Microwave Angiography by Ultra-Wideband Sounding: A Preliminary Investigation
Abstract
:1. Introduction
2. Materials and Methods
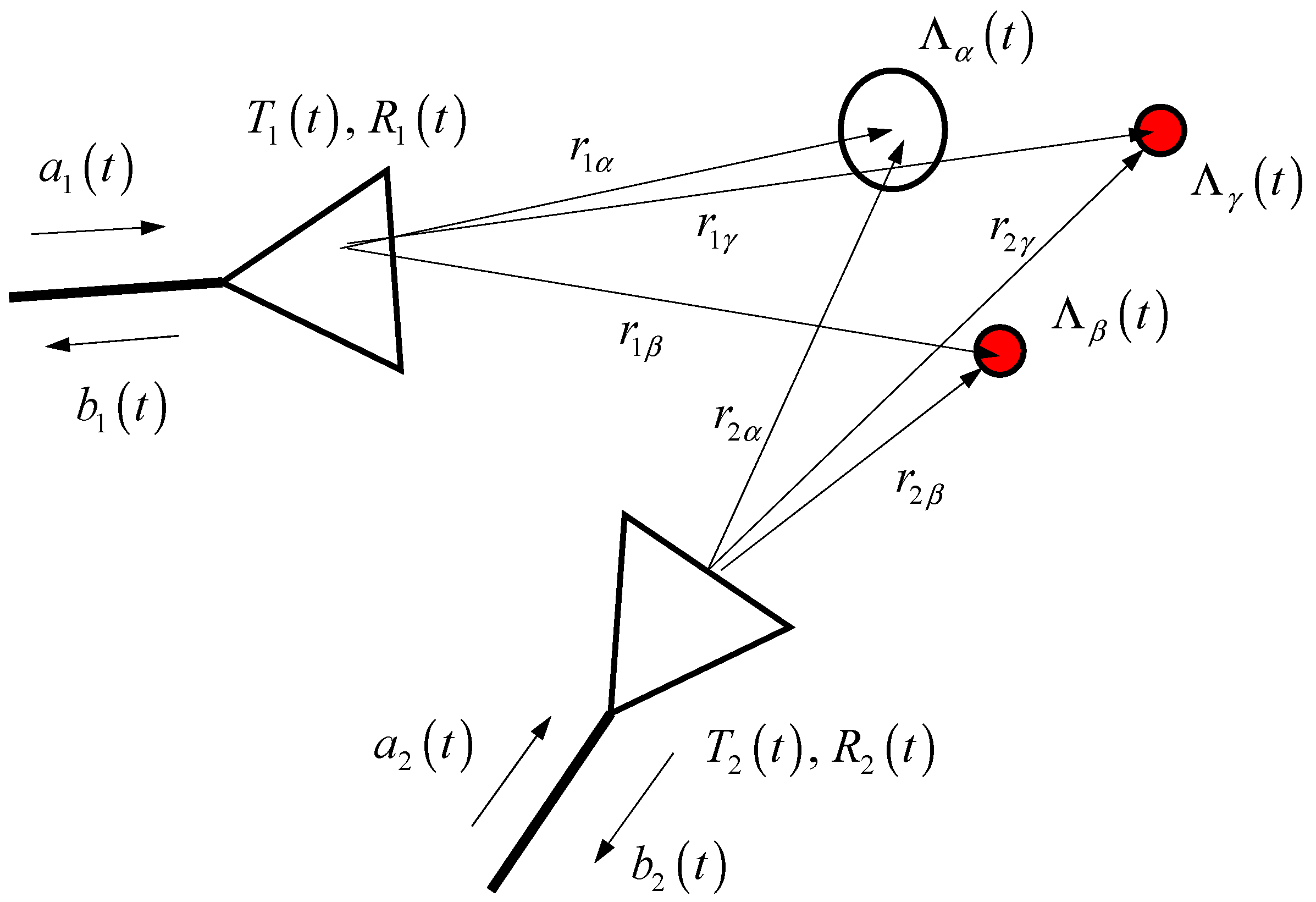
2.1. Scattering Model
2.1.1. Scattering from Static Target
2.1.2. Scattering from Pulsating Artery
2.2. Imaging
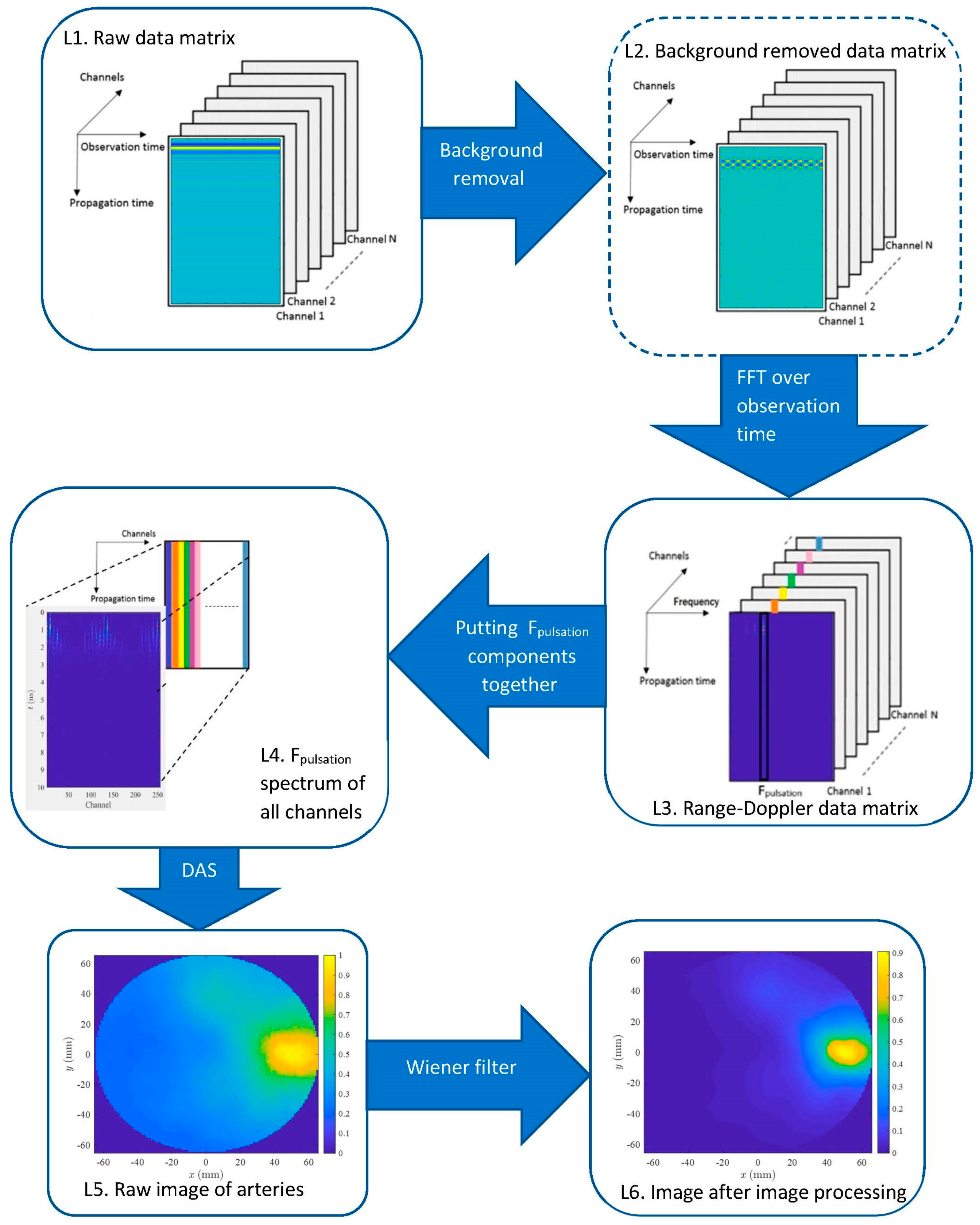
2.2.1. Preprocessing: Background Removal
2.2.2. 2D Range-Doppler Matrix Reconstruction
2.2.3. Delay and Sum of MIMO Signals at the Pulsation Frequency
- A.
- Conventional Delay and Sum
- B.
- Approximated Delay and Sum
2.2.4. Post-Processing: Wiener Filter
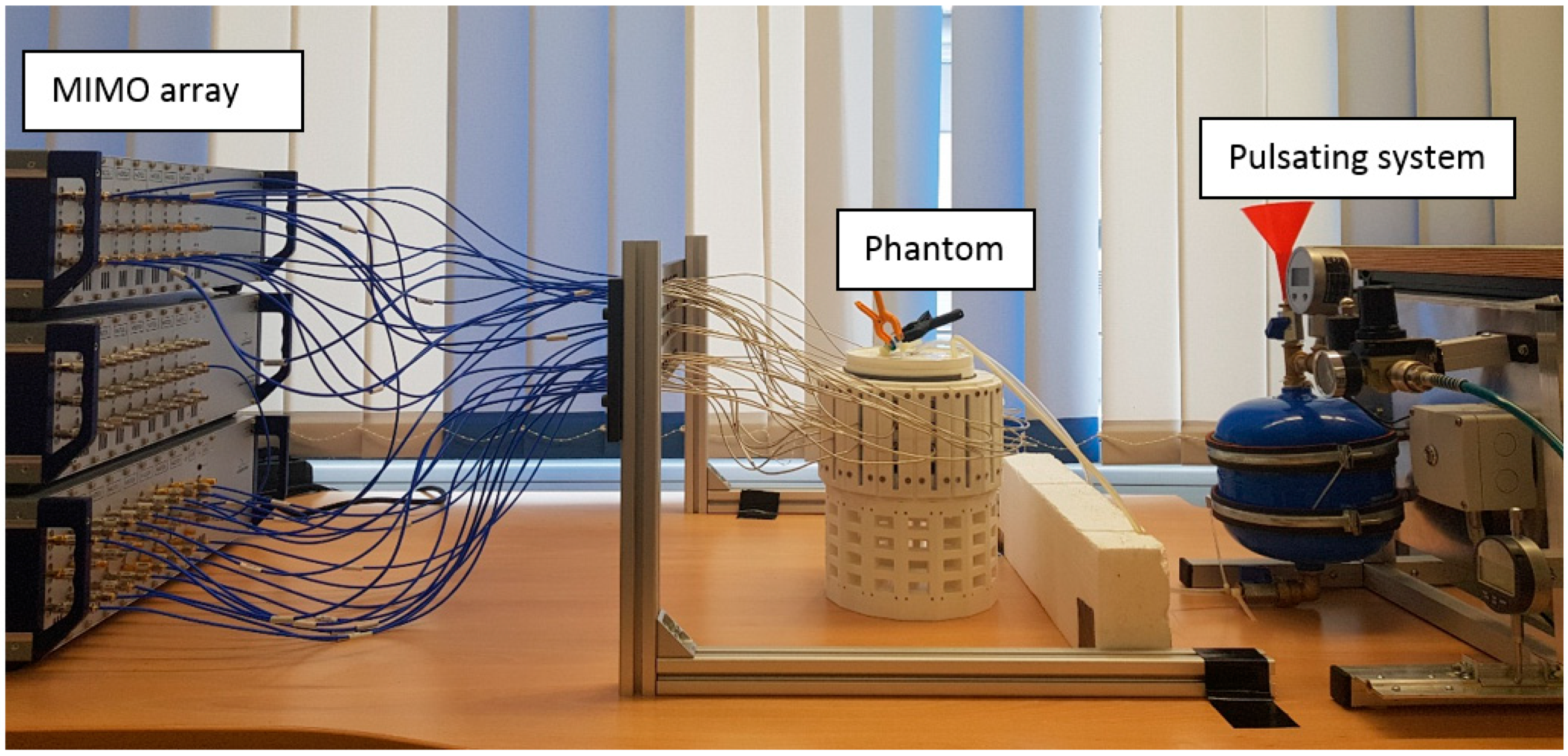
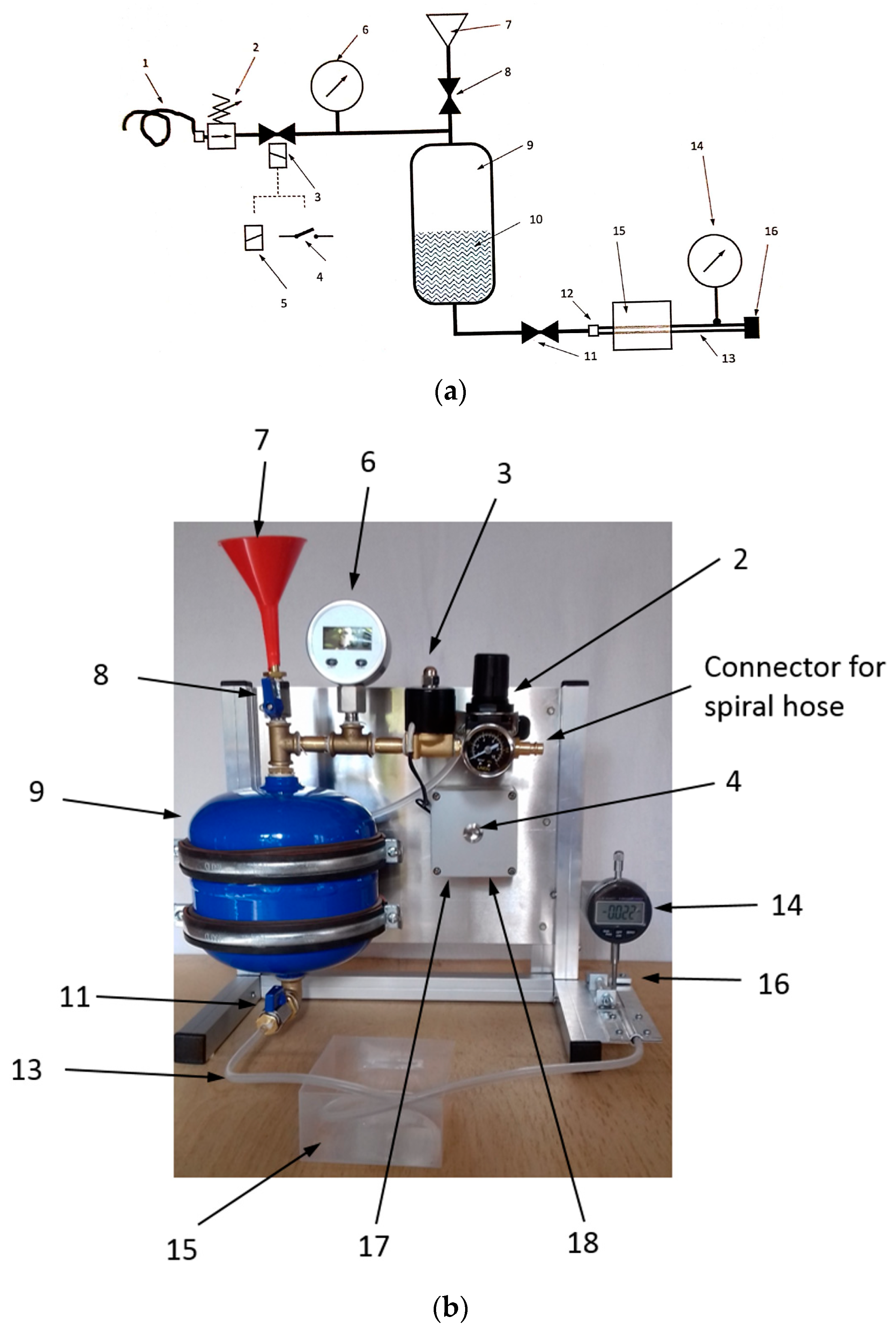
2.3. Experimental Setup
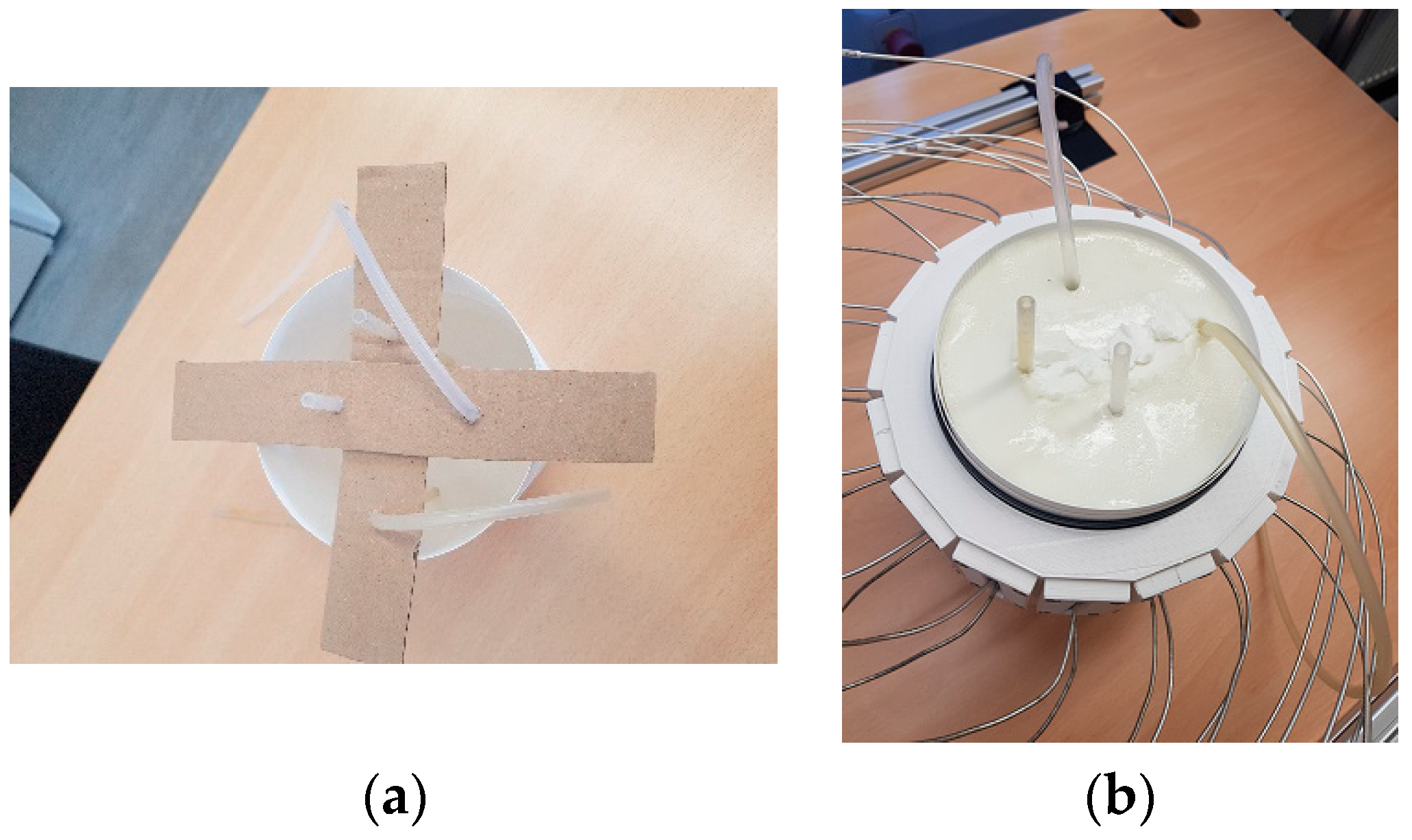
2.3.1. Phantom
2.3.2. MIMO Radar System
2.3.3. Pulsation System
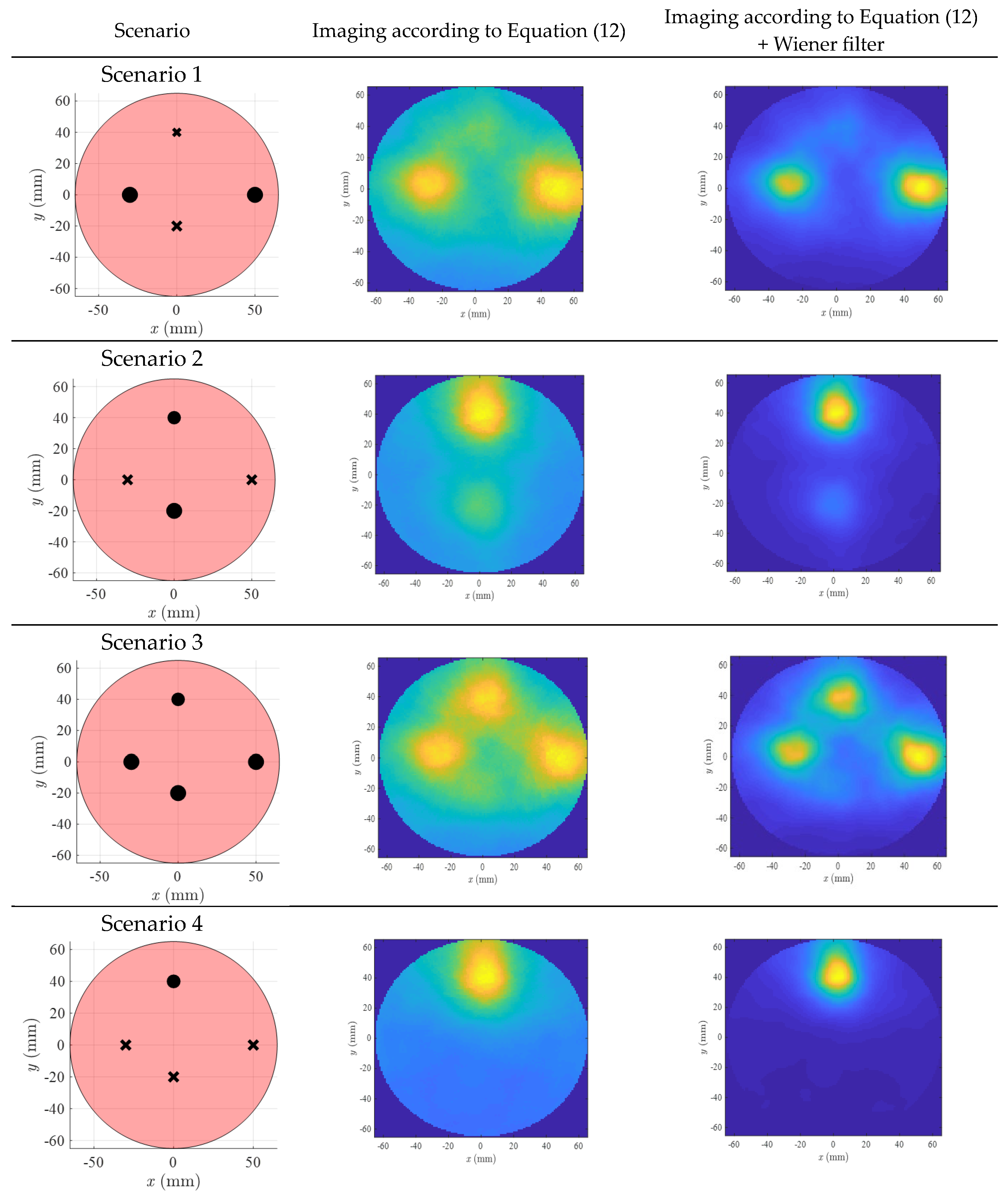
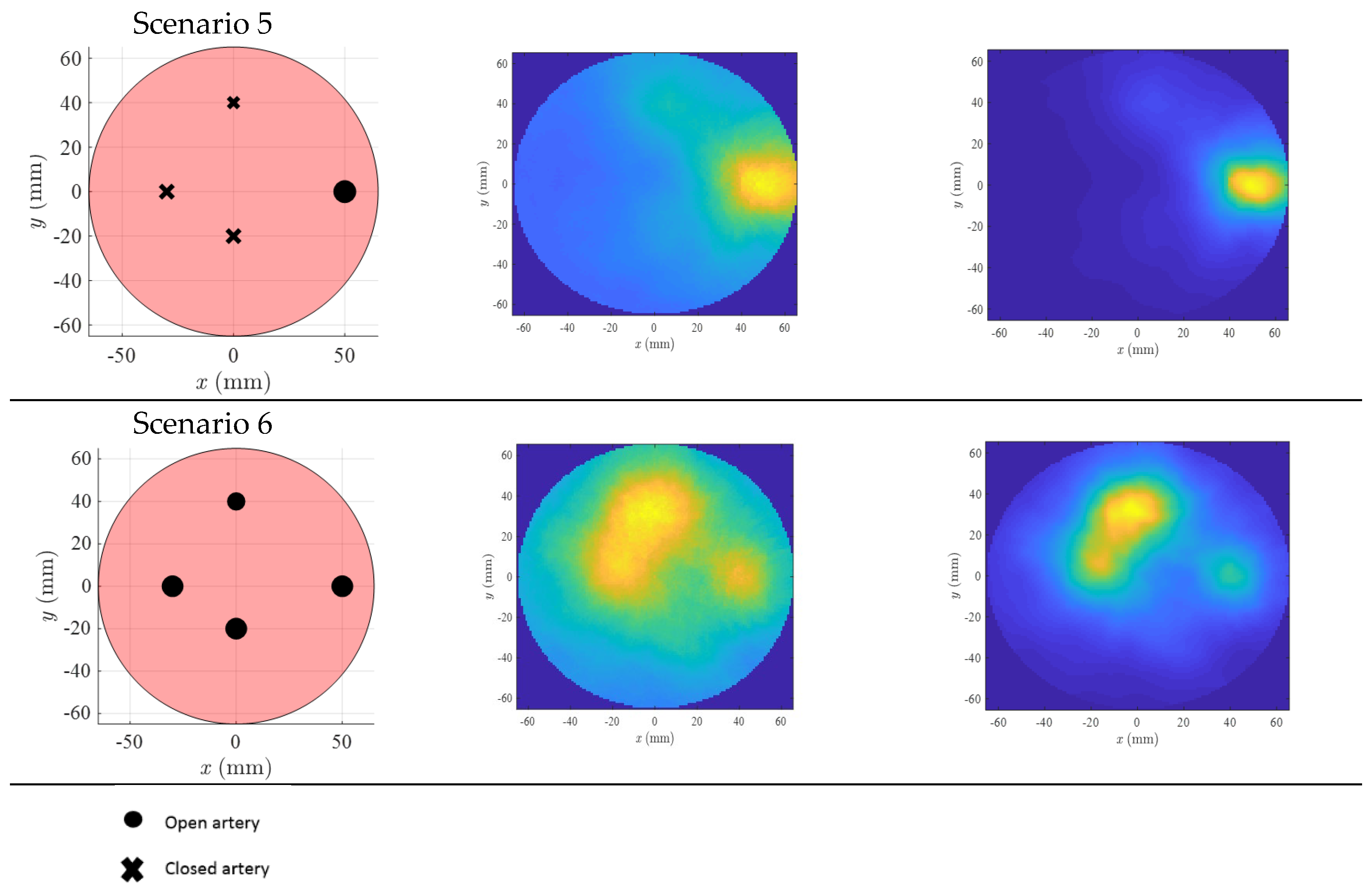
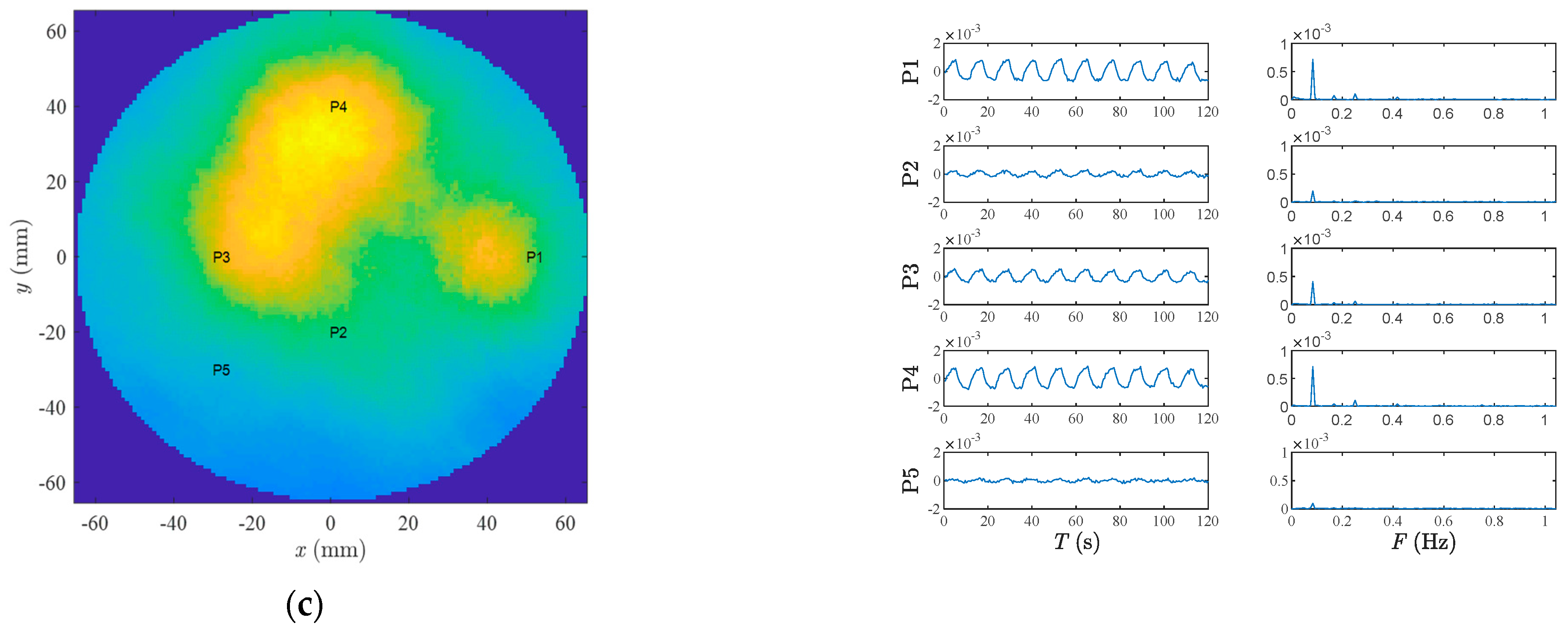
