Rare Solid Pancreatic Lesions on Cross-Sectional Imaging
Abstract
:1. Introduction
2. Benign Lesions (Table S1)
2.1. Intrapancreatic Splenic Tissue (Figure 1)

2.2. Pancreatic Tuberculosis (Figure 2)

2.3. Solid Serous Cystadenoma (Figure 3)

3. Potentially Malignant Lesions (Table S2)
3.1. Solid Pseudopapillary Tumour (Figure 4 and Figure 5)


3.2. Pancreatic Schwannoma (Figure 6)
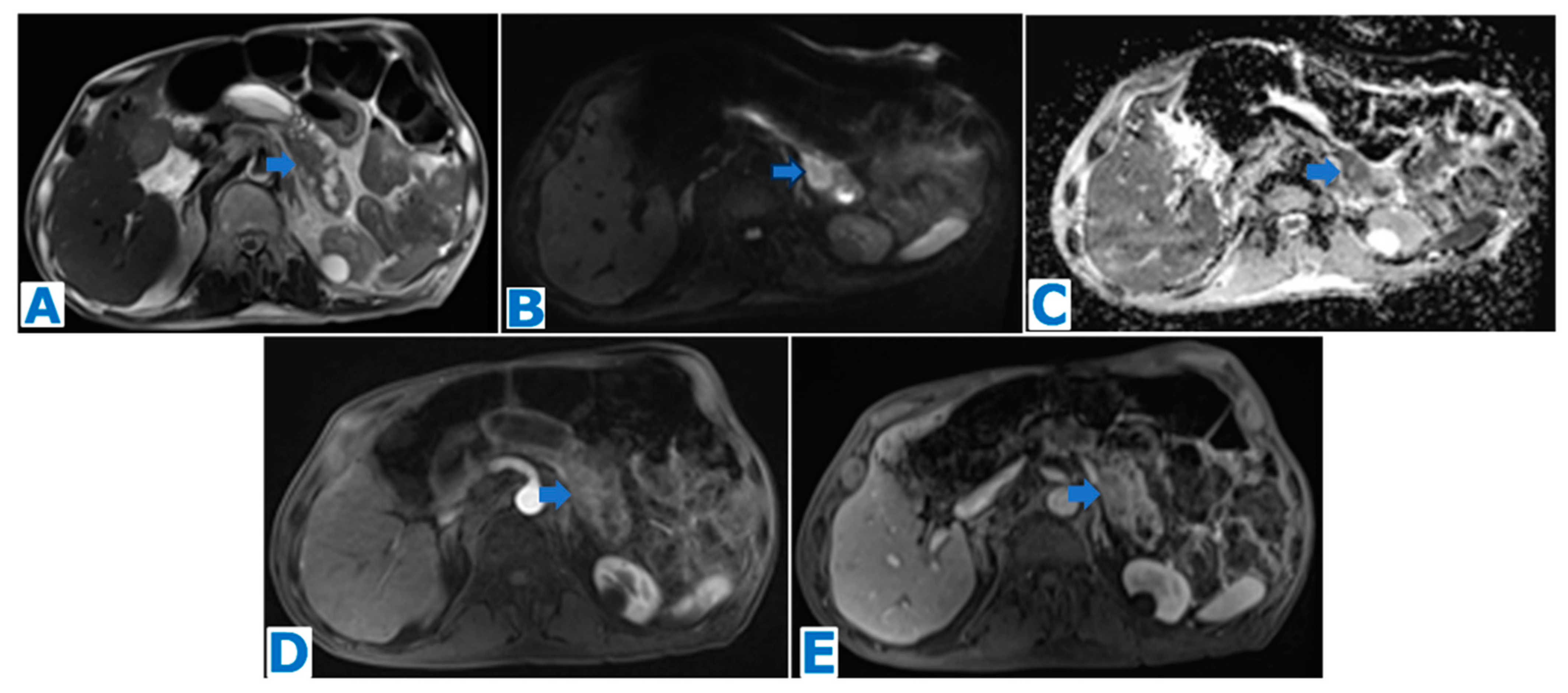
3.3. Purely Intraductal Pancreatic Neuroendocrine Tumour (Figure 7)

3.4. Pancreatic Solitary Fibrous Tumour (Figure 8)

4. Malignant Lesions (Table S3)
4.1. Acinar Cell Carcinoma (Figure 9 and Figure 10)


4.2. Undifferentiated Carcinoma with Osteoclastic-like Giant Cells (Figure 11)

4.3. Pancreatic Adenosquamous Carcinoma (Figure 12)

4.4. Colloid Carcinoma (Figure 13)

4.5. Primary Pancreatic Leiomyosarcoma (Figure 14 and Figure 15)


4.6. Primary and Secondary Pancreatic Lymphoma (Figure 16, Figure 17 and Figure 18)



4.7. Pancreatic Metastases (Figure 19, Figure 20, Figure 21 and Figure 22)




5. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Fletcher, J.G.; Wiersema, M.J.; Farrell, M.A.; Fidler, J.L.; Burgart, L.J.; Koyama, T.; Johnson, C.D.; Stephens, D.H.; Ward, E.M.; Harmsen, W.S. Pancreatic malignancy: Value of arterial, pancreatic, and hepatic phase imaging with multi-detector row CT. Radiology 2003, 229, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Dodds, W.J.; Taylor, A.J.; Erickson, S.J.; Stewart, E.T.; Lawson, T.L. Radiologic imaging of splenic anomalies. AJR Am. J. Roentgenol. 1990, 155, 805–810. [Google Scholar] [CrossRef]
- Lehtinen, S.J.; Schammel, C.M.; Devane, M.; Trocha, S.D. Intrapancreatic accessory spleen presenting as a pancreatic mass. J. Gastrointest. Oncol. 2013, 4, E23–E26. [Google Scholar]
- Lake, S.T.; Johnson, P.T.; Devane, M.; Trocha, S.D. CT of splenosis: Patterns and pitfalls. AJR Am. J. Roentgenol. 2012, 199, W686–W693. [Google Scholar] [CrossRef] [PubMed]
- White, J.D.; West, A.N.; Priebat, D.A. Splenosis mimicking an intra-abdominal malignancy. Am. J. Med. 1989, 87, 687–690. [Google Scholar] [CrossRef]
- Abu Hilal, M.; Harb, A.; Zeidan, B.; Steadman, B.; Primrose, J.N.; Pearce, N.W. Hepatic splenosis mimicking HCC in a patient with hepatitis C liver cirrhosis and mildly raised alpha feto protein; the important role of explorative laparoscopy. World J. Surg. Oncol. 2009, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Ren, Z.; Da Rold, A.; Guerriero, S.; Pariset, S.; Buffone, A.; Tedeschi, U. A rare diagnosis for a pancreatic mass: Splenosis. J. Gastrointest. Surg. 2004, 8, 915–916. [Google Scholar]
- Halpert, B.; Gyorkey, F. Lesions observed in accessory spleens of 311 patients. Am. J. Clin. Pathol. 1959, 32, 165–168. [Google Scholar] [CrossRef]
- Kim, S.H.; Lee, J.M.; Han, J.K.; Lee, J.Y.; Kim, K.W.; Cho, K.C.; Choi, B.I. Intrapancreatic accessory spleen: Findings on MR Imaging, CT, US and scintigraphy, and the pathologic analysis. Korean J. Radiol. 2008, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Varga, I.; Galfiova, P.; Adamkov, M.; Danisovic, L.; Polak, S.; Kubikova, E.; Galbavy, S. Congenital anomalies of the spleen from an embryological point of view. Med. Sci. Monit. 2009, 15, RA269–RA276. [Google Scholar] [PubMed]
- Kawamoto, S.; Johnson, P.T.; Hall, H.; Cameron, J.L.; Hruban, R.H.; Fishman, E.K. Intrapancreatic accessory spleen: CT appearance and differential diagnosis. Abdom. Imaging 2012, 37, 812–827. [Google Scholar] [CrossRef]
- Glazer, G.M.; Axel, L.; Goldberg, H.I.; Moss, A.A. Dynamic CT of the normal spleen. AJR Am. J. Roentgenol. 1981, 137, 343–346. [Google Scholar] [CrossRef]
- Ding, Q.; Ren, Z.; Wang, J.; Ma, X.; Zhang, J.; Sun, G.; Zuo, C.; Gu, H.; Jiang, H. Intrapancreatic accessory spleen: Evaluation with CT and MRI. Exp. Ther. Med. 2018, 16, 3623–3631. [Google Scholar] [CrossRef]
- Lin, W.C.; Lee, R.C.; Chiang, J.H.; Wei, C.J.; Chu, L.S.; Liu, R.S.; Chang, C.Y. MR features of abdominal splenosis. AJR Am. J. Roentgenol. 2003, 180, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Davidson, E.D.; Campbell, W.G.; Hersh, T. Epidermoid splenic cyst occurring in an intrapancreatic accessory spleen. Dig. Dis. Sci. 1980, 25, 964–967. [Google Scholar] [CrossRef]
- Hu, S.; Zhu, L.; Song, Q.; Chen, K. Epidermoid cyst in intrapancreatic accessory spleen: Computed tomography findings and clinical manifestation. Abdom. Imaging 2012, 37, 828–833. [Google Scholar] [CrossRef]
- Okura, N.; Mori, K.; Morishita, Y.; Oda, T.; Tanoi, T.; Minami, M. Inflammatory pseudotumor of the intrapancreatic accessory spleen: Computed tomography and magnetic resonance imaging findings. Jpn. J. Radiol. 2012, 30, 171–175. [Google Scholar] [CrossRef]
- Mariani, G.; Bruselli, L.; Kuwert, T.; Kim, E.E.; Flotats, A.; Israel, O.; Dondi, M.; Watanabe, N. A review on the clinical uses of SPECT/CT. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 1959–1985. [Google Scholar] [CrossRef]
- Barquilla-Cordero, P.; Chiquero-Palomo, M.; Martín-Noguerol, E.; Pacheco-Gómez, N.; Vinagre-Rodríguez, G.; Moyano-Calvente, S.L.; Molina-Infante, J. Tuberculosis pancreática primaria en un paciente inmunocompetente: Primer caso comunicado en España. Gastroenterol. Hepatol. 2010, 33, 582–585. [Google Scholar] [CrossRef]
- Panic, N.; Maetzel, H.; Bulajic, M.; Radovanovic, M.; Löhr, J.-M. Pancreatic tuberculosis: A systematic review of symptoms, diagnosis and treatment. United Eur. Gastroenterol. J. 2020, 8, 396–402. [Google Scholar] [CrossRef]
- De Backer, A.I.; Mortelé, K.J.; Bomans, P.; De Keulenaer, B.L.; Vanschoubroeck, I.J.; Kockx, M.M. Tuberculosis of the pancreas: MRI features. AJR Am. J. Roentgeno 2005, 184, 50–54. [Google Scholar] [CrossRef]
- Pandita, K.K.; Sarla, D.S. Isolated pancreatic tuberculosis. Indian. J. Med. Microbiol. 2009, 27, 259–260. [Google Scholar] [CrossRef] [PubMed]
- Baraboutis, I.; Skoutelis, A. Isolated tuberculosis of pancreas. J. Pancreas 2004, 5, 155–158. [Google Scholar]
- Xia, F.; Poon, R.T.; Wang, S.G.; Bie, P.; Huang, X.Q.; Dong, J.H. Tuberculosis of pancreas and peripancreatic lymph nodes in immunocompetent patients: Experience from China. World J. Gastroenterol. 2003, 9, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Nagar, A.M.; Raut, A.A.; Morani, A.C.; Sanghvi, D.A.; Desai, C.S.; Thapar, V.B. Pancreatic Tuberculosis: A Clinical and Imaging Review of 32 Cases. J. Comput. Assist. Tomogr. 2009, 33, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Knowles, K.F.; Saltman, D.; Robson, H.G.; Lalonde, R. Tuberculous pancreatitis. Tubercle 1990, 71, 65–68. [Google Scholar] [CrossRef]
- Radin, D.R. Intraabdominal Mycobacterium tuberculosis vs Mycobacterium avium-intracellulare infections in patients with AIDS: Distinction based on CT findings. AJR Am. J. Roentgenol. 1991, 156, 487–491. [Google Scholar] [CrossRef]
- Tetlezi, J.P.; Pisegna, J.R.; Barkin, J.S. Tuberculous pancreatic abscess as a manifestation of AIDS. Am. J. Gastroenterol. 1989, 84, 581–582. [Google Scholar]
- Levine, R.; Tenner, S.; Steinberg, W.; Ginsberg, A.; Borum, M.; Huntington, D. Tuberculous abscess of the pancreas. Case report and review of literature. Dig. Dis. Sci. 1992, 37, 141–144. [Google Scholar] [CrossRef]
- Puri, R.; Thandassery, R.B.; Eloubeidi, M.A.; Sud, R. Diagnosis of isolated pancreatic tuberculosis: The role of EUS-guided FNA cytology. Gastrointest. Endosc. 2012, 75, 900–904. [Google Scholar] [CrossRef]
- Pombo, F.; Díaz Candamio, M.J.; Rodriguez, E.; Pombo, S. Pancreatic tuberculosis. CT findings. Abdom. Imaging 1998, 23, 394–397. [Google Scholar] [CrossRef]
- Ladas, S.D.; Vaidakis, E.; Lariou, C.; Anastasiou, K.; Chalevelakis, G.; Kintzonidis, D.; Raptis, S.A. Pancreatic tuberculosis in non-immunocompromised patients: Reports of two cases and a literature review. Eur. J. Gastroenterol. Hepatol. 1998, 10, 973–976. [Google Scholar] [CrossRef]
- Dou, Y.; Liang, Z. Pancreatic tuberculosis: A computed tomography imaging review of thirteen cases. Radiol. Infect. Dis. 2019, 6, 31–37. [Google Scholar] [CrossRef]
- Fischer, G.; Spengler, U.; Nuebrand, M.; Sauerbruch, T. Isolated tuberculosis of the pancreas masquerading as a pancreatic mass. Am. J. Gastroenterol. 1995, 90, 2227–2230. [Google Scholar]
- Rana, S.S.; Sharma, V.; Sampath, S.; Sharma, R.; Mittal, B.R.; Bhasin, D.K. Vascular invasion does not discriminate between pancreatic tuberculosis and pancreatic malignancy: A case series. Ann. Gastroenterol. 2014, 27, 395–398. [Google Scholar]
- Sharma, V.; Rana, S.S.; Kumar, A.; Bhasin, D.K. Pancreatic tuberculosis. J. Gastroenterol. Hepatol. 2016, 31, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, G.F.; Al-Nakshabandi, N.A. Pancreatic tuberculosis: Role of multidetector computed tomography. Can. Assoc. Radiol. J. 2011, 62, 260–264. [Google Scholar] [CrossRef]
- D’Cruz, S.; Sachdev, A.; Kaur, L.; Handa, U.; Bhalla, A.; Lehl, S.S. Fine needle aspiration diagnosis of isolated pancreatic tuberculosis. A case report and review of literature. J. Pancreas 2003, 4, 158–162. [Google Scholar]
- Kimura, W.; Moriya, T.; Hirai, I.; Hanada, K.; Abe, H.; Yanagisawa, A.; Fukushima, N.; Ohike, N.; Shimizu, M.; Hatori, T.; et al. Multicenter study of serous cystic neoplasm of the Japan pancreas society. Pancreas 2012, 41, 380–387. [Google Scholar] [CrossRef]
