Analysis of Bilaterality and Symmetry of Posterior Staphyloma in High Myopia
Abstract
:1. Introduction
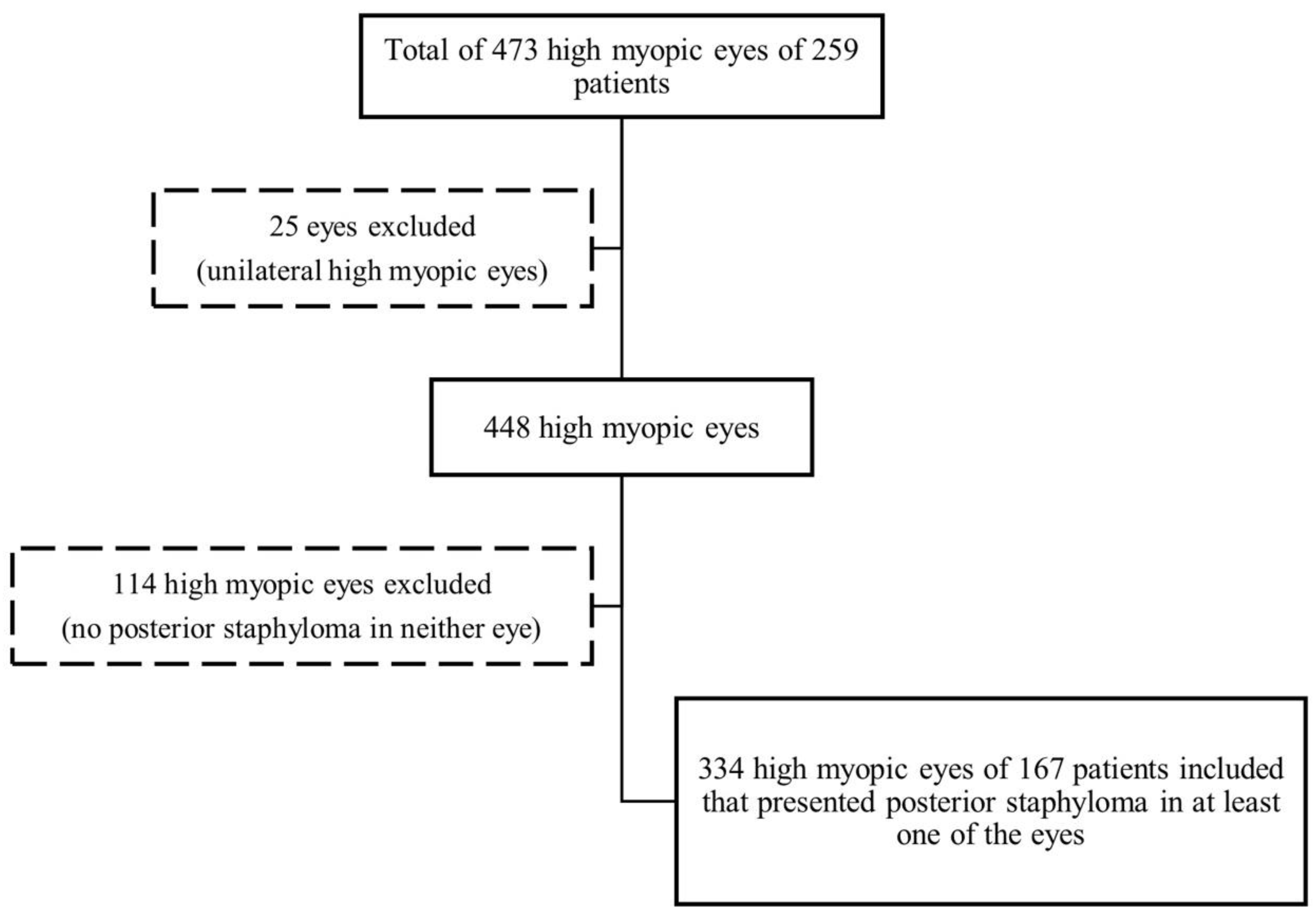
2. Materials & Methods
2.1. Multimodal Imaging
2.2. ATN Grading System
2.3. Pathologic Myopia and Severe Pathologic Myopia