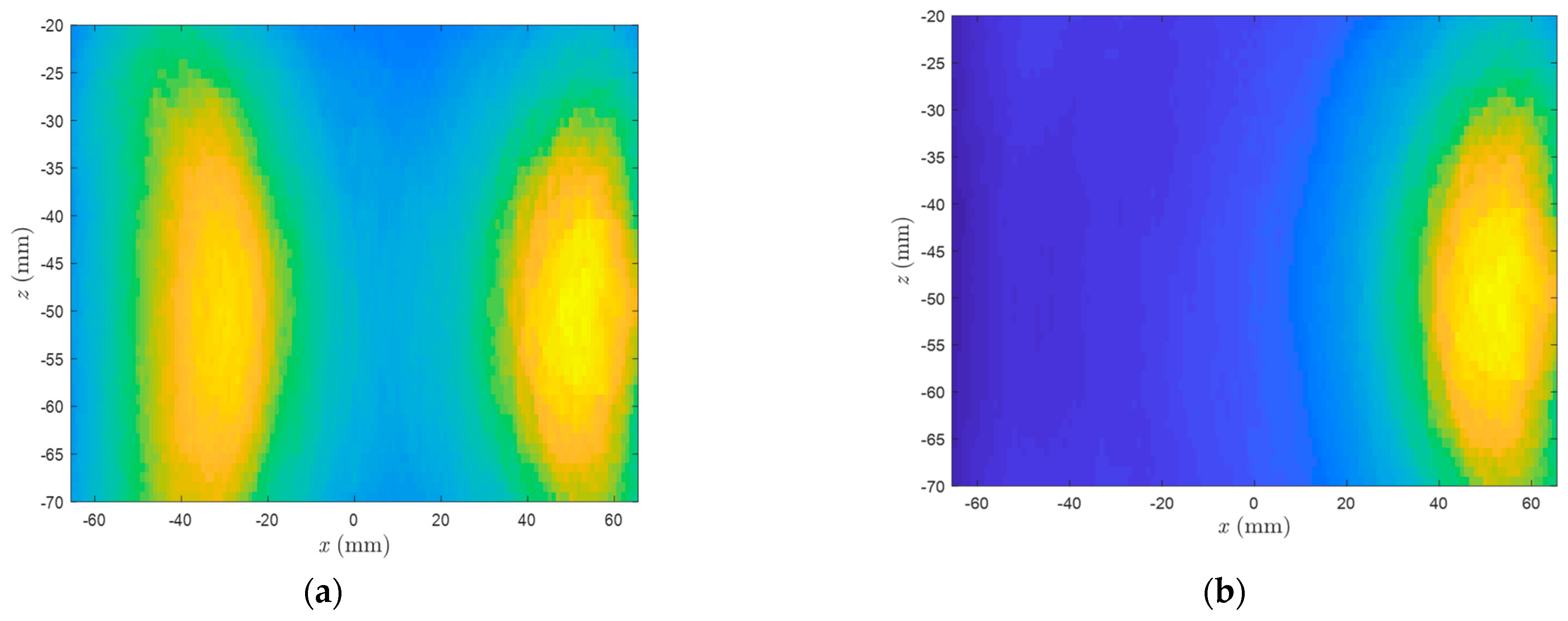
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Nishimiya, K.; Matsumoto, Y.; Shimokawa, H. Recent Advances in Vascular Imaging. Arterioscler. Thromb. Vasc. Biol. 2020, 40, e313–e321. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Sivasubramanian, K.; Mark, C.S.K.; Pramanik, M. Recent developments in vascular imaging techniques in tissue engineering and regenerative medicine. BioMed Res Int. 2015, 2015, 783983. [Google Scholar] [CrossRef] [PubMed]
- Schnell, S.; Wu, C.; Ansari, S.A. 4D MRI flow examinations in cerebral and extracerebral vessels. Ready for clinical routine? Curr. Opin. Neurol. 2016, 29, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Persson, M.; Fhager, A.; Trefná, H.D.; Yu, Y.; McKelvey, T.; Pegenius, G.; Karlsson, J.E.; Elam, M. Microwave-based stroke diagnosis making global prehospital thrombolytic treatment possible. IEEE Trans. Biomed. Eng. 2014, 61, 2806–2817. [Google Scholar] [CrossRef] [PubMed]