- Demesmaker, V.; Abou-Messaoud, F.; Parent, M.; Vanhoute, B.; Maassarani, F.; Kothonidis, K. Pancreatic solid serous cystadenoma: A rare entity that can lead to a futile surgery. J. Surg. Case Rep. 2019, 12, 360. [Google Scholar] [CrossRef]
- Compagno, J.; Oertel, J.E. Microcystic adenoma of the pancreas (glycogen-rich cystadenoma): A clinicopathologic study of 34 cases. Am. J. Clin. Pathol. 1978, 69, 289–298. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.C.; Singhi, A.D.; Haroun, R.R.; Hruban, R.H.; Fishman, E.K. The many faces of pancreatic serous cystadenoma: Radiologic and pathologic correlation. Diagn. Interv. Imaging 2017, 98, 191–202. [Google Scholar] [CrossRef]
- Jais, B.; Rebours, V.; Malleo, G.; Salvia, R.; Fontana, M.; Maggino, L.; Bassi, C.; Manfredi, R.; Moran, R.; Lennon, A.M.; et al. Serous cystic neoplasm of the pancreas: A multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut 2016, 65, 305–312. [Google Scholar] [CrossRef]
- Okumura, Y.; Noda, T.; Eguchi, H.; Iwagami, Y.; Yamada, D.; Asaoka, T.; Kawamoto, K.; Gotoh, K.; Kobayashi, S.; Umeshita, K.; et al. Middle segment pancreatectomy for a solid serous cystadenoma diagnosed by MRCP and review of the literature: A case report. Mol. Clin. Oncol. 2018, 8, 675–682. [Google Scholar] [CrossRef]
- Kishida, Y.; Matsubayashi, H.; Okamura, Y.; Uesaka, K.; Sasaki, K.; Sawai, H.; Imai, K.; Ono, H. A case of solid-type serous cystadenoma mimicking neuroendocrine tumor of the pancreas. J. Dig. Dis. 2014, 15, 211–215. [Google Scholar] [CrossRef]
- Sun, H.Y.; Kim, S.H.; Kim, M.A.; Lee, J.Y.; Han, J.K.; Choi, B.I. CT imaging spectrum of pancreatic serous tumors: Based on new pathologic classification. Eur. J. Radiol. 2010, 75, 45–55. [Google Scholar] [CrossRef]
- Stern, J.R.; Frankel, W.L.; Ellison, E.C.; Bloomston, M. Solid serous microcystic adenoma of the pancreas. World J. Surg. Oncol. 2007, 5, 26. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas: European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [CrossRef] [PubMed]
- Ganeshan, D.M.; Paulson, E.; Tamm, E.P.; Taggart, M.W.; Balachandran, A.; Bhosale, P. Solid pseudo-papillary tumors of the pancreas: Current update. Abdom. Imaging 2013, 38, 1373–1382. [Google Scholar] [CrossRef] [PubMed]
- De Robertis, R.; Marchegiani, G.; Catania, M.; Ambrosetti, M.C.; Capelli, P.; Salvia, R.; D’Onofrio, M. Solid Pseudopapillary Neoplasms of the Pancreas: Clinicopathologic and Radiologic Features According to Size. AJR Am. J. Roentgenol. 2019, 213, 1073–1080. [Google Scholar] [CrossRef]
- Canzonieri, V.; Berretta, M.; Buonadonna, A.; Libra, M.; Vasquez, E.; Barbagallo, E.; Bearz, A.; Berretta, S. Solid pseudopapillary tumour of the pancreas. Lancet Oncol. 2003, 4, 255–256. [Google Scholar] [CrossRef] [PubMed]
- Cienfuegos, J.A.; Lozano, M.D.; Rotellar, F.; Martí, P.; Pedano, N.; Arredondo, J.; Bellver, M.; Sola, J.J.; Pardo, F. Solid pseudopapillary tumor of the pancreas (SPPT). Still an unsolved enigma. Rev. Esp. Enferm. Dig. 2010, 102, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Kosmahl, M.; Seada, L.S.; Jänig, U.; Harms, D.; Klöppel, G. Solid-pseudopapillary tumor of the pancreas: Its origin revisited. Virchows Arch. 2000, 436, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.C.; Machado, M.A.; Bacchella, T.; Jukemura, J.; Almeida, J.L.; Cunha, J.E. Solid pseudopapillary neoplasm of the pancreas: Distinct patterns of onset, diagnosis, and prognosis for male versus female patients. Surgery 2008, 143, 29–34. [Google Scholar] [CrossRef]
- Papavramidis, T.; Papavramidis, S. Solid pseudopapillary tumors of the pancreas: Review of 718 patients reported in English literature. J. Am. Coll. Surg. 2005, 200, 965–972. [Google Scholar] [CrossRef]
- Orlando, C.A.; Bowman, R.L.; Loose, J.H. Multicentric papillary-cystic neoplasm of the pancreas. Arch. Pathol. Lab. Med. 1991, 115, 958–960. [Google Scholar]
- Salvia, R.; Bassi, C.; Festa, L.; Falconi, M.; Crippa, S.; Butturini, G.; Brighenti, A.; Capelli, P.; Pederzoli, P. Clinical and biological behavior of pancreatic solidpseudopapillary tumors: Report on 31 consecutive patients. J. Surg. Oncol. 2007, 95, 304–310. [Google Scholar] [CrossRef]
- Adams, A.L.; Siegal, G.P.; Jhala, N.C. Solid pseudopapillary tumor of the pancreas: A review of salient clinical and pathologic features. Adv. Anat. Pathol. 2008, 15, 39–45. [Google Scholar] [CrossRef]
- Huang, S.C.; Wu, T.H.; Chen, C.C.; Chen, T.C. Spontaneous rupture of solid pseudopapillary neoplasm of the pancreas during pregnancy. Obs. Gynecol. 2013, 121, 486–488. [Google Scholar] [CrossRef]
- Mirapoğlu, S.L.; Aydogdu, I.; Gucin, Z.; Yilmaz, T.F.; Umutoglu, T.; Kilincaslan, H. Traumatic rupture of solid pseudopapillary tumors of the pancreas in children: A case report. Mol. Clin. Oncol. 2016, 5, 587–589. [Google Scholar] [CrossRef]
- Hibi, T.; Ojima, H.; Sakamoto, Y.; Kosuge, T.; Shimada, K.; Sano, T.; Sakamoto, M.; Kitajima, M.; Yamasaki, S. A solid pseudopapillary tumor arising from the greater omentum followed by multiple metastases with increasing malignant potential. J. Gastroenterol. 2006, 41, 276–281. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zong, L.; Wang, P.; Liu, Y.; Zhang, H.; Chang, X.; Lu, Z.; Li, W.; Ma, Y.; Yu, S.; et al. Solid Pseudopapillary Neoplasms of the Pancreas: Clinicopathologic Analysis and a Predictive Model. Mod. Pathol. 2023, 36, 100141. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Huang, Y.F.; Liu, X.H.; Xu, M.H. Extrapancreatic solid pseudopapillary neoplasm followed by multiple metastases: Case report. World J. Gastrointest. Oncol. 2017, 9, 497–501. [Google Scholar] [CrossRef]
- Miyazaki, Y.; Miyajima, A.; Maeda, T.; Yuge, K.; Hasegawa, M.; Kosaka, T.; Kikuchi, E.; Kameyama, K.; Jinzaki, M.; Nakagawa, K.; et al. Extrapancreatic solid pseudopapillary tumor: Case report and review of the literature. Int. J. Clin. Oncol. 2012, 17, 165–168. [Google Scholar] [CrossRef]
- Deshpande, V.; Oliva, E.; Young, R.H. Solid pseudopapillary neoplasm of the ovary: A report of 3 primary ovarian tumors resembling those of the pancreas. Am. J. Surg. Pathol. 2010, 34, 1514–1520. [Google Scholar] [CrossRef] [PubMed]
- Walter, T.; Hommell-Fontaine, J.; Hervieu, V.; Adham, M.; Poncet, G.; Dumortier, J.; Lombard-Bohas, C.; Scoazec, J.Y. Primary malignant solid pseudopapillary tumors of the gastroduodenal area. Clin. Res. Hepatol. Gastroenterol. 2011, 35, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Buetow, P.C.; Buck, J.L.; Pantongrag-Brown, L.; Beck, K.G.; Ros, P.R.; Adair, C.F. Solid and papillary epithelial neoplasm of the pancreas: Imaging-pathologic correlation on 56 cases. Radiology 1996, 199, 707–711. [Google Scholar] [CrossRef]
- Alexandrescu, D.T.; O’Boyle, K.; Feliz, A.; Fueg, A.; Wiernik, P.H. Metastatic solid-pseudopapillary tumour of the pancreas: Clinico-biological correlates and management. Clin. Oncol. 2005, 17, 358–363. [Google Scholar] [CrossRef]
- Lee, J.H.; Yu, J.S.; Kim, H.; Kim, J.K.; Kim, T.H.; Kim, K.W.; Park, M.S.; Kim, J.H.; Kim, Y.B.; Park, C. Solid pseudopapillary carcinoma of the pancreas: Differentiation from benign solid pseudopapillary tumour using CT and MRI. Clin. Radiol. 2008, 63, 1006–1014. [Google Scholar] [CrossRef]
- Hassan, I.; Celik, I.; Nies, C.; Zielke, A.; Gerdes, B.; Moll, R.; Ramaswamy, A.; Wagner, H.J.; Bartsch, D.K. Successful treatment of solid-pseudopapillary tumor of the pancreas with multiple liver metastases. Pancreatology 2005, 5, 289–294. [Google Scholar] [CrossRef]
- Zhan, H.; Cheng, Y.; Wang, L.; Su, P.; Zhong, N.; Zhang, Z. Clinicopathological Features and Treatment Outcomes of Solid Pseudopapillary Neoplasms of the Pancreas: A 10-Year Case Series from a Single Center. J. Laparoendosc. Adv. Surg. Tech. 2019, 29, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Beltrame, V.; Pozza, G.; Dalla Bona, E.; Fantin, A.; Valmasoni, M.; Sperti, C. Solid-Pseudopapillary Tumor of the Pancreas: A Single Center Experience. Gastroenterol. Res. Pract. 2016, 2016, 4289736. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.J.; Javed, A.A.; Saunders, T.; Zhu, Y.; Burkhart, R.A.; Yu, J. Surgical Resection of 78 Pancreatic Solid Pseudopapillary Tumors: A 30-Year Single Institutional Experience. J. Gastrointest. Surg. 2020, 24, 874–881. [Google Scholar] [CrossRef]
- Ventriglia, A.; Manfredi, R.; Mehrabi, S.; Boninsegna, E.; Negrelli, R.; Pedrinolla, B.; Pozzi Mucelli, R. MRI features of solid pseudopapillary neoplasm of the pancreas. Abdom. Imaging 2014, 39, 1213–1220. [Google Scholar] [CrossRef]
- Chae, S.H.; Lee, J.M.; Baek, J.H.; Shin, C.I.; Yoo, M.H.; Yoon, J.H.; Kim, J.H.; Han, J.K.; Choi, B.I. Magnetic resonance imaging spectrum of solid pseudopapillary neoplasm of the pancreas. J. Comput. Assist. Tomogr. 2014, 38, 249–257. [Google Scholar] [CrossRef]
- Guerrache, Y.; Soyer, P.; Dohan, A.; Faraoun, S.A.; Laurent, V.; Tasu, J.P.; Aubé, C.; Cazejust, J.; Boudiaf, M.; Hoeffel, C. Solid-pseudopapillary tumor of the pancreas: MR imaging findings in 21 patients. Clin. Imaging 2014, 38, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K.M.; Doherty, M.C.; Bigler, S.A. Solid-pseudopapillary tumor of the pancreas. Radiographics 2003, 23, 1644–1648. [Google Scholar] [CrossRef]