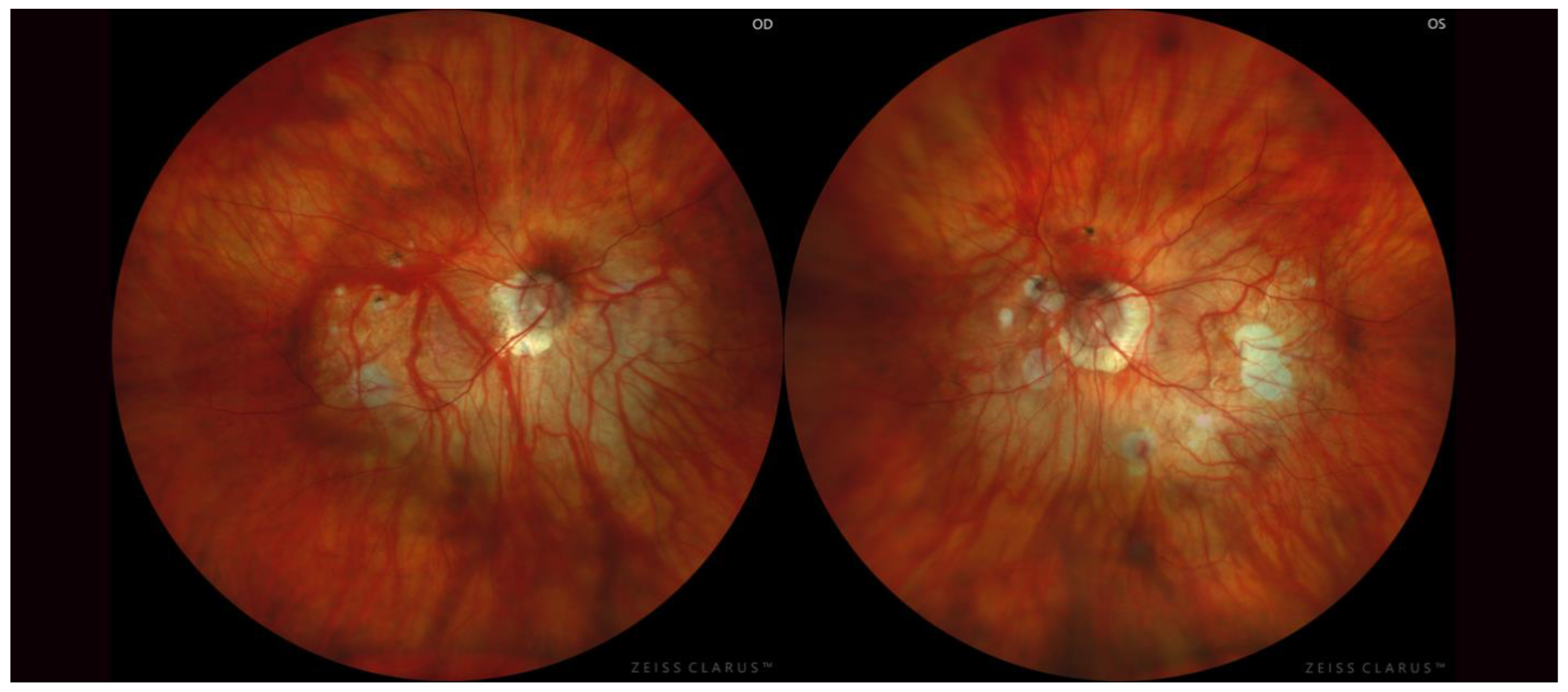
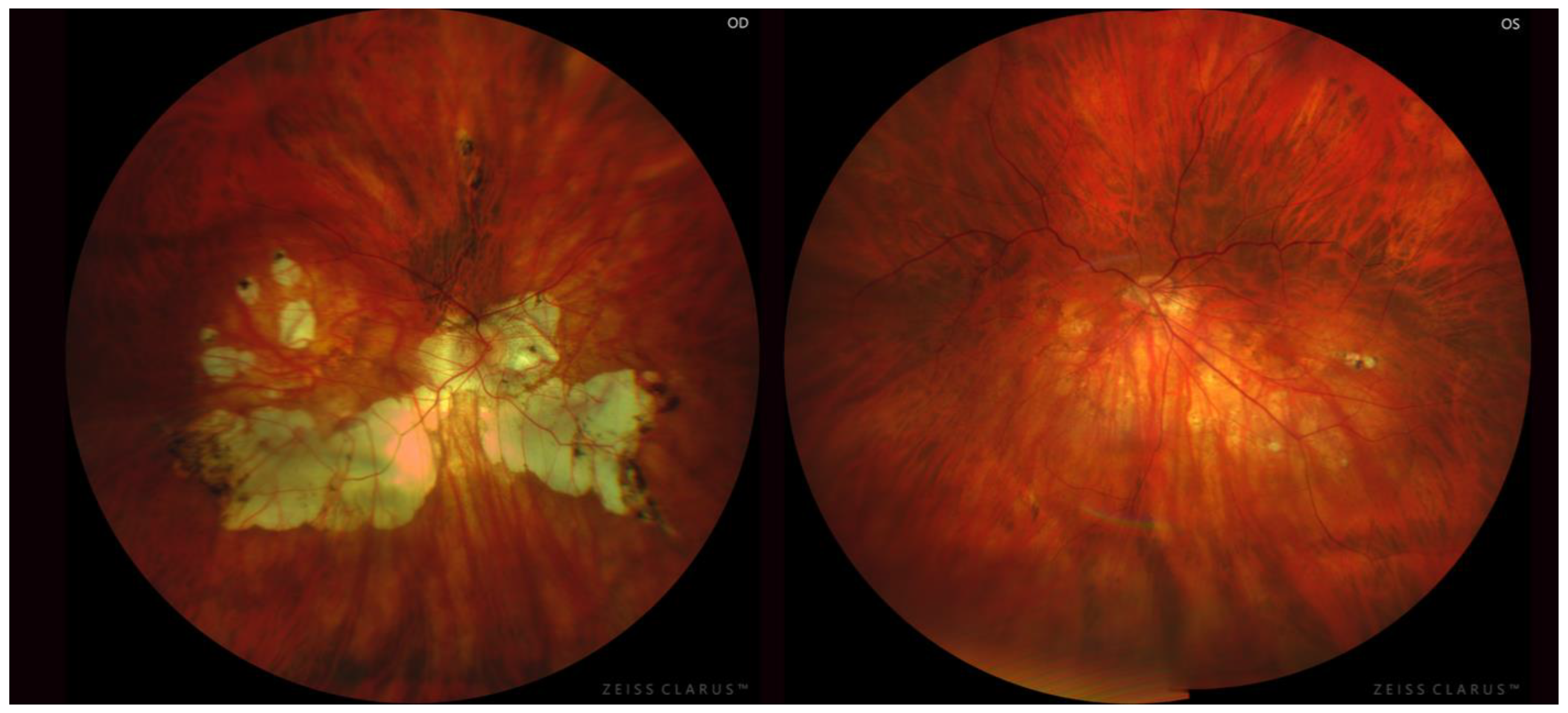
2.4. Posterior Staphyloma
- Curtin’s classification [4]: 10 types—five primary and five compound forms— based on their funduscopic appearance according to the area involved.
- The recent classification defined by Ohno-Matsui [6]: 6 types renamed according to their location and distribution. The first five types coincide with the same as Curtin’s classification and the sixth and last type named “others” where all the compound forms of staphylomas are grouped.
