The Value of Ultrasonic Elastography in Detecting Placental Stiffness for the Diagnosis of Preeclampsia: A Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. The Literature Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Data Analysis
3. Results
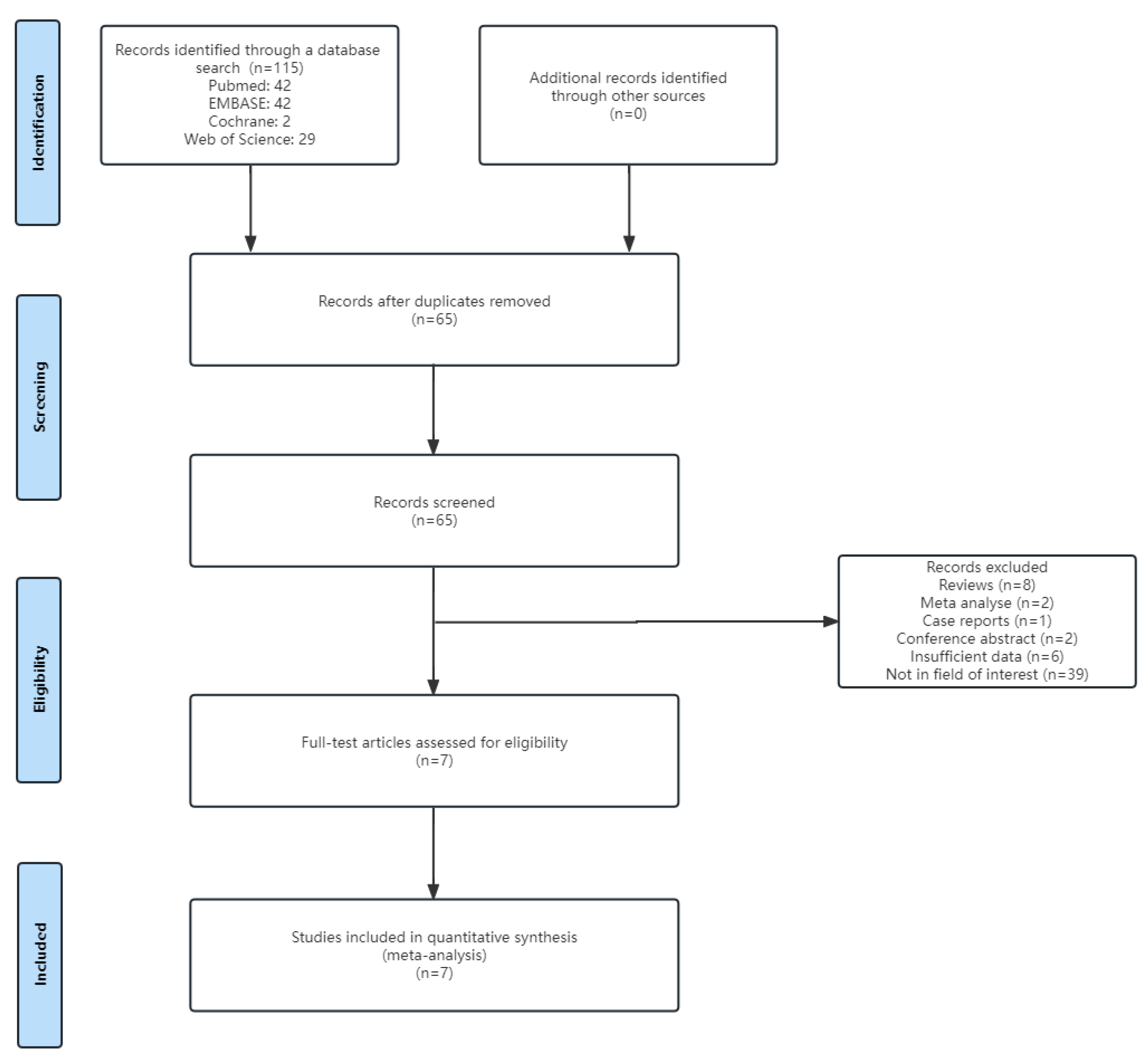
3.1. Literature Search
3.2. Basic Characteristics of Ultrasound Technology and Literature
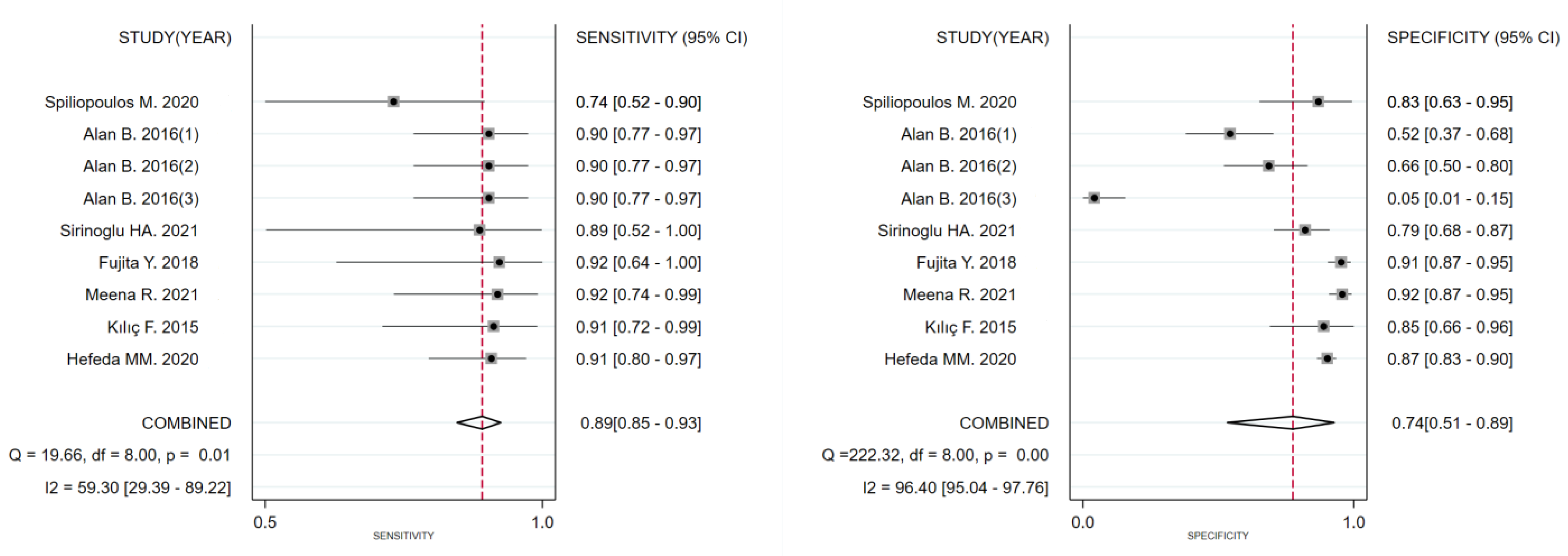
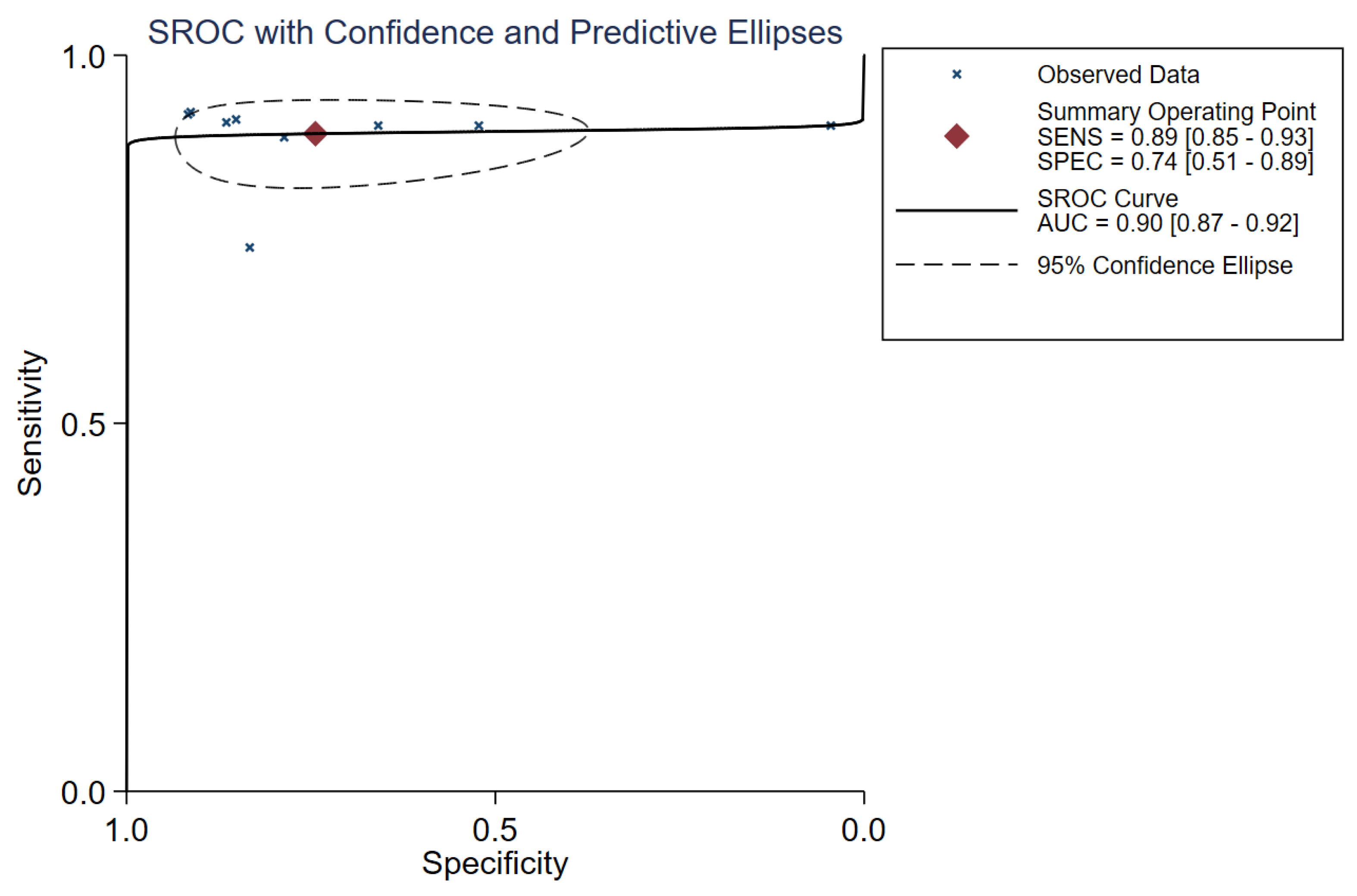
3.3. Diagnostic Performance
3.4. Sensitivity Analysis and Meta-Regression Analysis
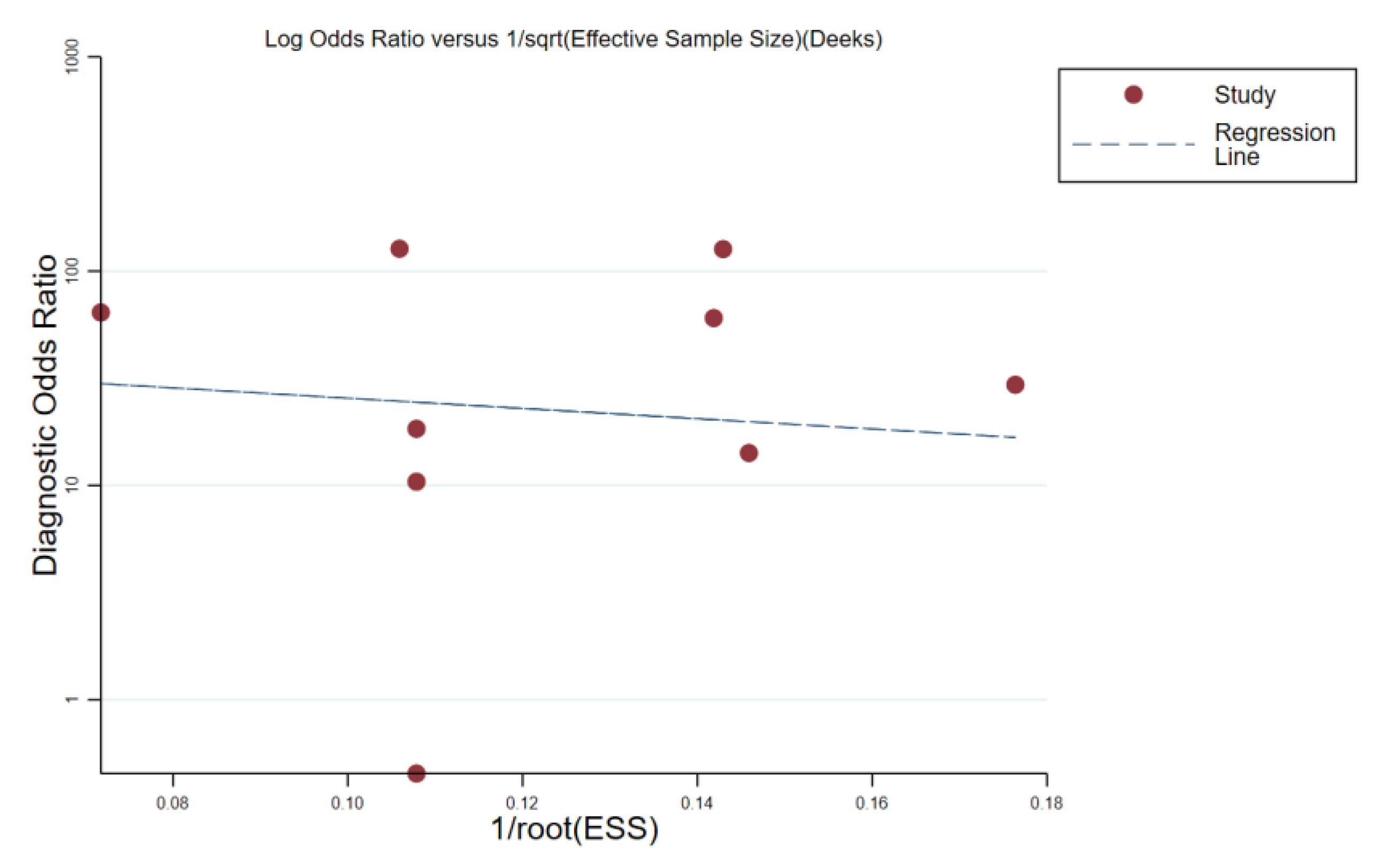
3.5. Publication Bias Assessment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Maltepe, E.; Bakardjiev, A.I.; Fisher, S.J. The placenta: Transcriptional, epigenetic, and physiological integration during development. J. Clin. Investig. 2010, 120, 1016–1025. [Google Scholar] [CrossRef]
- Maltepe, E.; Fisher, S.J. Placenta: The forgotten organ. Annu. Rev. Cell Dev. Biol. 2015, 31, 523–552. [Google Scholar] [CrossRef]
- Red-Horse, K.; Kapidzic, M.; Zhou, Y.; Feng, K.T.; Singh, H.; Fisher, S.J. EPHB4 regulates chemokine-evoked trophoblast responses: A mechanism for incorporating the human placenta into the maternal circulation. Development 2005, 132, 4097–4106. [Google Scholar] [CrossRef]
- Sato, Y. Endovascular trophoblast and spiral artery remodeling. Mol. Cell. Endocrinol. 2020, 503, 110699. [Google Scholar] [CrossRef] [PubMed]
- Redman, C.W.G.; Sargent, I.L. Immunology of pre-eclampsia. Am. J. Reprod. Immunol. 2010, 63, 534–543. [Google Scholar] [CrossRef]
- Red-Horse, K.; Rivera, J.; Schanz, A.; Zhou, Y.; Winn, V.; Kapidzic, M.; Maltepe, E.; Okazaki, K.; Kochman, R.; Vo, K.C.; et al. Cytotrophoblast induction of arterial apoptosis and lymphangiogenesis in an in vivo model of human placentation. J. Clin. Investig. 2010, 116, 2643–2652. [Google Scholar] [CrossRef]