- Baek, J.H.; Lee, J.M.; Kim, S.H.; Kim, S.J.; Kim, S.H.; Lee, J.Y.; Han, J.K.; Choi, B.I. Small (≤3 cm) Solid Pseudopapillary Tumors of the Pancreas at Multiphasic Multidetector CT 1. Radiology 2010, 257, 97–106. [Google Scholar] [CrossRef]
- Liu, M.; Liu, J.; Hu, Q.; Xu, W.; Liu, W.; Zhang, Z.; Sun, Q.; Qin, Y.; Yu, X.; Ji, S.; et al. Management of solid pseudopapillary neoplasms of pancreas: A single center experience of 243 consecutive patients. Pancreatology 2019, 19, 681–685. [Google Scholar] [CrossRef]
- Morito, A.; Eto, K.; Matsuishi, K.; Hamasaki, H.; Morita, K.; Ikeshima, S.; Horino, K.; Shimada, S.; Baba, H. A case of repeat hepatectomy for liver mastasis from solid pseudopapillary neoplasm of the pancreas: A case report. Surg. Case Rep. 2021, 7, 60. [Google Scholar] [CrossRef]
- Law, J.K.; Ahmed, A.; Singh, V.K.; Akshintala, V.S.; Olson, M.T.; Raman, S.P.; Ali, S.Z.; Fishman, E.K.; Kamel, I.; Canto, M.I.; et al. A systematic review of solid-pseudopapillary neoplasms: Are these rare lesions? Pancreas 2014, 43, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Jin, C.; Long, J.; Yu, X.J.; Xu, J.; Di, Y.; Li, J.; de Fu, L.; Ni, Q.X. Solid pseudopapillary tumor of the pancreas: A case series of 26 consecutive patients. Am. J. Surg. 2009, 198, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Devi, J.; Sathyalakshmi, R.; Chandramouleeswari, K.; Devi, N.R. Pancreatic schwannoma—A rare case report. J. Clin. Diagn. Res. 2014, 8, FD15–FD16. [Google Scholar]
- Antinheimo, J.; Sankila, R.; Carpén, O.; Pukkala, E.; Sainio, M.; Jääskeläinen, J. Population-based analysis of sporadic and type 2 neurofibromatosis-associated meningiomas and schwannomas. Neurology 2000, 54, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Siegelman, E.S.; Lee, M.K., 4th; Tondon, R. Pancreatic schwannoma, an extremely rare and challenging entity: Report of two cases and review of literature. Pancreatology 2019, 19, 729–737. [Google Scholar] [CrossRef]
- Varshney, V.K.; Yadav, T.; Elhence, P.; Sureka, B. Preoperative diagnosis of pancreatic schwannoma—Myth or reality. J. Cancer Res. Ther. 2020, 16, S222–S226. [Google Scholar] [CrossRef]
- Kinhal, V.A.; Ravishankar, T.H.; Melapure, A.I.; Jayaprakasha, G.; Range Gowda, B.C.; Manjunath. Pancreatic schwannoma: Report of a case and review of literature. Indian J. Surg. 2010, 72, 296–298. [Google Scholar] [CrossRef]
- Ma, Y.; Shen, B.; Jia, Y.; Luo, Y.; Tian, Y.; Dong, Z.; Chen, W.; Li, Z.P.; Feng, S.T. Pancreatic schwannoma: A case report and an updated 40-year review of the literature yielding 68 cases. BMC Cancer 2017, 14, 853. [Google Scholar]
- Wang, H.; Zhang, B.B.; Wang, S.F.; Zhong, J.J.; Zheng, J.M.; Han, H. Pancreatic schwannoma: Imaging features and pathological findings. Hepatobiliary Pancreat. Dis. Int. HBPD INT 2020, 19, 200–202. [Google Scholar] [CrossRef]
- Tofigh, A.M.; Hashemi, M.; Honar, B.N.; Solhjoo, F. Rare presentation of pancreatic schwannoma: A case report. J. Med. Case Rep. 2008, 12, 268. [Google Scholar] [CrossRef]
- Abu-Zaid, A.; Azzam, A.; Abou Al-Shaar, H.; Alshammari, A.M.; Amin, T.; Mohammed, S. Pancreatic tail schwannoma in a 44-year-old male: A case report and literature review. Case Rep. Oncol. Med. 2013, 2013, 416713. [Google Scholar] [CrossRef] [PubMed]
- Moriya, T.; Kimura, W.; Hirai, I.; Takeshita, A.; Tezuka, K.; Watanabe, T.; Mizutani, M.; Fuse, A. Pancreatic schwannoma: Case report and an updated 30-year review of the literature yielding 47 cases. World J. Gastroenterol. 2012, 18, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Novellas, S.; Chevallier, P.; Saint Paul, M.C.; Gugenheim, J.; Bruneton, J.N. MRI features of a pancreatic schwannoma. Clin. Imaging 2005, 29, 434–436. [Google Scholar] [CrossRef]
- Yu, R.S.; Sun, J.Z. Pancreatic schwannoma: CT findings. Abdom. Imaging 2006, 31, 103–105. [Google Scholar] [CrossRef] [PubMed]
- Hamada, K.; Ueda, T.; Higuchi, I.; Inoue, A.; Tamai, N.; Myoi, A.; Tomita, Y.; Aozasa, K.; Yoshikawa, H.; Hatazawa, J. Peripheral nerve schwannoma: Two cases exhibiting increased FDG uptake in early and delayed PET imaging. Skelet. Radiol. 2005, 34, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Birk, H.; Zygourakis, C.C.; Kliot, M. Developing an algorithm for cost-effective, clinically judicious management of peripheral nerve tumors. Surg. Neurol. Int. 2016, 7, 80. [Google Scholar]
- Wang, S.; Xing, C.; Wu, H.; Dai, M.; Zhao, Y. Pancreatic schwannoma mimicking pancreatic cystadenoma: A case report and literature review of the imaging features. Medicine 2019, 98, e16095. [Google Scholar] [CrossRef]
- Shimizu, K.; Shiratori, K.; Toki, F.; Suzuki, M.; Imaizumi, T.; Takasaki, K.; Kobayashi, M.; Hayashi, N. Nonfunctioning islet cell tumor with a unique pattern of tumor growth. Dig. Dis. Sci. 1999, 44, 547–551. [Google Scholar] [CrossRef]
- Akatsu, T.; Wakabayashi, G.; Aiura, K.; Suganuma, K.; Takigawa, Y.; Wada, M.; Kawachi, S.; Tanabe, M.; Ueda, M.; Shimazu, M.; et al. Intraductal growth of a nonfunctioning endocrine tumor of the pancreas. J. Gastroenterol. 2004, 39, 584–588. [Google Scholar] [CrossRef]
- Fassan, M.; Pizzi, S.; Pasquali, C.; Parenti, A.R. Pancreatic endocrine tumor in multiple endocrine neoplasia type 1syndrome with intraductal growth into the main pancreatic duct. Pancreas 2009, 38, 341–342. [Google Scholar] [CrossRef]
- Inagaki, M.; Watanabe, K.; Yoshikawa, D.; Suzuki, S.; Ishizaki, A.; Matsumoto, K.; Haneda, M.; Tokusashi, Y.; Miyokawa, N.; Sato, S.; et al. A malignant nonfunctioning pancreatic endocrine tumor with unique pattern of intraductal growth. J. Hepatobiliary Pancreat. Surg. 2007, 14, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, H.; Kuwatani, M.; Hirano, S.; Kondo, S.; Nakanishi, Y.; Itoh, T.; Asaka, M. Pancreatic endocrine tumors with intraductal growth into the main pancreatic duct and tumor thrombus within the portal vein: A case report and review of the literature. Int. Med. 2007, 46, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Kitami, C.E.; Shimizu, T.; Sato, O.; Kurosaki, I.; Mori, S.; Yanagisawa, Y.; Ajioka, Y.; Hatakeyama, K. Malignant islet cell tumor projecting into the main pancreatic duct. J. Hepatobiliary Pancreat. Surg. 2000, 7, 529–533. [Google Scholar] [CrossRef]
- Terada, T.; Kawaguchi, M.; Furukawa, K.; Sekido, Y.; Osamura, Y. Minute mixed ductal-endocrine carcinoma of the pancreas with predominant intraductal growth. Pathol. Int. 2002, 52, 740–746. [Google Scholar] [CrossRef]
- D’Onofrio, M.; Capelli, P.; Pederzoli, P. Imaging and Pathology of Pancreatic Neoplasms. A Pictorial Atlas, 1st ed.; Springer Science + Business Media: Milan, Italy, 2015. [Google Scholar]
- Yazawa, N.; Imaizumi, T.; Okada, K.; Matsuyama, M.; Dowaki, S.; Tobita, K.; Ohtani, Y.; Ogoshi, K.; Hirabayashi, K.; Makuuchi, H. Nonfunctioning pancreatic endocrine tumor with extension into the main pancreatic duct: Report of a case. Surg. Today 2011, 41, 737–740. [Google Scholar] [CrossRef] [PubMed]
- Chetty, R.; El-Shinnawy, I. Intraductal pancreatic neuroendocrine tumor. Endocr. Pathol. 2009, 20, 262–266. [Google Scholar] [CrossRef]
- Ciaravino, V.; De Robertis, R.; Tinazzi Martini, P.; Cardobi, N.; Cingarlini, S.; Amodio, A.; Landoni, L.; Capelli, P.; D’Onofrio, M. Imaging presentation of pancreatic neuroendocrine neoplasms. Insights Imaging 2018, 9, 943–953. [Google Scholar] [CrossRef]
- Klemperer, P.; Coleman, B.R. Primary neoplasms of the pleura. A report of five cases. Am. J. Ind. Med. 1992, 22, 4–31. [Google Scholar] [CrossRef]
- Manning, M.A.; Paal, E.E.; Srivastava, A.; Mortele, K.J. Nonepithelial Neoplasms of the Pancreas, Part 2: Malignant Tumors and Tumors of Uncertain Malignant Potential From the Radiologic Pathology Archives. Radiographics 2018, 38, 1047–1072. [Google Scholar] [CrossRef]
- Taguchi, Y.; Hara, T.; Tamura, H.; Ogiku, M.; Watahiki, M.; Takagi, T.; Harada, T.; Miyazaki, S.; Hayashi, T.; Kanai, T.; et al. Malignant solitary fibrous tumor of the pancreas: A case report. Surg. Case Rep. 2020, 136, 287. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Xiong, Y.; Xu, T.; Xu, J.; Li, Q.; Yang, G. Atypical/malignant solitary fibrous tumor of the pancreas with spleen vein invasion: Case report and literature review. Medicine 2020, 99, e19783. [Google Scholar] [CrossRef] [PubMed]
- Fung, E.C.; Crook, M.A. Doege-Potter syndrome and ‘big-IGF2’: A rare cause of hypoglycaemia. Ann. Clin. Biochem. 2011, 48 Pt 2, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Shanbhogue, A.K.; Prasad, S.R.; Takahashi, N.; Vikram, R.; Zaheer, A.; Sandrasegaran, K. Somatic and visceral solitary fibrous tumors in the abdomen and pelvis: Cross-sectional imaging spectrum. Radiographics 2011, 31, 393–408. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.C.; Huang, H.Y. Solitary fibrous tumor: An evolving and unifying entity with unsettled issues. Histol. Histopathol. 2019, 34, 313–334. [Google Scholar]