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spaide, R.F. Staphyloma: Part I. In Pathologic Myopia; Springer: New York, NY, USA, 2014; pp. 167–176. [Google Scholar]
- Ohno-Matsui, K.; Wu, P.C.; Yamashiro, K.; Vutipongsatorn, K.; Fang, Y.; Cheung, C.M.G.; Lai, T.Y.Y.; Ikuno, Y.; Cohen, S.Y.; Gaudric, A.; et al. IMI Pathologic Myopia. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef]
- Scarpa, A.; Briggs, J. Practical Observations on the Principal Diseases of the Eyes: Illustrated with Cases; Cadell, T., Davies, W., Eds.; Besleys Books: London, UK, 1806. [Google Scholar]
- Curtin, B.J. The Posterior Staphyloma of Pathologic Myopia. Trans. Am. Ophthalmol. Soc. 1977, 75, 67–86. [Google Scholar]
- Moriyama, M.; Ohno-Matsui, K.; Hayashi, K.; Shimada, N.; Yoshida, T.; Tokoro, T.; Morita, I. Topographic Analyses of Shape of Eyes with Pathologic Myopia by High-Resolution Three-Dimensional Magnetic Resonance Imaging. Ophthalmology 2011, 118, 1626–1637. [Google Scholar] [CrossRef]
- Ohno-Matsui, K. Proposed Classification of Posterior Staphylomas Based on Analyses of Eye Shape by Three-Dimensional Magnetic Resonance Imaging and Wide-Field Fundus Imaging. Ophthalmology 2014, 121, 1798–1809. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Kawasaki, R.; Jonas, J.B.; Cheung, C.M.G.; Saw, S.-M.; Verhoeven, V.J.M.; Klaver, C.C.W.; Moriyama, M.; Shinohara, K.; Kawasaki, Y.; et al. International Photographic Classification and Grading System for Myopic Maculopathy. Am. J. Ophthalmol. 2015, 159, 877–883.e7. [Google Scholar] [CrossRef]
- Fang, Y.; Yokoi, T.; Nagaoka, N.; Shinohara, K.; Onishi, Y.; Ishida, T.; Yoshida, T.; Xu, X.; Jonas, J.B.; Ohno-Matsui, K. Progression of Myopic Maculopathy during 18-Year Follow-Up. Ophthalmology 2018, 125, 863–877. [Google Scholar] [CrossRef]
- Hayashi, K.; Ohno-Matsui, K.; Shimada, N.; Moriyama, M.; Kojima, A.; Hayashi, W.; Yasuzumi, K.; Nagaoka, N.; Saka, N.; Yoshida, T.; et al. Long-Term Pattern of Progression of Myopic Maculopathy: A Natural History Study. Ophthalmology 2010, 117, 1595–1611.e4. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Jonas, J.B. Posterior Staphyloma in Pathologic Myopia. Prog. Retin. Eye Res. 2019, 70, 99–109. [Google Scholar] [CrossRef]
- Yan, Y.N.; Wang, Y.X.; Yang, Y.; Xu, L.; Xu, J.; Wang, Q.; Yang, J.Y.; Yang, X.; Zhou, W.J.; Ohno-Matsui, K.; et al. Ten-Year Progression of Myopic Maculopathy: The Beijing Eye Study 2001–2011. Ophthalmology 2018, 125, 1253–1263. [Google Scholar] [CrossRef] [Green Version]
- Maruko, I.; Iida, T.; Sugano, Y.; Oyamada, H.; Sekiryu, T. Morphologic Choroidal and Scleral Changes at the Macula in Tilted Disc Syndrome with Staphyloma Using Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2011, 52, 8763–8768. [Google Scholar] [CrossRef]
- Xu, X.; Fang, Y.; Yokoi, T.; Shinohara, K.; Hirakata, A.; Iwata, T.; Tsunoda, K.; Jonas, J.B.; Ohno-Matsui, K. Posterior Staphylomas in Eyes with Retinitis Pigmentosa Without High Myopia. Retina 2019, 39, 1299–1304. [Google Scholar] [CrossRef]
- Scott, A.; Kashani, S.; Towler, H.M.A. Progressive Myopia Due to Posterior Staphyloma in Type I Osteogenesis Imperfecta. Int. Ophthalmol. 2005, 26, 167–169. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Lai, T.Y.Y.; Lai, C.C.; Cheung, C.M.G. Updates of Pathologic Myopia. Prog. Retin. Eye Res. 2016, 52, 156–187. [Google Scholar] [CrossRef]