- Ridder, A.; Giorgione, V.; Khalil, A.; Thilaganathan, B. Preeclampsia: The relationship between uterine artery blood flow and trophoblast function. Int. J. Mol. Sci. 2019, 20, 3263. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.; Syngelaki, A.; Akolekar, R.; Poon, L.C.; Nicolaides, K.H. Competing risks model in screening for preeclampsia by maternal characteristics and medical history. Am. J. Obstet. Gynecol. 2015, 213, 62.e1–62.e10. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J. Recent biomarkers for the identification of patients at risk for preeclampsia: The role of uteroplacental ischemia. Expert. Opin. Med. Diag. 2012, 6, 121–130. [Google Scholar] [CrossRef]
- Cnossen, J.S.; Morris, R.K.; Ter Riet, G.; Mol, B.W.; Van Der Post, J.A.; Coomarasamy, A.; Zwinderman, A.H.; Robson, S.C.; Bindels, P.J.E.; Kleijnen, J.; et al. Use of uterine artery doppler ultrasonography to predict preeclampsia and intrauterine growth restriction: A systematic review and bivariable meta-analysis. Can. Med. Assoc. J. 2008, 178, 701–711. [Google Scholar] [CrossRef]
- Zhu, Y.C.; Zhang, Y.; Shan, J.; Deng, S.H.; Shi, X.R.; Jiang, Q. Added value of superb microvascular imaging and virtual touch imaging quantification in assisting thyroid cancer classification. Ultrasound Med. Biol. 2021, 47, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Berzigotti, A.; Tsochatzis, E.; Boursier, J.; Castera, L.; Cazzagon, N.; Friedrich-Rust, M.; Petta, S.; Thiele, M. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis—2021 update. J. Hepatol. 2021, 75, 659–689. [Google Scholar] [CrossRef]
- Tekcan Sanli, D.E.; Yildirim, D.; Kandemirli, S.G.; Sanli, A.N.; Aribal, E. Evaluation of multiparametric shear wave elastography indices in malignant and benign breast lesions. Acad. Radiol. 2022, 29 (Suppl. S1), S50–S61. [Google Scholar] [CrossRef]
- Ferraioli, G.; Wong, V.W.-S.; Castera, L.; Berzigotti, A.; Sporea, I.; Dietrich, C.F.; Choi, B.I.; Wilson, S.R.; Kudo, M.; Barr, R.G. Liver ultrasound elastography: An update to the world federation for ultrasound in medicine and biology guidelines and recommendations. Ultrasound Med. Biol. 2018, 44, 2419–2440. [Google Scholar] [CrossRef]