- Ginat, D.T.; Bokhari, A.; Bhatt, S.; Dogra, V. Imaging features of solitary fibrous tumors. AJR Am. J. Roentgenol. 2011, 196, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Martin-Broto, J.; Mondaza-Hernandez, J.L.; Moura, D.S.; Hindi, N. A Comprehensive Review on Solitary Fibrous Tumor: New Insights for New Horizons. Cancers 2021, 13, 2913. [Google Scholar] [CrossRef]
- Srinivasan, V.D.; Wayne, J.D.; Rao, M.S.; Zynger, D.L. Solitary fibrous tumor of the pancreas: Case report with cytologic and surgical pathology correlation and review of the literature. J. Pancreas 2008, 9, 526–530. [Google Scholar]
- Davanzo, B.; Emerson, R.E.; Lisy, M.; Koniaris, L.G.; Kays, J.K. Solitary fibrous tumor. Transl. Gastroenterol. Hepatol. 2018, 3, 94. [Google Scholar] [CrossRef]
- Kayani, B.; Sharma, A.; Sewell, M.D.; Platinum, J.; Olivier, A.; Briggs, T.W.R.; Eastwood, D.M. A Review of the Surgical Management of Extrathoracic Solitary Fibrous Tumors. Am. J. Clin. Oncol. 2018, 41, 687–694. [Google Scholar] [CrossRef]
- Robinson, L.A. Solitary fibrous tumor of the pleura. Cancer Control 2006, 13, 264–269. [Google Scholar] [CrossRef]
- Ordóñez, N.G. Pancreatic acinar cell carcinoma. Adv. Anat. Pathol. 2001, 8, 144–159. [Google Scholar] [PubMed]
- Toll, A.D.; Hruban, R.H.; Ali, S.Z. Acinar cell carcinoma of the pancreas: Clinical and cytomorphologic characteristics. Korean J. Pathol. 2013, 47, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, P. Acinar Cell Carcinoma of the Pancreas: A Literature Review and Update. Indian J. Surg. 2015, 77, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Lack, E.E.; Cassady, J.R.; Levey, R.; Vawter, G.F. Tumors of the exocrine pancreas in children and adolescents. A clinical and pathologic study of eight cases. Am. J. Surg. Pathol. 1983, 7, 319–327. [Google Scholar] [CrossRef]
- Bhosale, P.; Balachandran, A.; Wang, H.; Wei, W.; Hwang, R.F.; Fleming, J.B.; Varadhachary, G.; Charnsangavej, C.; Tamm, E. CT imaging features of acinar cell carcinoma and its hepatic metastases. Abdom. Imaging 2013, 38, 1383–1390. [Google Scholar] [CrossRef]
- Klimstra, D.S.; Heffess, C.S.; Oertel, J.E.; Rosai, J. Acinar cell carcinoma of the pancreas. A clinicopathologic study of 28 cases. Am. J. Surg. Pathol. 1992, 16, 815–837. [Google Scholar] [CrossRef]
- Hsu, M.Y.; Pan, K.T.; Chu, S.Y.; Hung, C.F.; Wu, R.C.; Tseng, J.H. CT and MRI features of acinar cell carcinoma of the pancreas with pathological correlations. Clin. Radiol. 2010, 65, 223–229. [Google Scholar] [CrossRef]
- Fabre, A.; Sauvanet, A.; Flejou, J.F.; Belghiti, J.; Palazzo, L.; Ruszniewski, P.; Degott, C.; Terris, B. Intraductal acinar cell carcinoma of the pancreas. Virchows Arch. 2001, 438, 312–315. [Google Scholar] [CrossRef]
- Calimano-Ramirez, L.F.; Daoud, T.; Gopireddy, D.R.; Morani, A.C.; Waters, R.; Gumus, K.; Klekers, A.R.; Bhosale, P.R.; Virarkar, M.K. Pancreatic acinar cell carcinoma: A comprehensive review. World J. Gastroenterol. 2022, 28, 5827–5844. [Google Scholar] [CrossRef]
- Borowicz, J.; Morrison, M.; Hogan, D.; Miller, R. Subcutaneous fat necrosis/panniculitis and polyarthritis associated with acinar cell carcinoma of the pancreas: A rare presentation of pancreatitis, panniculitis and polyarthritis syndrome. J. Drugs Dermatol. 2010, 9, 1145–1150. [Google Scholar]
- Radin, D.R.; Colletti, P.M.; Forrester, D.M.; Tang, W.W. Pancreatic acinar cell carcinoma with subcutaneous and intraosseous fat necrosis. Radiology 1986, 158, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Burns, W.A.; Matthews, M.J.; Hamosh, M.; Weide, G.V.; Blum, R.; Johnson, F.B. Lipase-secreting acinar cell carcinoma of the pancreas with polyarthropathy. A light and electron microscopic, histochemical, and biochemical study. Cancer 1974, 33, 1002–1009. [Google Scholar] [CrossRef] [PubMed]
- Cingolani, N.; Shaco-Levy, R.; Farruggio, A.; Klimstra, D.S.; Rosai, J. Alpha-fetoprotein production by pancreatic tumors exhibiting acinar cell differentiation: Study of five cases, one arising in a mediastinal teratoma. Hum. Pathol. 2000, 31, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Raman, S.P.; Hruban, R.H.; Cameron, J.L.; Wolfgang, C.L.; Fishman, E.K. Pancreatic imaging mimics: Part 2, pancreatic neuroendocrine tumors and their mimics. AJR Am. J. Roentgenol. 2012, 199, 309–318. [Google Scholar] [CrossRef]
- Raman, S.P.; Hruban, R.H.; Cameron, J.L.; Wolfgang, C.L.; Kawamoto, S.; Fishman, E.K. Acinar cell carcinoma of the pancreas: Computed tomography features—A study of 15 patients. Abdom. Imaging 2013, 38, 137–143. [Google Scholar] [CrossRef]
- Chiou, Y.Y.; Chiang, J.H.; Hwang, J.I.; Yen, C.H.; Tsay, S.H.; Chang, C.Y. Acinar cell carcinoma of the pancreas: Clinical and computed tomography manifestations. J. Comput. Assist. Tomogr. 2004, 28, 180–186. [Google Scholar] [CrossRef]
- Thompson, E.D.; Wood, L.D. Pancreatic Neoplasms With Acinar Differentiation: A Review of Pathologic and Molecular Features. Arch. Pathol. Lab. Med. 2020, 144, 808–815. [Google Scholar] [CrossRef]
- Tatli, S.; Mortele, K.J.; Levy, A.D.; Glickman, J.N.; Ros, P.R.; Banks, P.A.; Silverman, S.G. CT and MRI features of pure acinar cell carcinoma of the pancreas in adults. AJR Am. J. Roentgenol. 2005, 184, 511–519. [Google Scholar] [CrossRef]
- Javadi, S.; Menias, C.O.; Korivi, B.R.; Shaaban, A.M.; Patnana, M.; Alhalabi, K.; Elsayes, K.M. Pancreatic Calcifications and Calcified Pancreatic Masses: Pattern Recognition Approach on CT. Am. J. Roentgenol. 2017, 209, 77–87. [Google Scholar] [CrossRef]
- Klöppel, G.; Kosmahl, M. Cystic lesions and neoplasms of the pancreas. The features are becoming clearer. Pancreatology 2001, 1, 648–655. [Google Scholar] [CrossRef]
- Mergo, P.J.; Helmberger, T.K.; Buetow, P.C.; Helmberger, R.C.; Ros, P.R. Pancreatic neoplasms: MR imaging and pathologic correlation. Radiographics 1997, 17, 281–301. [Google Scholar] [CrossRef] [PubMed]
- Cantisani, V.; Mortele, K.J.; Levy, A.; Glickman, J.N.; Ricci, P.; Passariello, R.; Ros, P.R.; Silverman, S.G. MR imaging features of solid pseudopapillary tumor of the pancreas in adult and pediatric patients. Am. J. Roentgenol. 2003, 181, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Solcia, E.; Capella, C.; Kloppel, G. Tumors of the exocrine pancreas. In Atlas of Tumor Pathology: Tumors of the Pancreas; Rosai, J., Sorbin, L., Eds.; Armed Forces Institute of Pathology: Washington, DC, USA, 1997; pp. 31–144. [Google Scholar]
- Distler, M.; Rückert, F.; Dittert, D.D.; Stroszczynski, C.; Dobrowolski, F.; Kersting, S.; Grützmann, R. Curative resection of a primarily unresectable acinar cell carcinoma of the pancreas after chemotherapy. World J. Surg. Oncol. 2009, 7, 22. [Google Scholar] [CrossRef] [PubMed]
- Mortenson, M.M.; Katz, M.H.; Tamm, E.P.; Bhutani, M.S.; Wang, H.; Evans, D.B.; Fleming, J.B. Current diagnosis and management of unusual pancreatic tumors. Am. J. Surg. 2008, 196, 100–113. [Google Scholar] [CrossRef]
- Matos, J.M.; Schmidt, C.M.; Turrini, O.; Agaram, N.P.; Niedergethmann, M.; Saeger, H.D.; Merchant, N.; Johnson, C.S.; Lillemoe, K.D.; Grützmann, R. Pancreatic acinar cell carcinoma: A multi-institutional study. J. Gastrointest. Surg. 2009, 13, 1495–1502. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Zhou, X.; Zhou, H.; Cui, Y.; Li, Q.; Zhang, L. Acinar cell carcinoma: A report of 19 cases with a brief review of the literature. World J. Surg. Oncol. 2016, 14, 172. [Google Scholar] [CrossRef]
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Muraki, T.; Reid, M.D.; Basturk, O.; Jang, K.T.; Bedolla, G.; Bagci, P.; Mittal, P.; Memis, B.; Katabi, N.; Bandyopadhyay, S.; et al. Undifferentiated Carcinoma With Osteoclastic Giant Cells of the Pancreas: Clinicopathologic Analysis of 38 Cases Highlights a More Protracted Clinical Course Than Currently Appreciated. Am. J. Surg. Pathol. 2016, 40, 1203–1216. [Google Scholar] [CrossRef]
- Jo, S. Huge undifferentiated carcinoma of the pancreas with osteoclast-like giant cells. World J. Gastroenterol. 2014, 20, 2725–2730. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The basics of epithelial-mesenchymal transition. J. Clin. Investig. 2010, 119, 1420–1428. [Google Scholar] [CrossRef]
- Nieto, M.A.; Huang, R.Y.; Jackson, R.A.; Thiery, J.P. EMT: 2016. Cell 2016, 166, 21–45. [Google Scholar] [CrossRef] [PubMed]
- Lambert, A.W.; Pattabiraman, D.R.; Weinberg, R.A. Emerging Biological Principles of Metastasis. Cell 2017, 168, 670–691. [Google Scholar] [CrossRef] [PubMed]
- Joo, Y.E.; Heo, T.; Park, C.H.; Lee, W.S.; Kim, H.S.; Kim, J.C.; Koh, Y.S.; Choi, S.K.; Cho, C.K.; Rew, J.S.; et al. A case of osteoclast-like giant cell tumor of the pancreas with ductal adenocarcinoma: Histopathological, immunohistochemical, ultrastructural and molecular biological studies. J. Korean Med. Sci. 2005, 20, 516–520. [Google Scholar] [CrossRef] [PubMed]
- Nagtegaal, I.D.; Odze, R.D.; Klimstra, D.; Paradis, V.; Rugge, M.; Schirmacher, P.; Washington, K.M.; Carneiro, F.; Cree, I.A. WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020, 76, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, E.; Schena, N.; Serino, G.; Lantone, G.; Armentano, R. Assessment and management of undifferentiated carcinoma with osteoclastic like giant cells of the pancreas: A case report and revision of literature. BMC Gastroenterol. 2021, 21, 247. [Google Scholar] [CrossRef]