- Ruiz-Moreno, J.M.; Puertas, M.; Flores-Moreno, I.; Almazán-Alonso, E.; García-Zamora, M.; Ruiz-Medrano, J. Posterior Staphyloma as Determining Factor for Myopic Maculopathy. Am. J. Ophthalmol. 2023, 252, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Vongphanit, J.; Mitchell, P.; Wang, J.J. Prevalence and Progression of Myopic Retinopathy in an Older Population. Ophthalmology 2002, 109, 704–711. [Google Scholar] [CrossRef]
- Zheng, F.; Wong, C.W.; Sabanayagam, C.; Cheung, Y.B.; Matsumura, S.; Chua, J.; Man, R.E.K.; Ohno-Matsui, K.; Wong, T.Y.; Cheng, C.Y.; et al. Prevalence, Risk Factors and Impact of Posterior Staphyloma Diagnosed from Wide-Field Optical Coherence Tomography in Singapore Adults with High Myopia. Acta Ophthalmol. 2021, 99, e144–e153. [Google Scholar] [CrossRef]
- Gözüm, N.; Çakir, M.; Gücukoglu, A.; Sezen, F. Relationship between Retinal Lesions and Axial Length, Age and Sex in High Myopia. Eur. J. Ophthalmol. 1997, 7, 277–282. [Google Scholar] [CrossRef]
- Hsiang, H.W.; Ohno-Matsui, K.; Shimada, N.; Hayashi, K.; Moriyama, M.; Yoshida, T.; Tokoro, T.; Mochizuki, M. Clinical Characteristics of Posterior Staphyloma in Eyes with Pathologic Myopia. Am. J. Ophthalmol. 2008, 146, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medrano, J.; Montero, J.A.; Flores-Moreno, I.; Arias, L.; García-Layana, A.; Ruiz-Moreno, J.M. Myopic Maculopathy: Current Status and Proposal for a New Classification and Grading System (ATN). Prog. Retin. Eye Res. 2019, 69, 80–115. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Medrano, J.; Flores-Moreno, I.; Ohno-Matsui, K.; Cheung, C.M.G.; Silva, R.; Ruiz-Moreno, J.M. Validation of the Recently Developed ATN Classification and Grading System for Myopic Maculopathy. Retina 2020, 40, 2113–2118. [Google Scholar] [CrossRef]
- Ruiz-Medrano, J.; Flores-Moreno, I.; Ohno-Matsui, K.; Cheung, C.M.G.; Silva, R.; Ruiz-Moreno, J.M. Correlation between ATN Grade for Myopic Maculopathy and Clinical Severity. Retina 2021, 41, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Gisev, N.; Bell, J.S.; Chen, T.F. Interrater Agreement and Interrater Reliability: Key Concepts, Approaches, and Applications. Res. Soc. Adm. Pharm. 2013, 9, 330–338. [Google Scholar] [CrossRef]
- Landis, J.; Koch, G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Shimada, N.; Yasuzumi, K.; Hayashi, K.; Yoshida, T.; Kojima, A.; Moriyama, M.; Tokoro, T. Long-Term Development of Significant Visual Field Defects in Highly Myopic Eyes. Am. J. Ophthalmol. 2011, 152, 256–265.e1. [Google Scholar] [CrossRef]
- Curtin, B.J.; Karlin, D.B. Axial Length Measurements and Fundus Changes of the Myopic Eye. I. The Posterior Fundus. Trans. Am. Ophthalmol. Soc. 1970, 68, 312–334. [Google Scholar]
- Morgan, I.G.; French, A.N.; Ashby, R.S.; Guo, X.; Ding, X.; He, M.; Rose, K.A. The Epidemics of Myopia: Aetiology and Prevention. Prog. Retin. Eye Res. 2018, 62, 134–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guan, H.; Yu, N.N.; Wang, H.; Boswell, M.; Shi, Y.; Rozelle, S.; Congdon, N. Impact of Various Types of near Work and Time Spent Outdoors at Different Times of Day on Visual Acuity and Refractive Error among Chinese School-Going Children. PLoS ONE 2019, 14, e0215827. [Google Scholar] [CrossRef]