- Tapper, E.B.; Loomba, R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Bora Makal, G.; Aslan, A. The diagnostic value of the American College of Radiology thyroid imaging reporting and data system classification and Shear-Wave elastography for the differentiation of thyroid nodules. Ultrasound Med. Biol. 2021, 47, 1227–1234. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Wei, Z.T.; Yan, R.L.; Zhang, Y.L. Detection of placenta elasticity modulus by quantitative real-time shear wave imaging. Clin. Exp. Obstet. Gynecol. 2012, 39, 470–473. [Google Scholar]
- Sugitani, M.; Fujita, Y.; Yumoto, Y.; Fukushima, K.; Takeuchi, T.; Shimokawa, M.; Kato, K. A new method for measurement of placental elasticity: Acoustic radiation force impulse imaging. Placenta 2013, 34, 1009–1013. [Google Scholar] [CrossRef] [PubMed]
- Edwards, C.; Cavanagh, E.; Kumar, S.; Clifton, V.; Fontanarosa, D. The use of elastography in placental research—A literature review. Placenta 2020, 99, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Abeysekera, J.M.; Ma, M.; Pesteie, M.; Terry, J.; Pugash, D.; Hutcheon, J.A.; Mayer, C.; Lampe, L.; Salcudean, S.; Rohling, R. SWAVE imaging of placental elasticity and viscosity: Proof of concept. Ultrasound Med. Biol. 2017, 43, 1112–1124. [Google Scholar] [CrossRef]
- Deeba, F.; Hu, R.; Lessoway, V.; Terry, J.; Pugash, D.; Hutcheon, J. SWAVE 2.0 imaging of placental elasticity and viscosity: Potential biomarkers for placenta-mediated disease detection. Ultrasound Med. Biol. 2022, 48, 2486–2501. [Google Scholar] [CrossRef]
- Sigrist, R.M.S.; Liau, J.; Kaffas, A.E.; Chammas, M.C.; Willmann, J.K. Ultrasound elastography: Review of techniques and clinical applications. Theranostics 2017, 7, 1303–1329. [Google Scholar] [CrossRef]
- Bamber, J.; Cosgrove, D.; Dietrich, C.F.; Fromageau, J.; Bojunga, J.; Calliada, F.; Cantisani, V.; Correas, J.M.; D’Onofrio, M.; Drakonaki, E.E.; et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography. Part 1: Basic principles and technology. Ultraschall Med. 2013, 34, 169–184. [Google Scholar] [CrossRef]