- Yang, K.Y.; Choi, J.I.; Choi, M.H.; Park, M.Y.; Rha, S.E.; Byun, J.Y.; Jung, E.S.; Lall, C. Magnetic resonance imaging findings of undifferentiated carcinoma with osteoclast-like giant cells of pancreas. Clin. Imaging 2016, 40, 148–151. [Google Scholar] [CrossRef]
- Maksymov, V.; Khalifa, M.A.; Bussey, A.; Carter, B.; Hogan, M. Undifferentiated (anaplastic) carcinoma of the pancreas with osteoclast-like giant cells showing various degree of pancreas duct involvement. A case report and literature review. JOP 2011, 12, 170–176. [Google Scholar]
- Yazawa, T.; Watanabe, A.; Araki, K.; Segawa, A.; Hirai, K.; Kubo, N.; Igarashi, T.; Tsukagoshi, M.; Ishii, N.; Hoshino, K.; et al. Complete resection of a huge pancreatic undifferentiated carcinoma with osteoclast-like giant cells. Int. Cancer Conf. J. 2017, 6, 193–196. [Google Scholar] [CrossRef]
- Gao, H.Q.; Yang, Y.M.; Zhuang, Y.; Liu, P. Locally advanced undifferentiated carcinoma with osteoclast-like giant cells of the pancreas. World J. Gastroenterol. 2015, 21, 694–698. [Google Scholar] [CrossRef]
- Sakhi, R.; Hamza, A.; Khurram, M.S.; Ibrar, W.; Mazzara, P. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells reported in an asymptomatic patient: A rare case and literature review. Autops. Case Rep. 2017, 7, 51–57. [Google Scholar] [CrossRef]
- Togawa, Y.; Tonouchi, A.; Chiku, T.; Sano, W.; Doki, T.; Yano, K.; Uno, H.; Muronoi, T.; Kaneoya, K.; Shinagawa, T.; et al. A case report of undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and literature review. Clin. J. Gastroenterol. 2010, 3, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Federle, M.P.; Ohba, S.; Ohtomo, K.; Sugiyama, A.; Fujimoto, H.; Haradome, H.; Araki, T. Atypical exocrine and endocrine pancreatic tumors (anaplastic, small cell, and giant cell types): CT and pathologic features in 14 patients. Abdom. Imaging 2000, 25, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Wada, T.; Itano, O.; Oshima, G.; Chiba, N.; Ishikawa, H.; Koyama, Y.; Du, W.; Kitagawa, Y. A male case of an undifferentiated carcinoma with osteoclast-like giant cells originating in an indeterminate mucin-producing cystic neoplasm of the pancreas. A case report and review of the literature. World J. Surg. Oncol. 2011, 9, 100. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Urakawa, H.; Sakamoto, K.; Ito, E.; Hamada, Y.; Yoshimitsu, K. Undifferentiated carcinoma of the pancreas with osteoclast-like giant cells showing intraductal growth and intratumoral hemorrhage: MRI features. Radiol. Case Rep. 2019, 14, 1283–1287. [Google Scholar] [CrossRef] [PubMed]
- Oehler, U.; Jurs, M.; Kloppel, G.; Helpap, B. Osteoclast-like giant cell tumour of the pancreas presenting as a pseudocyst-like lesion. Virchows Arch. 1997, 431, 215–218. [Google Scholar] [CrossRef]
- Molberg, K.H.; Heffess, C.; Delgado, R.; Albores-Saavedra, J. Undifferentiated carcinoma with osteoclast-like giant cells of the pancreas and periampullary region. Cancer 1998, 82, 1279–1287. [Google Scholar] [CrossRef]
- Zou, X.P.; Yu, Z.L.; Li, Z.S.; Zhou, G.Z. Clinicopathological features of giant cell carcinoma of the pancreas. Hepatobiliary Pancreat. Dis. Int. 2004, 3, 300–302. [Google Scholar]
- Paal, E.; Thompson, L.D.; Frommelt, R.A.; Przygodzki, R.M.; Heffess, C.S. A clinicopathologic and immunohistochemical study of 35 anaplastic carcinomas of the pancreas with a review of the literature. Ann. Diagn. Pathol. 2001, 5, 129–140. [Google Scholar] [CrossRef]
- Hirano, H.; Morita, K.; Tachibana, S.; Okimura, A.; Fujisawa, T.; Ouchi, S.; Nakasho, K.; Ueyama, S.; Nishigami, T.; Terada, N. Undifferentiated carcinoma with osteoclast-like giant cells arising in a mucinous cystic neoplasm of the pancreas. Pathol. Int. 2008, 58, 383–389. [Google Scholar] [CrossRef]
- Demetter, P.; Maréchal, R.; Puleo, F.; Delhaye, M.; Debroux, S.; Charara, F.; Gomez Galdon, M.; Van Laethem, J.L.; Verset, L. Undifferentiated Pancreatic Carcinoma with Osteoclast-like Giant Cells: What Do We Know So Far? Front. Oncol. 2021, 11, 630086. [Google Scholar] [CrossRef]
- Luchini, C.; Pea, A.; Lionheart, G.; Mafficini, A.; Nottegar, A.; Veronese, N.; Chianchiano, P.; Brosens, L.A.; Noë, M.; Offerhaus, G.J.A.; et al. Pancreatic undifferentiated carcinoma with osteoclast-like giant cells is genetically similar to, but clinically distinct from, conventional ductal adenocarcinoma. J. Pathol. 2017, 243, 148–154. [Google Scholar] [CrossRef]
- Moore, J.C.; Bentz, J.S.; Hilden, K.; Adler, D.G. Osteoclastic and pleomorphic giant cell tumors of the pancreas: A review of clinical, endoscopic, and pathologic features. World J. Gastrointest. Endosc. 2010, 2, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Boyd, C.A.; Benarroch-Gampel, J.; Sheffield, K.M.; Cooksley, C.D.; Riall, T.S. 415 patients with adenosquamous carcinoma of the pancreas: A population-based analysis of prognosis and survival. J. Surg. Res. 2012, 174, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Borazanci, E.; Millis, S.Z.; Korn, R.; Han, H.; Whatcott, C.J.; Gatalica, Z.; Barrett, M.T.; Cridebring, D.; Von Hoff, D.D. Adenosquamous carcinoma of the pancreas: Molecular characterization of 23 patients along with a literature review. World J. Gastrointest. Oncol. 2015, 7, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Simone, C.G.; Zuluaga Toro, T.; Chan, E.; Feely, M.M.; Trevino, J.G.; George, T.J., Jr. Characteristics and outcomes of adenosquamous carcinoma of the pancreas. Gastrointest. Cancer Res. 2013, 6, 75–79. [Google Scholar] [CrossRef]
- Herxheimer, G. Über heterologe Cancroide. Beitr. Pathol. Anat. 1907, 41, 348–412. [Google Scholar]
- Hruban, R.H.; Pitman, M.B.; Klimstra, D.S. Tumors of the Pancreas, Afip Atlas of Tumor Pathology, 4th Series Fascicle 6, 6th ed.; American Registry of Pathology: Washington, DC, USA, 2007. [Google Scholar]
- Voong, K.R.; Davison, J.; Pawlik, T.M.; Uy, M.O.; Hsu, C.C.; Winter, J.; Hruban, R.H.; Laheru, D.; Rudra, S.; Swartz, M.J.; et al. Resected pancreatic adenosquamous carcinoma: Clinicopathologic review and evaluation of adjuvant chemotherapy and radiation in 38 patients. Hum. Pathol. 2010, 41, 113–122. [Google Scholar] [CrossRef]
- Kardon, D.E.; Thompson, L.D.; Przygodzki, R.M.; Heffess, C.S. Adenosquamous carcinoma of the pancreas: A clinicopathologic series of 25 cases. Mod. Pathol. 2001, 14, 443–451. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Enjoji, M. Adenosquamous carcinoma of the pancreas: A clinicopathologic study. J. Surg. Oncol. 1991, 47, 109–116. [Google Scholar] [CrossRef]
- Murakami, Y.; Yokoyama, T.; Yokoyama, Y.; Kanehiro, T.; Uemura, K.; Sasaki, M.; Morifuji, M.; Sueda, T. Adenosquamous carcinoma of the pancreas: Preoperative diagnosis and molecular alterations. J. Gastroenterol. 2003, 38, 1171–1175. [Google Scholar] [CrossRef]
- Trikudanathan, G.; Dasanu, C.A. Adenosquamous carcinoma of the pancreas: A distinct clinicopathologic entity. South. Med. J. 2010, 103, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Momosaki, S.; Yano, H.; Kojiro, M. Establishment of a human hepatic adenosquamous carcinoma cell line (KMC-2) and its response to cytokines. Pathol. Int. 1995, 45, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Madura, J.A.; Jarman, B.T.; Doherty, M.G.; Yum, M.N.; Howard, T.J. Adenosquamous carcinoma of the pancreas. Arch. Surg. 1999, 134, 599–603. [Google Scholar] [CrossRef] [PubMed]
- Kovi, J. Adenosquamous carcinoma of the pancreas: A light and electron microscopic study. Ultrastruct. Pathol. 1982, 3, 17–23. [Google Scholar] [CrossRef]
- Xiong, Q.; Zhang, Z.; Xu, Y.; Zhu, Q. Pancreatic Adenosquamous Carcinoma: A Rare Pathological Subtype of Pancreatic Cancer. J. Clin. Med. 2022, 11, 7401. [Google Scholar] [CrossRef]
- Yin, Q.; Wang, C.; Wu, Z.; Wang, M.; Cheng, K.; Zhao, X.; Yuan, F.; Tang, Y.; Miao, F. Adenosquamous carcinoma of the pancreas: Multidetector-row computed tomographic manifestations and tumor characteristics. J. Comput. Assist. Tomogr. 2013, 37, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Nagao, S.; Tajima, H.; Okudaira, K.; Hashiguchi, K.; Miyazaki, J.; Matsuzaki, K.; Tsuzuki, Y.; Kawaguchi, A.; Itoh, K.; et al. Adenosquamous pancreatic cancer producing parathyroid hormone-related protein. J. Gastroenterol. 2004, 39, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, N.; Higurashi, T.; Iida, H.; Mawatari, H.; Endo, H.; Nozaki, Y.; Tomimoto, A.; Yoneda, K.; Akiyama, T.; Fujita, K.; et al. Adenosquamous carcinoma of the pancreas associated with humoral hypercalcemia of malignancy (HHM). J. Hepatobiliary Pancreat. Surg. 2008, 15, 531–535. [Google Scholar] [CrossRef]
- López-Tomassetti-Fernández, E.M.; Favre-Rizzo, J.; Delgado-Plasencia, L.; Hernández-Hernández, J.R. Hypercalcemia associated with adenosquamous pancreatic carcinoma: A reason to initiate palliative treatment. Rev. Esp. Enferm. Dig. 2013, 105, 425–428. [Google Scholar] [CrossRef]
- Hsu, J.T.; Chen, H.M.; Wu, R.C.; Yeh, C.N.; Yeh, T.S.; Hwang, T.L.; Jan, Y.Y.; Chen, M.F. Clinicopathologic features and outcomes following surgery for pancreatic adenosquamous carcinoma. World J. Surg. Oncol. 2008, 6, 95. [Google Scholar] [CrossRef]