- Curtin, B.J. Ocular Findings and Complications. In The Myopias: Basic Science and Clinical Management; Harper and Row: New York, NY, USA, 1985; pp. 277–382. [Google Scholar]
- Ohno-Matsui, K.; Moriyama, M. Staphyloma II: Analyses of Morphological Features of Posterior Staphyloma in Pathologic Myopia Analyzed by a Combination of Wide-View Fundus Observation and 3D MRI Analyses. In Pathologic Myopia; Springer: New York, NY, USA, 2014; pp. 177–185. [Google Scholar] [CrossRef]
- Okisaka, S. Clinicopathology of Pathologic Myopia. In Myopia; Hosaka, A., Ed.; Kanehara Shuppan: Tokyo, Japan, 1987; pp. 110–121. [Google Scholar]
- Fujiwara, T.; Imamura, Y.; Margolis, R.; Slakter, J.S.; Spaide, R.F. Enhanced Depth Imaging Optical Coherence Tomography of the Choroid in Highly Myopic Eyes. Am. J. Ophthalmol. 2009, 148, 445–450. [Google Scholar] [CrossRef]
- Spaide, R.F. The Choroid. Pathol. Myopia 2014, 113–131. [Google Scholar] [CrossRef]
- Jonas, J.B.; Ohno-Matsui, K.; Jiang, W.J.; Panda-Jonas, S. Bruch membrane and the mechanism of myopization: A New Theory. Retina 2017, 37, 1428–1440. [Google Scholar] [CrossRef]
- Jonas, J.B.; Holbach, L.; Panda-Jonas, S. Bruch′s Membrane Thickness in High Myopia. Acta Ophthalmol. 2014, 92, 470–474. [Google Scholar] [CrossRef]
- Jonas, J.B.; Panda-Jonas, S. Secondary Bruch′s Membrane Defects and Scleral Staphyloma in Toxoplasmosis. Acta Ophthalmol. 2016, 94, e664–e666. [Google Scholar] [CrossRef] [PubMed]
- Jonas, J.B.; Ohno-Matsui, K.; Holbach, L.; Panda-Jonas, S. Histology of Myopic Posterior Scleral Staphylomas. Acta Ophthalmol. 2020, 98, e856–e863. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Jonas, J.B.; Yokoi, T.; Cao, K.; Shinohara, K.; Ohno-Matsui, K. Macular Bruch’s Membrane Defect and Dome-Shaped Macula in High Myopia. PLoS ONE 2017, 12, e0178998. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-Year Clinical and Epidemiologic Study of Keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Barr, J.T.; Zadnik, K.; Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study Group. Collaborative Longitudinal Evaluation of Keratoconus (CLEK) Study: Methods and Findings to Date. Cont. Lens Anterior Eye 2007, 30, 223–232. [Google Scholar] [CrossRef] [Green Version]
- Romero-Jiménez, M.; Santodomingo-Rubido, J.; Wolffsohn, J.S. Keratoconus: A Review. Contact Lens Anterior Eye 2010, 33, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Zadnik, K.; Steger-May, K.; Fink, B.A.; Joslin, C.E.; Nichols, J.J.; Rosenstiel, C.E.; Tyler, J.A.; Yu, J.A.; Raasch, T.W.; Schechtman, K.B. Between-Eye Asymmetry in Keratoconus. Cornea 2002, 21, 671–679. [Google Scholar] [CrossRef]
- Nichols, J.J.; Steger-May, K.; Edrington, T.B.; Zadnik, K. The Relation between Disease Asymmetry and Severity in Keratoconus. Br. J. Ophthalmol. 2004, 88, 788–791. [Google Scholar] [CrossRef] [Green Version]
- Burns, D.M.; Johnston, F.M.; Frazer, D.G.; Patterson, C. Keratoconus: An Analysis of Corneal Asymmetry. Br. J. Ophthalmol. 2004, 88, 1252–1255. [Google Scholar] [CrossRef]