- Fhager, A.; Candefjord, S.; Elam, M.; Persson, M. 3D Simulations of itracerebral hemorrhage detection using broadband microwave technology. Sensors 2019, 19, 3482. [Google Scholar] [CrossRef] [PubMed]
- Mobashsher, A.T.; Abbosh, A.M. On-site Rapid Diagnosis of Intracranial Hematoma using Portable Multi-slice Microwave Imaging System. Sci. Rep. 2016, 6, 36720. [Google Scholar] [CrossRef] [PubMed]
- Poltschak, S.; Freilinger, M.; Feger, R.; Stelzer, A.; Hamidipour, A.; Henriksson, T.; Hopfer, M.; Planas, R.; Semenov, S. A multiport vector network analyzer with high-precision and realtime capabilities for brain imaging and stroke detection. Int. J. Microw. Wirel. Technol. 2018, 10, 605–612. [Google Scholar] [CrossRef]
- Razzicchia, E.; Sotiriou, I.; Cano-Garcia, H.; Kallos, E.; Palikaras, G.; Kosmas, P. Feasibility study of enhancing microwave brain imaging using metamaterials. Sensors 2019, 19, 5472. [Google Scholar] [CrossRef]
- Lauteslager, T.; Tømmer, M.; Lande, T.S.; Constandinou, T.G. Coherent UWB Radar-on-Chip for In-Body Measurement of Cardiovascular Dynamics. IEEE Trans. Biomed. Circuits Syst. 2019, 13, 814–824. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Lauteslager, T. UWB radar for non-contact heart rate variability monitoring and mental state classification. In Proceedings of the 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019. [Google Scholar] [CrossRef]
- Brovoll, S.; Berger, T.; Paichard, Y.; Aardal, O.; Lande, T.S.; Hamran, S.E. Time-lapse imaging of human heart motion with switched array UWB radar. IEEE Trans. Biomed. Circuits Syst. 2014, 8, 704–715. [Google Scholar] [CrossRef] [PubMed]
- Lauteslager, T. Coherent Ultra-Wideband Radar-on-Chip for Biomedical Sensing and Imaging Timo Lauteslager. Ph.D. Thesis, Imperial College London, London, UK, 2020. [Google Scholar]
