Sanchez, W. Anti-diabetic medications and the risk of hepatocellular cancer: A systematic review and meta-analysis. Am. J. Gastroenterol. 2013, 108, 881–892. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Poly, T.N.; Walther, B.A.; Yang, H.C.; Jack Li, Y.C. Statin Use and the Risk of Hepatocellular Carcinoma: A Meta-Analysis of Observational Studies. Cancers 2020, 12, 671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Facciorusso, A.; Abd El Aziz, M.A.; Singh, S.; Pusceddu, S.; Milione, M.; Giacomelli, L.; Sacco, R. Statin Use Decreases the Incidence of Hepatocellular Carcinoma: An Updated Meta-Analysis. Cancers 2020, 12, 874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Single Nucleotide Polymorphism | Association in NAFLD | Mechanism | Ref. |
|---|---|---|---|
| Patatin-like phospholipase domain containing 3 (PNPLA3) The rs738409 C > G single nucleotide polymorphism | increased risk of NASH and fibrosis and is an independent risk factor for the development of HCC | Accumulation of TG in hepatocytes HSCs activation Mitochondrial dysfunction | [12,13] |
| rs58542926 C > T missense variant in the Transmembrane 6 superfamily member 2 (TM6SF2) E167K | steatosis, inflammation, ballooning and fibrosis but it conferred protection against cardiovascular events Fibrosis and HCC (controversial) | impaired VLDL secretion and fat accumulation in hepatocytes | [14,15] |
| TM6SF2 silencing in HepG2 (TM6SF2−/−) by (CRISPR/Cas9) | NASH, fibrosis and, HCC | Mitochondrial dysfunction | [16,17,18] |
| rs641738 C > T variant in the TMC4/MBOAT7 locus | MBOAT7 rs641738 confers risk of fibrotic progression in NAFLD and independently associated with the development of HCC even in the absence of cirrhosis | impaired hepatic MBOAT7 function | [19,20] |
| The PDCD-1 gene encodes an inhibitory cell surface receptor involved in the regulation of T cell functions during immune responses/tolerance PDCD-1 rs7421861 | NAFLD HCC | remodeling of the immune cell population | [21] |
| Dysregulated Non-Coding RNA | Association | Mechanism | References |
|---|---|---|---|
| Decreased levels of micro RNA122, 192 and 194 (in tissues) | Associated with
| Interfere with c-Myc pathway epithelial mesenchymal transition pathway | [25,26] |
| Micro RNA 21, 155, 375 and 16 in tissues and serum as cell free RNA | HCC development | [27] | |
| Highly upregulated in liver cancer (HULC) long non-coding RNA | NAFLD progression HCC proliferation and metastases | Mitogen activated protein kinase (MAPK) signaling epithelial mesenchymal transition pathway | [28,29,30,31,32] |
| Metastases associated lung adenocarcinoma transcript 1 (MALAT1) long non-coding RNA | NAFLD fibrosis HCC development | Silent information regulator 1(SIRT1) and TGFbeta 1 Wnt signalling activation | [30,33] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salaheldin, M.; Aly, H.; Lau, L.; Afify, S.; El-Kassas, M. Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: The Next Threat after Viral Hepatitis. Diagnostics 2023, 13, 2631. https://doi.org/10.3390/diagnostics13162631
Salaheldin M, Aly H, Lau L, Afify S, El-Kassas M. Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: The Next Threat after Viral Hepatitis. Diagnostics. 2023; 13(16):2631. https://doi.org/10.3390/diagnostics13162631
Chicago/Turabian StyleSalaheldin, Mohamed, Heba Aly, Louis Lau, Shimaa Afify, and Mohamed El-Kassas. 2023. "Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: The Next Threat after Viral Hepatitis" Diagnostics 13, no. 16: 2631. https://doi.org/10.3390/diagnostics13162631
APA StyleSalaheldin, M., Aly, H., Lau, L., Afify, S., & El-Kassas, M. (2023). Nonalcoholic Fatty Liver Disease-Related Hepatocellular Carcinoma: The Next Threat after Viral Hepatitis. Diagnostics, 13(16), 2631. https://doi.org/10.3390/diagnostics13162631