- Verhoeven, V.J.M.; Hysi, P.G.; Wojciechowski, R.; Fan, Q.; Guggenheim, J.A.; Höhn, R.; Macgregor, S.; Hewitt, A.W.; Nag, A.; Cheng, C.Y.; et al. Genome-Wide Meta-Analyses of Multiancestry Cohorts Identify Multiple New Susceptibility Loci for Refractive Error and Myopia. Nat. Genet. 2013, 45, 314–318. [Google Scholar] [CrossRef]
- Tedja, M.S.; Wojciechowski, R.; Hysi, P.G.; Eriksson, N.; Furlotte, N.A.; Verhoeven, V.J.M.; Iglesias, A.I.; Meester-Smoor, M.A.; Tompson, S.W.; Fan, Q.; et al. Genome-Wide Association Meta-Analysis Highlights Light-Induced Signaling as a Driver for Refractive Error. Nat. Genet. 2018, 50, 834–848. [Google Scholar] [CrossRef] [PubMed]
- Hysi, P.G.; Choquet, H.; Khawaja, A.P.; Wojciechowski, R.; Tedja, M.S.; Yin, J.; Simcoe, M.J.; Patasova, K.; Mahroo, O.A.; Thai, K.K.; et al. Meta-Analysis of 542,934 Subjects of European Ancestry Identifies New Genes and Mechanisms Predisposing to Refractive Error and Myopia. Nat. Genet. 2020, 52, 401–407. [Google Scholar] [CrossRef]
- Hosoda, Y.; Yoshikawa, M.; Miyake, M.; Tabara, Y.; Shimada, N.; Zhao, W.; Oishi, A.; Nakanishi, H.; Hata, M.; Akagi, T.; et al. CCDC102B Confers Risk of Low Vision and Blindness in High Myopia. Nat. Commun. 2018, 9, 1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyake, M.; Yamashiro, K.; Akagi-Kurashige, Y.; Oishi, A.; Tsujikawa, A.; Hangai, M.; Yoshimura, N. Analysis of Fundus Shape in Highly Myopic Eyes by Using Curvature Maps Constructed from Optical Coherence Tomography. PLoS ONE 2014, 9, e107923. [Google Scholar] [CrossRef] [PubMed]
- Cases, O.; Joseph, A.; Obry, A.; Santin, M.D.; Ben-Yacoub, S.; Pâques, M.; Amsellem-Levera, S.; Bribian, A.; Simonutti, M.; Augustin, S.; et al. Foxg1-Cre Mediated Lrp2 Inactivation in the Developing Mouse Neural Retina, Ciliary and Retinal Pigment Epithelia Models Congenital High Myopia. PLoS ONE 2015, 10, e0129518. [Google Scholar] [CrossRef]
| Primaries (%, n) | Compounds (%, n) | ||
|---|---|---|---|
| 72.6% (225/310) | 27.4% (85/310) | ||
| Type I | 10.6% (33/310) | Type VI | 0.7% (2/310) |
| Type II | 16.8% (52/310) | Type VII | 10.3% (32/310) |
| Type III | 20% (62/310) | Type VIII | 2.9% (9/310) |
| Type IV | 8.1% (25/310) | Type IX | 8.7% (27/310) |
| Type V | 17.1% (53/310) | Type X | 4.8% (15/310) |
| Bilateral | Unilateral | p | |
|---|---|---|---|
| n (patients, eyes) | 85.6% (n = 143/167, n = 286/334) | 14.4% (n = 24/167, n = 24/334) | |
| Age (years-old) | 66.28 ± 11.49 | 63.50 ± 9.93 | p > 0.05 * |
| BCVA (decimal) | 0.51 ± 0.33 | 0.47 ± 0.35 | p > 0.05 * |
| AL (mm) | 30.27 ± 2.58 | 28.45 ± 2.04 | p < 0.01 * |
| AL-difference (Right eye AL—left eye AL) | 0.21 ± 1.35 | 1.25 ± 2.05 | p < 0.01 * |
| A component | 2.58 ± 0.74 | 2.04 ± 0.87 | p < 0.01 ** |
| T component | 0.88 ± 1.05 | 0.77 ± 1.04 | p > 0.05 ** |
| N component | 0.63 ± 0.89 | 0.25 ± 0.67 | p < 0.01 ** |
| Severe pathologic myopia | 60.84% (n = 174/286) | 37.5% (n = 9/24) | p < 0.05 *** |
| Symmetric | Asymmetric | |
|---|---|---|
| Curtin’s classification (%, patients) | 55.2% (n = 79/143) | 44.8% (n = 64/143) |
| Ohno-Matsui’s classification (%, patients) | 58.7% (n = 84/143) | 41.3% (n = 59/143) |
| Simple/compound subtypes (%, patients) | 80.4% (n = 115/143) | 19.6% (n = 28/143) |
| Symmetric Subtype | Asymmetric Subtype | p | ||
|---|---|---|---|---|
| N (patients/eyes) | Curtin’s classification | 55.2% (n = 79/143, n = 158/286) | 58.7% (n = 84/143, n = 168/286) | |