- Lauteslager, T.; Tømmer, M.; Lande, T.S.; Constandinou, T.G. Dynamic Microwave Imaging of the Cardiovascular System Using Ultra-Wideband Radar-on-Chip Devices. IEEE Trans. Biomed. Eng. 2022, 69, 2935–2946. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, M.; Madsen, E.L.; Frank, G.R.; Hagness, S.C. Tissue-mimicking phantom materials for narrowband and ultrawideband microwave applications. Phys. Med. Biol. 2005, 50, 4245–4258. [Google Scholar] [CrossRef] [PubMed]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of Ultrasound in Medicine. Acta Inform. Medica 2011, 19, 168–171. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J.; Ley, S.; Just, T.; Chamaani, S.; Helbig, M. Differential ultra-wideband microwave imaging: Principle application challenges. Sensors 2018, 18, 2136. [Google Scholar] [CrossRef]
- Zamir, M. Hemo-Dynamics; Springer: Cham, Switzerland, 2016; pp. 123–130. [Google Scholar] [CrossRef]
- Nikolova, N.K. Introduction to Microwave Imaging; Cambridge University Press: Cambridge, UK, 2017; pp. 182–235. [Google Scholar] [CrossRef]
- Ilmsens GmbH. Available online: https://www.ilmsens.com/ (accessed on 27 August 2023).
- Prokhorova, A.; Fiser, O.; Vrba, J.; Helbig, M. Microwave Ultra-Wideband Imaging for Non-invasive Temperature Monitoring During Hyperthermia Treatment. In Electromagnetic Imaging for a Novel Generation of Medical Devices; Lecture Notes in Bioengineering; Vipiana, F., Crocco, L., Eds.; Springer: Cham, Switzerland, 2023; pp. 293–330. [Google Scholar] [CrossRef]
- Helbig, M.; Dahlke, K.; Hilger, I.; Kmec, M.; Sachs, J. Design and test of an imaging system for uwb breast cancer detection. Frequenz 2012, 66, 387–394. [Google Scholar] [CrossRef]