- Spiliopoulos, M.; Kuo, C.-Y.; Eranki, A.; Jacobs, M.; Rossi, C.T.; Iqbal, S.N.; Fisher, J.P.; Fries, M.H.; Kim, P.C.W. Characterizing placental stiffness using ultrasound Shear-Wave elastography in healthy and preeclamptic pregnancies. Arch. Gynecol. Obstet. 2020, 302, 1103–1112. [Google Scholar] [CrossRef]
- Alan, B.; Tunç, S.; Agacayak, E.; Bilici, A. Diagnosis of pre-eclampsia and assessment of severity through examination of the placenta with acoustic radiation force impulse elastography. Int. J. Gynecol. Obstet. 2016, 135, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Sirinoglu, H.A.; Uysal, G.; Nazik, H.; Cingillioglu, B.; Genc, S.; Pekin, O. Efficacy of shear wave elastography in predicting preeclampsia in the first trimester. Rev. Assoc. Med. Bras. 2021, 67, 1558–1563. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Nakanishi, T.O.; Sugitani, M.; Kato, K. Placental elasticity as a new non-invasive predictive marker of pre-eclampsia. Ultrasound Med. Biol. 2019, 45, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Meena, R.; Malik, A.; Jain, S.; Batra, A. Placental elastography in second trimester preeclampsia prediction: A prospective study. Ultrasound 2022, 30, 228–235. [Google Scholar] [CrossRef]
- Kilic, F.; Kayadibi, Y.; Yuksel, M.A.; Adaletli, I.; Ustabasioglu, F.E.; Oncul, M.; Madazli, R.; Yilmaz, M.H.; Mihmanli, I.; Kantarci, F. Shear wave elastography of placenta: In vivo quantitation of placental elasticity in preeclampsia. Diagn. Interv. Radiol. 2015, 21, 202–207. [Google Scholar] [CrossRef]
- Hefeda, M.M.; Zakaria, A. Shear wave velocity by quantitative acoustic radiation force impulse in the placenta of normal and high-risk pregnancy. Egypt. J. Radiol. Nucl. Med. 2020, 51, 131. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gotzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ 2009, 339, b2700. [Google Scholar] [CrossRef]