| Ohno-Matsui’s classification | 58.7% (n = 84/143, n = 168/286) | 55.2% (n = 79/143, n = 158/286) | ||
| Primary/Compound | 80.4% (n = 115/143, n = 230/286) | 19.6% (n = 28/143, n = 56/286) | ||
| Age (years) | Curtin’s classification | 67.06 ± 11.31 | 65.31 ± 11.67 | p > 0.05 * |
| Ohno-Matsui’s classification | 66.82 ± 11.30 | 65.51 ± 11.76 | ||
| Primary/Compound | 66.66 ± 11.30 | 64.71 ± 12.21 | ||
| BCVA (decimal) | Curtin’s classification | 0.53 ± 0.31 | 0.46 ± 0.34 | p < 0.01 * |
| Ohno-Matsui’s classification | 0.53 ± 0.31 | 0.46 ± 0.35 | ||
| Primary/Compound | 0.53 ± 0.31 | 0.46 ± 0.34 | ||
| AL (mm) | Curtin’s classification | 29.77 ± 2.66 | 30.88 ± 2.35 | p < 0.01 * |
| Ohno-Matsui’s classification | 29.97 ± 2.77 | 30.68 ± 2.22 | ||
| Primary/Compound | 30.00 ± 2.57 | 31.35 ± 2.34 | ||
| AL difference RE—LE (mm) | Curtin’s classification | 0.09 ± 1.21 | 0.36 ± 1.49 | p > 0.05 * |
| Ohno-Matsui’s classification | 0.16 ± 1.23 | 0.29 ± 1.49 | ||
| Primary/Compound | 0.15 ± 1.36 | 0.48 ± 1.29 | ||
| A component | Curtin’s classification | 2.45 ± 0.76 | 2.74 ± 0.60 | p < 0.01 ** |
| Ohno-Matsui’s classification | 2.47 ± 0.76 | 2.74 ± 0.69 | ||
| Primary/Compound | 2.53 ± 0.76 | 2.80 ± 0.62 | ||
| T component | Curtin’s classification | 0.87 ± 1.09 | 0.89 ± 0.99 | p > 0.05 ** |
| Ohno-Matsui’s classification | 0.89 ± 1.12 | 0.86 ± 0.94 | ||
| Primary/Compound | 0.87 ± 1.06 | 0.93 ± 1.02 | ||
| N component | Curtin’s classification | 0.61 ± 0.89 | 0.66 ± 0.91 | p > 0.05 ** |
| Ohno-Matsui’s classification | 0.61 ± 0.89 | 0.66 ± 0.91 | ||
| Primary/Compound | 0.61 ± 0.89 | 0.71 ± 0.91 | ||
| PM (%, eyes) | Curtin’s classification | 91.14% (n = 144/158) | 99.22% (n = 127/128) | p < 0.05 *** |
| Ohno-Matsui’s classification | 91.67% (n = 154/168) | 99.15% (n = 117/118) | ||
| Primary/Compound | 93.48% (n = 215/230) | 100% (n = 56/56) | ||
| Severe PM (%, eyes) | Curtin’s classification | 56.33.14% (n = 89/158) | 66.41% (n = 85/128) | p < 0.05 *** |
| Ohno-Matsui’s classification | 57.14% (n = 96/168) | 66.10% (n = 78/118) | ||
| Primary/Compound | 57.83% (n = 133/230) | 73.21% (n = 41/56) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruiz-Moreno, J.M.; Puertas, M.; Flores-Moreno, I.; Almazán-Alonso, E.; García-Zamora, M.; Ruiz-Medrano, J. Analysis of Bilaterality and Symmetry of Posterior Staphyloma in High Myopia. Diagnostics 2023, 13, 2680. https://doi.org/10.3390/diagnostics13162680
Ruiz-Moreno JM, Puertas M, Flores-Moreno I, Almazán-Alonso E, García-Zamora M, Ruiz-Medrano J. Analysis of Bilaterality and Symmetry of Posterior Staphyloma in High Myopia. Diagnostics. 2023; 13(16):2680. https://doi.org/10.3390/diagnostics13162680
Chicago/Turabian StyleRuiz-Moreno, José M., Mariluz Puertas, Ignacio Flores-Moreno, Elena Almazán-Alonso, María García-Zamora, and Jorge Ruiz-Medrano. 2023. "Analysis of Bilaterality and Symmetry of Posterior Staphyloma in High Myopia" Diagnostics 13, no. 16: 2680. https://doi.org/10.3390/diagnostics13162680
APA StyleRuiz-Moreno, J. M., Puertas, M., Flores-Moreno, I., Almazán-Alonso, E., García-Zamora, M., & Ruiz-Medrano, J. (2023). Analysis of Bilaterality and Symmetry of Posterior Staphyloma in High Myopia. Diagnostics, 13(16), 2680. https://doi.org/10.3390/diagnostics13162680