- Ducreux, M.; Cuhna, A.S.; Caramella, C.; Hollebecque, A.; Burtin, P.; Goéré, D.; Seufferlein, T.; Haustermans, K.; Van Laethem, J.L.; Conroy, T.; et al. ESMO Guidelines Committee. Cancer of the pancreas: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26 (Suppl. 5), v56–v68. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Gonzalez, J.J.; Devarkonda, V.; Saint-Phard, T.; Singh, T.; Adekolujo, O.S. Squamous cell carcinoma-A rare pancreatic exocrine malignancy. Cancer Biol. Ther. 2019, 20, 593–596. [Google Scholar] [CrossRef] [PubMed]
- Itani, K.M.; Karni, A.; Green, L. Squamous cell carcinoma of the pancreas. J. Gastrointest. Surg. 1999, 3, 512–515. [Google Scholar] [CrossRef] [PubMed]
- Horino, K.; Takamori, H.; Ikuta, Y.; Nakahara, O.; Chikamoto, A.; Ishiko, T.; Beppu, T.; Baba, H. Cutaneous metastases secondary to pancreatic cancer. World J. Gastrointest. Oncol. 2012, 4, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.Y.; Wang, X.B.; Gao, F.; Bu, B.; Zhang, S.; Wang, Z. Cutaneous metastasis from pancreatic cancer: A case report and systematic review of the literature. Oncol. Lett. 2014, 8, 2654–2660. [Google Scholar] [CrossRef]
- Miyahara, M.; Hamanaka, Y.; Kawabata, A.; Sato, Y.; Tanaka, A.; Yamamoto, A.; Ueno, T.; Nishihara, K.; Suzuki, T. Cutaneous metastases from pancreatic cancer. Int. J. Pancreatol. 1996, 20, 127–130. [Google Scholar] [CrossRef]
- Ding, Y.; Zhou, J.; Sun, H.; He, D.; Zeng, M.; Rao, S. Contrast-enhanced multiphasic CT and MRI findings of adenosquamous carcinoma of the pancreas. Clin. Imaging 2013, 37, 1054–1060. [Google Scholar] [CrossRef]
- Toshima, F.; Inoue, D.; Yoshida, K.; Yoneda, N.; Minami, T.; Kobayashi, S.; Ikdeda, H.; Matsui, O.; Gabata, T. Adenosquamous carcinoma of pancreas: CT and MR imaging features in eight patients, with pathologic correlations and comparison with adenocarcinoma of pancreas. Abdom. Radiol. 2016, 41, 508–520. [Google Scholar] [CrossRef]
- De Moura, D.T.H.; Coronel, M.; Chacon, D.A.; Tanigawa, R.; Chaves, D.M.; Matuguma, S.E.; Dos Santos, M.E.L.; Jukemura, J.; De Moura, E.G.H. Primary adenosquamous cell carcinoma of the pancreas: The use of endoscopic ultrasound guided—Fine needle aspiration to establish a definitive cytologic diagnosis. Rev. Gastroenterol. Peru 2017, 37, 370–373. [Google Scholar]
- Martínez de Juan, F.; Reolid Escribano, M.; Martínez Lapiedra, C.; Maia de Alcantara, F.; Caballero Soto, M.; Calatrava Fons, A.; Machado, I. Pancreatic adenosquamous carcinoma and intraductal papillary mucinous neoplasm in a CDKN2A germline mutation carrier. World J. Gastrointest. Oncol. 2017, 9, 390–396. [Google Scholar] [CrossRef]
- Paramythiotis, D.; Kyriakidis, F.; Karlafti, E.; Didangelos, T.; Oikonomou, I.M.; Karakatsanis, A.; Poulios, C.; Chamalidou, E.; Vagionas, A.; Michalopoulos, A. Adenosquamous carcinoma of the pancreas: Two case reports and review of the literature. J. Med. Case Rep. 2022, 16, 395. [Google Scholar] [CrossRef] [PubMed]
- Liszka, L.; Zielinska-Pajak, E.; Pajak, J.; Gołka, D. Colloid carcinoma of the pancreas: Review of selected pathological and clinical aspects. Pathology 2008, 40, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Adsay, N.V.; Pierson, C.; Sarkar, F. Colloid (mucinous noncystic) carcinoma of the pancreas. Am. J. Surg. Pathol. 2001, 25, 26–42. [Google Scholar] [CrossRef]
- Aaltonen, L.A.; Hamilton, S.R. Pathology and Genetics of Tumours of the Digestive System; World Health Organization, International Agency for Research on Cancer: Lyon, France, 2000; p. 314. [Google Scholar]
- Waters, J.A.; Schnelldorfer, T.; Aguilar-Saavedra, J.R. Survival after resection for invasive intraductal papillary mucinous neoplasm and for pancreatic adenocarcinoma: A multi-institutional comparison according to American Joint Committee on Cancer Stage. J. Am. Coll. Surg. 2011, 213, 275–283. [Google Scholar] [CrossRef]
- Whang, E.E.; Danial, T.; Dunn, J.C.; Ashley, S.W.; Reber, H.A.; Lewin, T.J.; Tompkins, R.K. The spectrum of mucin-producing adenocarcinoma of the pancreas. Pancreas 2000, 21, 147–151. [Google Scholar] [CrossRef]
- Yoon, M.A.; Lee, J.M.; Kim, S.H.; Lee, J.Y.; Han, J.K.; Choi, B.I.; Choi, J.Y.; Park, S.H.; Lee, M.W. MRI features of pancreatic colloid carcinoma. AJR Am. J. Roentgenol. 2009, 193, W308–W313. [Google Scholar] [CrossRef]
- Nakahashi, G.; Yamaguchi, R.; Watanabe, S. A case of rapid growing mucinous carcinoma of the pancreas without intraductal papillary mucinous neoplasm. J. Biliary Tract. Pancreas 2017, 38, 885–890. [Google Scholar]
- Raut, C.P.; Cleary, K.R.; Staerkel, G.A.; Abbruzzese, J.L.; Wolff, R.A.; Lee, J.H.; Vauthey, J.N.; Lee, J.E.; Pisters, P.W.; Evans, D.B. Intraductal papillary mucinous neoplasms of the pancreas: Effect of invasion and pancreatic margin status on recurrence and survival. Ann. Surg. Oncol. 2006, 13, 582–594. [Google Scholar] [CrossRef]
- Poultsides, G.A.; Reddy, S.; Cameron, J.L.; Hruban, R.H.; Pawlik, T.M.; Ahuja, N.; Jain, A.; Edil, B.H.; Iacobuzio-Donahue, C.A.; Schulick, R.D.; et al. Histopathologic basis for the favorable survival after resection of intraductal papillary mucinous neoplasm-associated invasive adenocarcinoma of the pancreas. Ann. Surg. 2010, 251, 470–476. [Google Scholar] [CrossRef]
- Ren, F.Y.; Shao, C.W.; Zuo, C.J.; Lu, J.P. CT features of colloid carcinomas of the pancreas. Chin. Med. J. 2010, 123, 1329–1332. [Google Scholar]
- Adsay, N.V.; Merati, K.; Nassar, H.; Shia, J.; Sarkar, F.; Pierson, C.R.; Cheng, J.D.; Visscher, D.W.; Hruban, R.H.; Klimstra, D.S. Pathogenesis of colloid (pure mucinous) carcinoma of exocrine organs: Coupling of gel-forming mucin (MUC2) production with altered cell polarity and abnormal cell-stroma interaction may be the key factor in the morphogenesis and indolent behavior of colloid carcinoma in the breast and pancreas. Am. J. Surg. Pathol. 2003, 27, 571–578. [Google Scholar] [PubMed]
- Song, S.J.; Lee, J.M.; Kim, Y.J.; Kim, S.H.; Lee, J.Y.; Han, J.K.; Choi, B.I. Differentiation of intraductal papillary mucinous neoplasms from other pancreatic cystic masses: Comparison of multirow-detector CT and MR imaging using ROC analysis. J. Magn. Reson. Imaging 2007, 26, 86–93. [Google Scholar] [CrossRef]
- Plerhoples, T.A.; Ahdoot, M.; DiMaio, M.A.; Pai, R.K.; Park, W.G.; Poultsides, G.A. Colloid carcinoma of the pancreas. Dig. Dis. Sci. 2011, 56, 1295–1298. [Google Scholar] [CrossRef] [PubMed]
- Flor-de-Lima, B.S.; Freitas, P.; Couto, N.; Castillo-Martin, M.; Santiago, I. Pancreatic intraductal papillary mucinous neoplasm associated colloid carcinoma. Radiol. Case Rep. 2021, 16, 2989–2992. [Google Scholar] [CrossRef]
- Schawkat, K.; Manning, M.A.; Glickman, J.N.; Mortele, K.J. Pancreatic Ductal Adenocarcinoma and Its Variants: Pearls and Perils. Radiographics 2020, 40, 1219–1239. [Google Scholar] [CrossRef] [PubMed]
- Parwani, A.V.; Ali, S.Z. Pathologic quiz case: A 52-year-old woman with jaundice and history of necrotizing pancreatitis. Primary colloid carcinoma of the pancreas. Arch. Pathol. Lab. Med. 2005, 129, 255–256. [Google Scholar] [CrossRef]
- Hirono, S.; Yamaue, H. Surgical strategy for intraductal papillary mucinous neoplasms of the pancreas. Surg. Today 2020, 50, 50–55. [Google Scholar] [CrossRef]
- Marchegiani, G.; Andrianello, S.; Dal Borgo, C.; Secchettin, E.; Melisi, D.; Malleo, G.; Bassi, C.; Salvia, R. Adjuvant chemotherapy is associated with improved postoperative survival in specific subtypes of invasive intraductal papillary mucinous neoplasms (IPMN) of the pancreas: It is time for randomized controlled data. HPB Oxf. 2019, 21, 596–603. [Google Scholar] [CrossRef]
- Sohn, T.A.; Yeo, C.J.; Cameron, J.L.; Koniaris, L.; Kaushal, S.; Abrams, R.A.; Sauter, P.K.; Coleman, J.; Hruban, R.H.; Lillemoe, K.D. Resected adenocarcinoma of the pancreas—616 patients: Results, outcomes, and prognostic indicators. J. Gastrointest. Surg. 2000, 4, 567–579. [Google Scholar] [CrossRef]
- Youngwirth, L.M.; Freischlag, K.; Nussbaum, D.P.; Benrashid, E.; Blazer, D.G. Primary sarcomas of the pancreas: A review of 253 patients from the National Cancer Data Base. Surg. Oncol. 2018, 27, 676–680. [Google Scholar] [CrossRef]
- Baylor, S.M.; Berg, J.W. Cross-classification and survival characteristics of 5,000 cases of cancer of the pancreas. J. Surg. Oncol. 1973, 5, 335–358. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Song, J.S.; Park, H.; Byun, J.H.; Song, K.B.; Kim, K.P.; Kim, S.C.; Hong, S.M. Primary mesenchymal tumors of the pancreas: Single-center experience over 16 years. Pancreas 2014, 43, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Papalampros, A.; Vailas, M.G.; Deladetsima, I.; Moris, D.; Sotiropoulou, M.; Syllaios, A.; Petrou, A.; Felekouras, E. Irreversible electroporation in a case of pancreatic leiomyosarcoma: A novel weapon versus a rare malignancy? World J. Surg. Oncol. 2019, 17, 6. [Google Scholar] [CrossRef] [PubMed]
- Søreide, J.A.; Undersrud, E.S.; Al-Saiddi, M.S.; Tholfsen, T.; Søreide, K. Primary Leiomyosarcoma of the Pancreas-a Case Report and a Comprehensive Review. J. Gastrointest. Cancer 2016, 47, 358–365. [Google Scholar] [CrossRef]