- Whiting, P.F.; Rutjes, A.W.S.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.G.; Sterne, J.A.C.; Bossuyt, P.M.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Deeks, J.J.; Macaskill, P.; Irwig, L. The performance of tests of publication bias and other sample size effects in systematic reviews of diagnostic test accuracy was assessed. J. Clin. Epidemiol. 2005, 58, 882–893. [Google Scholar] [CrossRef] [PubMed]
- Phipps, E.A.; Thadhani, R.; Benzing, T.; Karumanchi, S.A. Pre-eclampsia: Pathogenesis, novel diagnostics and therapies. Nat. Rev. Nephrol. 2019, 15, 275–289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Meikle, S.; Trumble, A. Severe maternal morbidity associated with hypertensive disorders in pregnancy in the United States. Hypertens. Pregnancy 2003, 22, 203–212. [Google Scholar] [CrossRef]
- Hall, T.J.; Milkowski, A.; Garra, B.; Carson, P.; Palmeri, M.; Nightingale, K. RSNA/QIBA: Shear wave speed as a biomarker for liver fibrosis staging. In Proceedings of the 2013 IEEE International Ultrasonics Symposium (IUS), Prague, Czech Republic, 21–25 July 2013; pp. 397–400. [Google Scholar]
- Edwards, C.; Cavanagh, E.; Kumar, S.; Clifton, V.L.; Borg, D.J.; Priddle, J.; Marie-Luise, W.; Drovandi, C.; Fontanarosa, D. Relationship between placental elastography, maternal pre-pregnancy body mass index and gestational weight gain. Placenta 2022, 121, 1–6. [Google Scholar] [CrossRef]
- Wu, S.; Nan, R.; Li, Y.; Cui, X.; Liang, X.; Zhao, Y. Measurement of elasticity of normal placenta using the Virtual Touch quantification technique. Ultrasonography 2016, 35, 253–257. [Google Scholar] [CrossRef]
| Number | Author | Year | Country | Study Design | Study Period | Position of Placenta | Trimester of Pregnancy | Group | n | Mean/Median Age | Age Range | BMI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Spiliopoulos M. [21] | 2020 | USA | NA | Jan 2017 to Jun 2017 | Not posterior placental position | Second and third trimesters | PE group | 23 | 21 ± 2 | NA | 35 ± 2 |
| Control group | 24 | 29 ± 1 | NA | 33 ± 1 | ||||||||