- Vo, H.; Lieb, W.; Lorbeer, R.; Grotz, A.; Do, M. Reference values of vessel diameters, stenosis prevalence, and arterial variations of the lower limb arteries in a male population sample using contrast-enhanced MR angiography. PLoS ONE 2018, 20, e0197559. [Google Scholar] [CrossRef]
- Roman, M.J.; Naqvi, T.Z.; Gardin, J.M.; Gerhard-Herman, M.; Jaff, M.; Mohler, E. Clinical Application of Noninvasive Vascular Ultrasound in Cardiovascular Risk Stratification: A Report from the American Society of Echocardiography and the Society of Vascular Medicine and Biology. J. Am. Soc. Echocardiogr. 2006, 19, 943–954. [Google Scholar] [CrossRef] [PubMed]
- Sachs, J. Handbook of Ultra-Wideband Short-Range Sensing—Theory, Sensors, Applications; Wiley-VCH: Berlin, Germany, 2012; pp. 391–404. [Google Scholar]
- Bowman, J.J.; Senior, T.B.A.; Uslenghi, P.L.E. Electromagnetic and Acoustic Scattering by Simple Shapes, Revised Ed.; Hemisph Publishing Corporation: New York, NY, USA, 1987; Volume 747, p. 109. [Google Scholar]
- Tseng, C.C.; Pei, S.C.; Hsia, S.C. Computation of fractional derivatives using Fourier transform and digital FIR differentiator. Signal Process. 2000, 80, 151–159. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chamaani, S.; Sachs, J.; Prokhorova, A.; Smeenk, C.; Wegner, T.E.; Helbig, M. Microwave Angiography by Ultra-Wideband Sounding: A Preliminary Investigation. Diagnostics 2023, 13, 2950. https://doi.org/10.3390/diagnostics13182950
Chamaani S, Sachs J, Prokhorova A, Smeenk C, Wegner TE, Helbig M. Microwave Angiography by Ultra-Wideband Sounding: A Preliminary Investigation. Diagnostics. 2023; 13(18):2950. https://doi.org/10.3390/diagnostics13182950
Chicago/Turabian StyleChamaani, Somayyeh, Jürgen Sachs, Alexandra Prokhorova, Carsten Smeenk, Tim Erich Wegner, and Marko Helbig. 2023. "Microwave Angiography by Ultra-Wideband Sounding: A Preliminary Investigation" Diagnostics 13, no. 18: 2950. https://doi.org/10.3390/diagnostics13182950