- Zalatnai, A.; Kovács, M.; Flautner, L.; Sipos, B.; Sarkady, E.; Bocsi, J. Pancreatic leiomyosarcoma. Case report with immunohistochemical and flow cytometric studies. Virchows Arch. 1998, 432, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Asanuma, Y.; Nanjo, H.; Arakawa, A.; Kusano, T.; Koyama, K.; Shindo, M. A resected case of giant leiomyosarcoma of the pancreas. J. Gastroenterol. 1994, 29, 223–227. [Google Scholar] [CrossRef]
- Muhammad, S.U.; Azam, F.; Zuzana, S. Primary pancreatic leiomyosarcoma: A case report. Cases J. 2008, 1, 280. [Google Scholar] [CrossRef]
- Singla, V.; Arora, A.; Tyagi, P.; Bansal, R.K.; Sharma, P.; Bansal, N.; Kumar, A. Rare cause of recurrent acute pancreatitis due to leiomyosarcoma. Trop. Gastroenterol. 2016, 37, 70–72. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, T.; Wang, T.; You, L.; Zhao, Y. Clinical characteristics and prognosis of primary leiomyosarcoma of the pancreas: A systematic review. World J. Surg. Oncol. 2013, 11, 290. [Google Scholar] [CrossRef]
- Aleshawi, A.J.; Allouh, M.Z.; Heis, F.H.; Tashtush, N.; Heis, H.A. Primary Leiomyosarcoma of the Pancreas: A Comprehensive Analytical Review. J. Gastrointest. Cancer 2020, 51, 433–438. [Google Scholar] [CrossRef]
- Zhang, H.; Jensen, M.H.; Farnell, M.B.; Smyrk, T.C.; Zhang, L. Primary leiomyosarcoma of the pancreas: Study of 9 cases and review of literature. Am. J. Surg. Pathol. 2010, 34, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Barral, M.; Faraoun, S.A.; Fishman, E.K.; Dohan, A.; Pozzessere, C.; Berthelin, M.A.; Bazeries, P.; Barat, M.; Hoeffel, C.; Soyer, P. Imaging features of rare pancreatic tumors. Diagn. Interv. Imaging 2016, 97, 1259–1273. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, D.N.; Batra, A.; Thulkar, S.; Julka, P.K. Leiomyosarcoma of pancreas: Imaging features. Indian. J. Gastroenterol. 2000, 19, 187–188. [Google Scholar]
- Ishikawa, O.; Matsui, Y.; Aoki, Y.; Iwanaga, T.; Terasawa, T.; Wada, A. Leiomyosarcoma of the pancreas. Report of a case and review of the literature. Am. J. Surg. Pathol. 1981, 5, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Riddle, N.D.; Quigley, B.C.; Browarsky, I.; Bui, M.M. Leiomyosarcoma arising in the pancreatic duct: A case report and review of the current literature. Case Rep. Med. 2010, 2010, 252364. [Google Scholar] [CrossRef]
- Kocakoc, E.; Havan, N.; Bilgin, M.; Atay, M. Primary pancreatic leiomyosarcoma. Iran. J. Radiol. 2014, 11, e4880. [Google Scholar] [CrossRef]
- Machado, M.C.; Cunha, J.E.; Penteado, S.; Bacchella, T.; Jukemura, J.; Costa, A.C.; Halpern-Salomon, I. Preoperative diagnosis of pancreatic leiomyosarcoma. Int. J. Pancreatol. 2000, 28, 97–100. [Google Scholar] [CrossRef]
- de la Santa, L.G.; Retortillo, J.A.; Miguel, A.C.; Klein, L.M. Radiology of pancreatic neoplasms: An update. World J. Gastrointest. Oncol. 2014, 6, 330–343. [Google Scholar] [CrossRef]
- Viúdez, A.; De Jesus-Acosta, A.; Carvalho, F.L.; Vera, R.; Martín-Algarra, S.; Ramírez, N. Pancreatic neuroendocrine tumors: Challenges in an underestimated disease. Crit. Rev. Oncol. Hematol. 2016, 101, 193–206. [Google Scholar] [CrossRef]
- Okun, S.D.; Lewin, D.N. Non-neoplastic pancreatic lesions that may mimic malignancy. Semin. Diagn. Pathol. 2016, 33, 31–42. [Google Scholar] [CrossRef]
- Konstantinidis, I.T.; Dursun, A.; Zheng, H.; Wargo, J.A.; Thayer, S.P.; Fernandez-del Castillo, C.; Warshaw, A.L.; Ferrone, C.R. Metastatic tumors in the pancreas in the modern era. J. Am. Coll. Surg. 2010, 211, 749–753. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Masuda, D.; Kurisu, Y.; Miyamoto, Y.; Hayashi, M.; Imoto, A.; Takii, M.; Takeuchi, T.; Inoue, T.; Tokioka, S.; et al. Multiple metastatic leiomyosarcoma of the pancreas: A first case report and review of the literature. Intern. Med. 2013, 52, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Wang, L.; Sheng, J.; Zhang, D.; Guan, L.; Zhao, K.; Zhang, X. Transdifferentiation of pancreatic stromal tumor into leiomyosarcoma with metastases to liver and peritoneum: A case report. BMC Cancer 2016, 16, 947. [Google Scholar] [CrossRef] [PubMed]
- Hébert-Magee, S.; Varadarajulu, S.; Frost, A.R.; Ramesh, J. Primary pancreatic leiomyosarcoma: A rare diagnosis obtained by EUS-FNA cytology. Gastrointest. Endosc. 2014, 80, 361–362. [Google Scholar] [CrossRef]
- Aihara, H.; Kawamura, Y.J.; Toyama, N.; Mori, Y.; Konishi, F.; Yamada, S. A small leiomyosarcoma of the pancreas treated by local excision. HPB 2002, 4, 145–148. [Google Scholar] [CrossRef]
- Milanetto, A.C.; Liço, V.; Blandamura, S.; Pasquali, C. Primary leiomyosarcoma of the pancreas: Report of a case treated by local excision and review of the literature. Surg. Case Rep. 2015, 1, 98. [Google Scholar] [CrossRef]
- Hur, Y.H.; Kim, H.H.; Park, E.K.; Seoung, J.S.; Kim, J.W.; Jeong, Y.Y.; Lee, J.H.; Koh, Y.S.; Kim, J.C.; Kim, H.J.; et al. Primary leiomyosarcoma of the pancreas. J. Korean Surg. Soc. 2011, 81 (Suppl. 1), S69–S73. [Google Scholar] [CrossRef]
- Izumi, H.; Okada, K.; Imaizumi, T.; Hirabayashi, K.; Matsuyama, M.; Dowaki, S.; Tobita, K.; Makuuchi, H. Leiomyosarcoma of the pancreas: Report of a case. Surg. Today 2011, 41, 1556–1561. [Google Scholar] [CrossRef]
- Battula, N.; Srinivasan, P.; Prachalias, A.; Rela, M.; Heaton, N. Primary pancreatic lymphoma: Diagnostic and therapeutic dilemma. Pancreas 2006, 33, 192–194. [Google Scholar] [CrossRef]
- Kloppel, G.; Solcia, E.; Longnecker, D.S.; Capella, C.; Sobin, L.H. Histological Typing of Tumours of the Exocrine Pancreas; World Health Organization International Classification of Tumours; Springer: Berlin, Germany, 1996; pp. 11–20. [Google Scholar]
- Sadot, E.; Yahalom, J.; Do, R.K.; Teruya-Feldstein, J.; Allen, P.J.; Gönen, M.; D’Angelica, M.I.; Kingham, T.P.; Jarnagin, W.R.; DeMatteo, R.P. Clinical features and outcome of primary pancreatic lymphoma. Ann. Surg. Oncol. 2015, 22, 1176–1184. [Google Scholar] [CrossRef]
- Jones, W.F.; Sheikh, M.Y.; McClave, S.A. AIDS-related non-Hodgkin’s lymphoma of the pancreas. Am. J. Gastroenterol. 1997, 92, 335–338. [Google Scholar]
- Facchinelli, D.; Sina, S.; Boninsegna, E.; Borin, A.; Tisi, M.C.; Piazza, F.; Scapinello, G.; Maiolo, E.; Hohaus, S.; Zamò, A.; et al. Primary pancreatic lymphoma: Clinical presentation, diagnosis, treatment, and outcome. Eur. J. Haematol. 2020, 105, 468–475. [Google Scholar] [CrossRef]
- Rad, N.; Khafaf, A.; Mohammad Alizadeh, A.H. Primary pancreatic lymphoma: What we need to know. J. Gastrointest. Oncol. 2017, 8, 749–757. [Google Scholar] [CrossRef]
- Mishra, M.V.; Keith, S.W.; Shen, X.; Ad, V.B.; Champ, C.E.; Biswas, T. Primary pancreatic lymphoma. Am. J. Clin. Oncol. 2013, 36, 38–43. [Google Scholar] [CrossRef]
- Fujinaga, Y.; Lall, C.; Patel, A.; Matsushita, T.; Sanyal, R.; Kadoya, M. MR features of primary and secondary malignant lymphoma of the pancreas: A pictorial review. Insights Imaging 2013, 4, 321–329. [Google Scholar] [CrossRef]
- Merkle, E.M.; Bender, G.N.; Brambs, H.J. Imaging findings in pancreatic lymphoma: Differential aspects. Am. J. Roentgenol. 2000, 174, 671–675. [Google Scholar] [CrossRef]
- Segaran, N.; Sandrasegaran, K.; Devine, C.; Wang, M.X.; Shah, C.; Ganeshan, D. Features of primary pancreatic lymphoma: A bi-institutional review with an emphasis on typical and atypical imaging features. World J. Clin. Oncol. 2021, 12, 823–832. [Google Scholar] [CrossRef]
- Low, G.; Panu, A.; Millo, N.; Leen, E. Multimodality imaging of neoplastic and nonneoplastic solid lesions of the pancreas. RadioGraphics 2011, 31, 993–1015. [Google Scholar] [CrossRef]
- Amodio, J.; Brodsky, J.E. Pediatric Burkitt lymphoma presenting as acute pancreatitis: MRI characteristics. Pediatr. Radiol. 2010, 40, 770–772. [Google Scholar] [CrossRef]
- Boninsegna, E.; Zamboni, G.A.; Facchinelli, D.; Triantopoulou, C.; Gourtsoyianni, S.; Ambrosetti, M.C.; Veneri, D.; Ambrosetti, A.; Pozzi Mucelli, R. CT imaging of primary pancreatic lymphoma: Experience from three referral centres for pancreatic diseases. Insights Imaging 2018, 9, 17–24. [Google Scholar] [CrossRef]
- Anand, D.; Lall, C.; Bhosale, P.; Ganeshan, D.; Qayyum, A. Current update on primary pancreatic lymphoma. Abdom. Radiol. 2016, 41, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.; Dang, N.; Dhawan, M.; Kulkarni, A. Diagnosis of a patient with primary pancreatic lymphoma. Gastroenterol. Hepatol. 2012, 8, 850–852. [Google Scholar]
- Saif, M.W. Primary pancreatic lymphomas. J. Pancreas 2006, 7, 262–273. [Google Scholar]
- Behrns, K.E.; Sarr, M.G.; Strickler, J.G. Pancreatic lymphoma: Is it a surgical disease? Pancreas 1994, 9, 662–667. [Google Scholar] [CrossRef]
- Sadaf, S.; Loya, A.; Akhtar, N.; Yusuf, M.A. Role of endoscopic ultrasound guided-fine needle aspiration biopsy in the diagnosis of lymphoma of the pancreas: A clinicopathological study of nine cases. Cytopathology 2017, 28, 536–541. [Google Scholar] [CrossRef]
- Freeman, C.; Berg, J.W.; Cutler, S.J. Occurrence and prognosis of extranodal lymphomas. Cancer 1972, 29, 252–260. [Google Scholar] [CrossRef]