| 2 | Alan B. [22] | 2016 | Turkey | Prospective study | Aug 2014 to Mar 2015 | Not posterior placental position | Second and third trimesters | PE group | 42 | 32 | 28–38 | NA |
| Control group | 44 | 31 | 29–37 | NA | ||||||||
| 3 | Sirinoglu HA. [23] | 2021 | Turkey | Prospective study | May 2019 to Dec 2019 | NA | First trimester | PE group | 9 | 27 ± 7 | NA | 26 ± 4 |
| Control group | 75 | 30 ± 4 | NA | 25 ± 3 | ||||||||
| 4 | Fujita Y. [24] | 2018 | Japan | NA | Feb 2014 to Mar 2017 | Anterior wall | Second and third trimesters | PE group | 13 | NA | NA | NA |
| Control group | 208 | NA | NA | NA | ||||||||
| 5 | Meena R. [25] | 2021 | India | Prospective study | Oct 2016 to Jun 2018 | Not posterior placental position | Second trimester | PE group | 25 | 24 | NA | NA |
| Control group | 205 | 25 | NA | NA | ||||||||
| 6 | Kılıç F. [26] | 2015 | Turkey | NA | Jan 2013 to Jun 2013 | Not posterior placental position | Second and third trimesters | PE group | 23 | 34 ± 5 | 22–45 | NA |
| Control group | 27 | 33. ± 5 | 26–42 | NA | ||||||||
| 7 | Hefeda MM. [27] | 2020 | Egypt | Prospective study | Oct 2018 to Mar 2020 | No restriction | Second trimester | PE group | 9 | 25 ± 7 | 18–37 | NA |
| Control group | 151 | 23 ± 6 | NA | |||||||||
| Third trimester | PE group | 46 | 27 ± 6 | 19–42 | NA | |||||||
| Control group | 264 | 24 ± 2 | NA |
| Number | Author | Test Device | Technique | Probe | No. of Measurements | Measurer Blinding | Area of ROI | Depth of Target Tissue |
|---|---|---|---|---|---|---|---|---|
| 1 | Spiliopoulos M. [21] | Aixplorer | 2D-SWE | XC6-1 (1–6 MHz) | NA | YES | NA | NA |
| 2 | Alan B. [22] | ACUSON S2000 | P-SWE | 4C1 (2–5 MHz) | 4 | NA | 10 × 6 mm | <8 cm |
| 3 | Sirinoglu HA. [23] | Samsung HS70A ultrasound system | 2D-SWE | CA1-7A (1–7 MHz) | 1 | NA | 10 × 10 mm | NA |
| 4 | Fujita Y. [24] | ACUSON S2000 | P-SWE | 4C1 (2–5 MHz) | NA | NA | 10 × 5 mm | 1–6 cm |
| 5 | Meena R. [25] | ElastPQ | 2D-SWE | ElastPQ (2–5 MHz) | 1 | NA | NA | <8 cm |
| 6 | Kılıç F. [26] | Aixplorer | 2D-SWE | XC6-1 (1–6 MHz) | 1 | YES | 5 × 5 mm | NA |
| 7 | Hefeda MM. [27] | ACUSON S3000 | P-SWE | 4C1 (2–5 MHz) | NA | NA | 10 × 5 mm | 2–8 cm |
| Number | Author | Gestational Weeks | Trimester of Pregnancy | Technique | Representative Values | PE Group | Control Group | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | PSM | Range | n | PSM | Range | ||||||
| 1 | Spiliopoulos M. [21] | >20 | Second and third trimesters | 2D-SWE | Mean | 23 | 22 ± 3 kPa | NA | 24 | 11 ± 2 kPa | NA |
| 2 | Alan B. [22] | 27–35 | Second and third trimesters | P-SWE | Mean | 42 | 1.4 m/s | 1.3–1.5 | 44 | 1.1 m/s | 1.00–1.1 |
| 3 | Sirinoglu HA. [23] | 23–37 | First trimester | 2D-SWE | Mean | 9 | 6 ± 2 kPa | 2–14 | 75 | 9 ± 3 kPa | 3–12 |
| 4 | Fujita Y. [24] | 16–32 | Second and third trimesters | P-SWE | Median | 13 | 1.4 m/s | 1.1–2.4 | 208 | NA | NA |
| 5 | Meena R. [25] | 16–20 | Second trimester | 2D-SWE | Mean | 25 | 5 kPa | NA | 205 | 3 kPa | NA |
| 6 | Kılıç F. [26] | 23–37 | Second and third trimesters | 2D-SWE | Median | 23 | 21 kPa | 2–71 | 27 | 4 kPa | 2–14 |
| 7 | Hefeda MM. [27] | >18 | Second trimester | P-SWE | Mean | 9 | 2.1 ± 1.5 m/s | NA | 46 | 0.9 ± 0.4 m/s | NA |
| Third trimester | P-SWE | Mean | 46 | 2.2 ± 1.5 m/s | NA | 94 | 0.9 ± 0.6 m/s | NA | |||
| Number | Author | Technique Value | Cutoff Value | AUROC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Spiliopoulos M. [21] | mean SWE value | 16 kPa | 0.82 | 75 | 83 | 82 | 76 |
| 2 | Alan B. [22] | min SWV value | 1.0 m/s | 0.86 | 91 | 47 | 65 | 83 |
| max SWV value | 1.7 m/s | 0.88 | 91 | 67 | 74 | 88 | ||
| mean SWV value | 1.5 m/s | 0.99 | 91 | 5 | 50 | 33 | ||
| 3 | Sirinoglu HA. [23] | mean SWE value | 7 kPa | 0.82 | 89 | 79 | 81 | 88 |
| 4 | Fujita Y. [24] | mean SWV value | 1.2 m/s | 0.91 | 92 | 91 | 40 | 99 |
| 5 | Meena R. [25] | mean SWE value | 3 kPa | 0.97 | 92 | 92 | 58 | 99 |
| 6 | Kılıç F. [26] | median SWE value | 7 kPa | 0.90 | 90 | 86 | 82 | 92 |
| 7 | Hefeda MM. [27] | mean SWV value | 1.4 m/s | 0.91 | 91 | 86 | 73 | 75 |