- Vitolo, U.; Seymour, J.F.; Martelli, M.; Illerhaus, G.; Illidge, T.; Zucca, E.; Campo, E.; Ladetto, M.; ESMO Guidelines Committee. Extranodal diffuse large B-cell lymphoma (DLBCL) and primary mediastinal B-cell lymphoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27 (Suppl. 5), 91–102. [Google Scholar] [CrossRef] [PubMed]
- Ballarin, R.; Spaggiari, M.; Cautero, N.; De Ruvo, N.; Montalti, R.; Longo, C.; Pecchi, A.; Giacobazzi, P.; De Marco, G.; D’Amico, G.; et al. Pancreatic metastases from renal cell carcinoma: The state of the art. World J. Gastroenterol. 2011, 17, 4747–4756. [Google Scholar] [CrossRef] [PubMed]
- Reddy, S.; Wolfgang, C.L. The role of surgery in the management of isolated metastases to the pancreas. Lancet Oncol. 2009, 10, 287–293. [Google Scholar] [CrossRef]
- Reddy, S.; Edil, B.H.; Cameron, J.L.; Pawlik, T.M.; Herman, J.M.; Gilson, M.M.; Campbell, K.A.; Schulik, R.D.; Ahuja, N.; Wolfgang, C.L. Pancreatic resection of isolated metastases from nonpancreatic primary cancers. Ann. Surg. Oncol. 2008, 15, 3199–3206. [Google Scholar] [CrossRef]
- Sweeny, A.D.; Fisher, W.E.; Wu, M.F.; Hilsenbeck, S.G.; Brunicardi, F.C. Value of pancreatic resection for cancer metastatic to the pancreas. J. Surg. Res. 2010, 160, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Showalter, S.L.; Hager, E.; Yeo, C.J. Metastatic disease to the pancreas and spleen. Semin. Oncol. 2008, 35, 160–171. [Google Scholar] [CrossRef] [PubMed]
- Rypens, F.; Van Gansbeke, D.; Lambilliotte, J.P.; Van Regemorter, G.; Verhest, A.; Struyven, J. Pancreatic metastasis from renal cell carcinoma. Br. J. Radiol. 1992, 65, 547–548. [Google Scholar] [CrossRef] [PubMed]
- Ferrozzi, F.; Bova, D.; Campodonico, F.; Chiara, F.D.; Passari, A.; Bassi, P. Pancreatic metastases: CT assessment. Eur. Radiol. 1997, 7, 241–245. [Google Scholar] [CrossRef] [PubMed]
- Muranaka, T.; Teshima, K.; Honda, H.; Nanjo, T.; Hanada, K.; Oshiumi, Y. Computed tomography and histologic appearance of pancreatic metastases from distant sources. Acta Radiol. 1989, 30, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.K.; Chuah, K.L. Metastatic renal cell carcinoma to the pancreas: A review. Arch. Pathol. Lab. Med. 2016, 140, 598–602. [Google Scholar] [CrossRef]
- Sikka, A.; Adam, S.Z.; Wood, C.; Hoff, F.; Harmath, C.B.; Miller, F.H. Magnetic resonance imaging of pancreatic metastases from renal cell carcinoma. Clin. Imaging 2015, 39, 945–953. [Google Scholar] [CrossRef]
- Maeno, T.; Satoh, H.; Ishikawa, H.; Yamashita, Y.T.; Naito, T.; Fujiwara, M.; Kamma, H.; Ohtsuka, M.; Hasegawa, S. Patterns of pancreatic metastasis from lung cancer. Anticancer Res. 1998, 18, 2881–2884. [Google Scholar]
- Woo, J.S.; Joo, K.R.; Woo, Y.S.; Jang, J.Y.; Chang, Y.W.; Lee, J.I.; Chang, R. Pancreatitis from metastatic small cell lung cancer: Successful treatment with endoscopic intrapancreatic stenting. Korean J. Intern. Med. 2006, 21, 256. [Google Scholar] [CrossRef]
- Cwik, G.; Wallner, G.; Skoczylas, T.; Ciechanski, A.; Zinkiewicz, K. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch. Surg. 2006, 141, 968–973. [Google Scholar] [CrossRef]
- Okamoto, T. Malignant biliary obstruction due to metastatic non-hepato-pancreato-biliary cancer. World J. Gastroenterol. 2022, 28, 985–1008. [Google Scholar] [CrossRef] [PubMed]
- Ascenti, G.; Visalli, C.; Genitori, A.; Certo, A.; Pitrone, A.; Mazziotti, S. Multiple hypervascular pancreatic metastases from renal cell carcinoma: Dynamic MR and spiral CT in three cases. Clin. Imaging 2004, 28, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Galia, M.; Albano, D.; Picone, D.; Terranova, M.C.; Agrusa, A.; Di Buono, G.; Licata, A.; Lo Re, G.; La Grutta, L.; Midiri, M. Imaging features of pancreatic metastases: A comparison with pancreatic ductal adenocarcinoma. Clin. Imaging 2018, 51, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Kelekis, N.L.; Semelka, R.C.; Siegelman, E.S. MRI of pancreatic metastases from renal cancer. J. Comput. Assist. Tomogr. 1996, 20, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Crippa, S.; Angelini, C.; Mussi, C.; Bonardi, C.; Romano, F.; Sartori, P.; Uggeri, F.; Bovo, G. Surgical treatment of metastatic tumors to the pancreas: A single center experience and re-view of the literature. World J. Surg. 2006, 30, 1536–1542. [Google Scholar] [CrossRef]
- Sperti, C.; Pasquali, C.; Liessi, G.; Pinciroli, L.; Decet, G.; Pedrazzoli, S. Pancreatic resection for metastatic tumors to the pancreas. J. Surg. Oncol. 2003, 83, 161–166. [Google Scholar] [CrossRef]
- Law, C.H.; Wei, A.C.; Hanna, S.S.; Al-Zahrani, M.; Taylor, B.R.; Greig, P.D.; Langer, B.; Gallinger, S. Pancreatic resection for metastatic renal cell carcinoma: Presentation, treatment, and outcome. Ann. Surg. Oncol. 2003, 10, 922–926. [Google Scholar] [CrossRef]
- Scatarige, J.C.; Horton, K.M.; Sheth, S.; Fishman, E.K. Pancreatic parenchymal metastases: Observations on helical CT. Am. J. Roentgenol. 2001, 176, 695–699. [Google Scholar] [CrossRef]
- Atlas, S.W.; Braffmann, B.H.; LoBrutto, R.; Elder, D.E.; Herlyn, D. Human malignant melanomas with varying degrees of melanin content in nude mice: MR imaging, histopathology, and electron paramagnetic resonance. J. Comput. Assist. Tomogr. 1990, 14, 547–554. [Google Scholar] [CrossRef]
- Wang, H.; Nie, P.; Dong, C.; Li, J.; Huang, Y.; Hao, D.; Xu, W. CT and MRI Findings of Soft Tissue Adult Fibrosarcoma in Extremities. Biomed. Res. Int. 2018, 2018, 6075705. [Google Scholar] [CrossRef]
- Adsay, N.V.; Andea, A.; Basturk, O.; Kilinc, N.; Nassar, H.; Cheng, J.D. Secondary tumors of the pancreas: An analysis of a surgical and autopsy database and review of the literature. Virchows Arch. 2004, 444, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.H.; Tamm, E.P.; Marcal, L.; Balachandran, A.; Charnsangavej, C.; Vikram, R.; Bhosale, P. Imaging features of hematogenous metastases to the pancreas: Pictorial essay. Cancer Imaging 2011, 11, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Palmowski, M.; Hacke, N.; Satzl, S.; Klauss, M.; Wente, M.N.; Neukamm, M.; Kleeff, J.; Hallscheidt, P. Metastasis to the pancreas: Characterization by morphology and contrast enhancement features on CT and MRI. Pancreatology 2008, 8, 199–203. [Google Scholar] [CrossRef]
- Bahra, M.; Jacob, D.; Langrehr, J.M.; Glanemann, M.; Schumacher, G.; Lopez-Hänninen, E.; Neuhaus, P. Metastasen im Pankreas. Wann ist eine Resektion sinnvoll? Chirurg 2008, 79, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Bassi, C.; Butturini, G.; Falconi, M.; Sargenti, M.; Mantovani, W.; Pederzoli, P. High recurrence rate after atypical resection for pancreatic metastases from renal cell carcinoma. Br. J. Surg. 2003, 90, 555–559. [Google Scholar] [CrossRef] [PubMed]
| Benign | Potentially Malignant | Malignant |
|---|---|---|
| Intrapancreatic splenic tissue | Solid pseudopapillary tumour | Acinar cell carcinoma |
| Tuberculosis | Schwannoma | Undifferentiated carcinoma with osteoclastic-like giant cells. |
| Solid serous cystadenoma | Purely intraductal neuroendocrine tumour | Adenosquamous carcinoma |
| Fibrous solitary tumour | Colloid carcinoma | |
| Primary leiomyosarcoma | ||
| Lymphoma (primary and secondary) | ||
| Metastases |
| Rare Solid Pancreatic Lesions | No. of Cases/Incidence/Prevalence |
|---|---|
| Intrapancreatic splenic tissue | 61 cases/3000 autopsies |
| Tuberculosis | 116 cases |
| Solid serous cystadenoma | 22 cases |
| Solid pseudopapillary tumour | 2% of all exocrine pancreatic neoplasms |
| Schwannoma | <80 cases reported |
| Purely intraductal neuroendocrine tumour | 7 cases reported |
| Fibrous solitary tumour | 29 cases reported |
| Acinar cell carcinoma | <2% of all primary pancreatic neoplasms |
| Undifferentiated carcinoma with osteoclasic-like giant cells | <1% of all malignant pancreatic neoplasms |
| Adenosquamous carcinoma | 0.38–10% prevalence |
| Colloid carcinoma | 1% of all pancreatic tumours |
| Primary leiomyosarcoma | 0.1% of malignant pancreatic neoplasms |
| Primary lymphoma | <0.5% of all primary pancreatic neoplasms, 1% of all extranodal lymphomas |
| Secondary lymphoma | 30% cases of extranodal lymphoma |
| Metastases | 2–5% of pancreatic malignancies |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veron Sanchez, A.; Santamaria Guinea, N.; Cayon Somacarrera, S.; Bennouna, I.; Pezzullo, M.; Bali, M.A. Rare Solid Pancreatic Lesions on Cross-Sectional Imaging. Diagnostics 2023, 13, 2719. https://doi.org/10.3390/diagnostics13162719
Veron Sanchez A, Santamaria Guinea N, Cayon Somacarrera S, Bennouna I, Pezzullo M, Bali MA. Rare Solid Pancreatic Lesions on Cross-Sectional Imaging. Diagnostics. 2023; 13(16):2719. https://doi.org/10.3390/diagnostics13162719
Chicago/Turabian StyleVeron Sanchez, Ana, Nuria Santamaria Guinea, Silvia Cayon Somacarrera, Ilias Bennouna, Martina Pezzullo, and Maria Antonietta Bali. 2023. "Rare Solid Pancreatic Lesions on Cross-Sectional Imaging" Diagnostics 13, no. 16: 2719. https://doi.org/10.3390/diagnostics13162719
APA StyleVeron Sanchez, A., Santamaria Guinea, N., Cayon Somacarrera, S., Bennouna, I., Pezzullo, M., & Bali, M. A. (2023). Rare Solid Pancreatic Lesions on Cross-Sectional Imaging. Diagnostics, 13(16), 2719. https://doi.org/10.3390/diagnostics13162719