| Subgroup | Number of Studies | Pooled Sensitivity (95% CI) | Pooled Specificity (95% CI) | Pooled DOR (95% CI) | Pooled AUROC (95% CI) | p-Value | |
|---|---|---|---|---|---|---|---|
| Representative values | Mean | 7 | 0.89 (0.84–0.92) | 0.68 (0.39–0.88) | 17 (5–59) | 0.89 (0.86–0.92) | 0.76 |
| Median | 2 | 0.92 (0.78–0.98) | 0.91 (0.86–0.94) | 82 (21–323) | NA | ||
| Publication year | ≥2020 | 4 | 0.88 (0.82–0.92) | 0.878 (0.830–0.914) | 52 (28–96) | 0.94 (0.91–0.96) | 0.31 |
| <2020 | 5 | 0.91 (0.85–0.95) | 0.60 (0.23–0.89) | 15 (3–88) | 0.91 (0.88–0.93) | ||
| Total number of cases | ≥100 | 3 | 0.91 (0.84–0.96) | 0.89 (0.87–0.91) | 83 (39–177) | NA | 0.14 |
| <100 | 6 | 0.88 (0.82–0.92) | 0.61 (0.30–0.85) | 12 (3–39) | 0.88 (0.85–0.91) | ||
| US elastography techniques | 2D-SWE | 4 | 0.86 (0.75–0.930) | 0.86 (0.78–0.91) | 38 (13–110) | 0.93 (0.90–0.95) | 0.64 |
| P-SWE | 5 | 0.91 (0.86–0.94) | 0.61 (0.23–0.89) | 15 (3–87) | 0.91 (0.88–0.93) | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, S.; Huang, Y.; Luo, W.; Li, S. The Value of Ultrasonic Elastography in Detecting Placental Stiffness for the Diagnosis of Preeclampsia: A Meta-Analysis. Diagnostics 2023, 13, 2894. https://doi.org/10.3390/diagnostics13182894
Su S, Huang Y, Luo W, Li S. The Value of Ultrasonic Elastography in Detecting Placental Stiffness for the Diagnosis of Preeclampsia: A Meta-Analysis. Diagnostics. 2023; 13(18):2894. https://doi.org/10.3390/diagnostics13182894
Chicago/Turabian StyleSu, Shanshan, Yanyan Huang, Weiwen Luo, and Shaohui Li. 2023. "The Value of Ultrasonic Elastography in Detecting Placental Stiffness for the Diagnosis of Preeclampsia: A Meta-Analysis" Diagnostics 13, no. 18: 2894. https://doi.org/10.3390/diagnostics13182894
APA StyleSu, S., Huang, Y., Luo, W., & Li, S. (2023). The Value of Ultrasonic Elastography in Detecting Placental Stiffness for the Diagnosis of Preeclampsia: A Meta-Analysis. Diagnostics, 13(18), 2894. https://doi.org/10.3390/diagnostics13182894






