Diagnostic Tools for Cutaneous Leishmaniasis Caused by Leishmania donovani: A Narrative Review
Abstract
:1. Introduction
2. The Diagnostic Tests Recommended by World Health Organization for Cutaneous Leishmaniasis
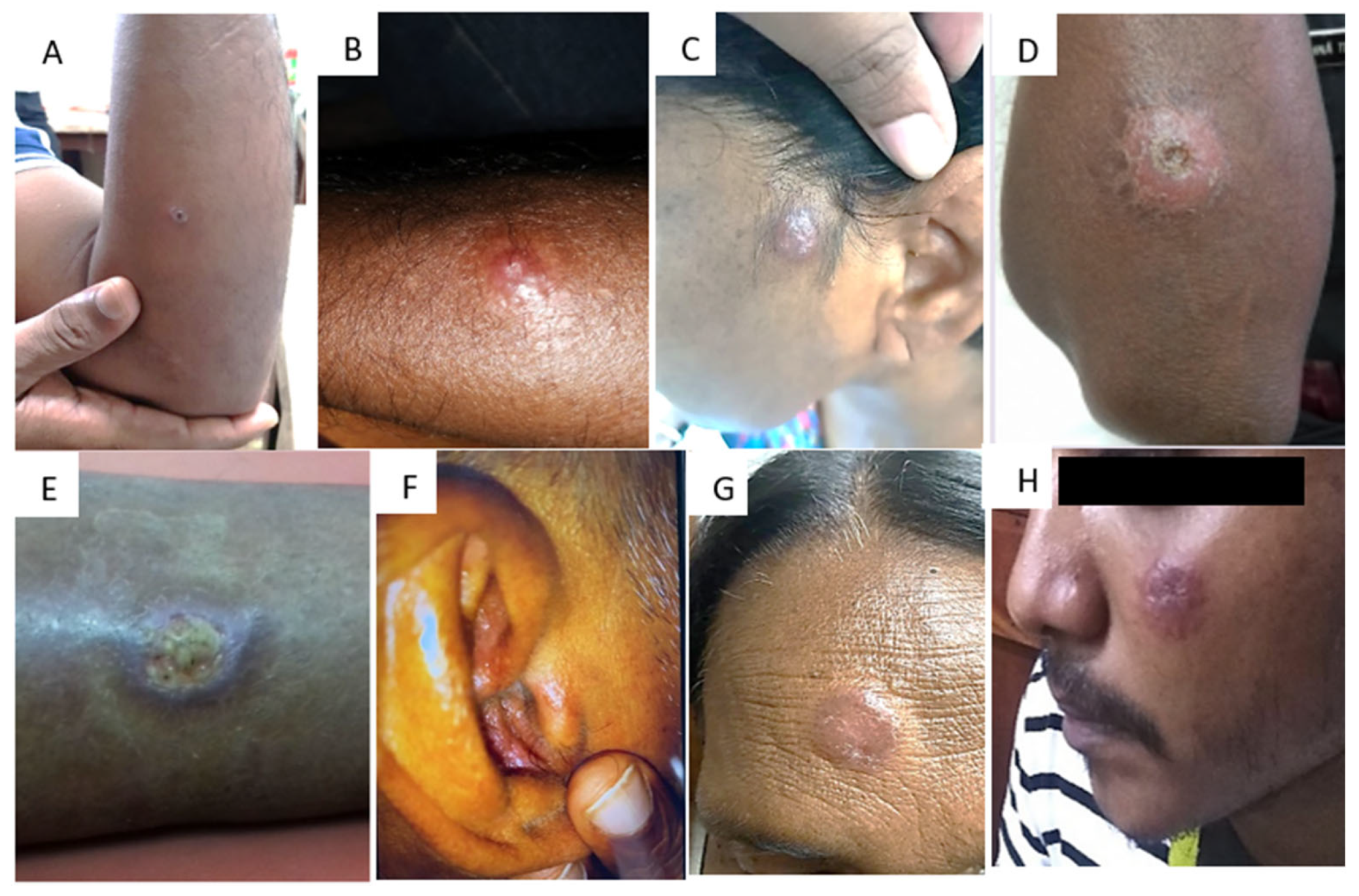
3. Clinical Identification
4. Direct Parasitological Methods for Diagnosis of CL
4.1. Direct Microscopy
4.2. Histopathology
4.3. Identification of Promastigotes by In Vitro Isolation (Parasite Cultures)
4.4. Supplementary Tests to Histopathology
5. Molecular Techniques for Identifying Leishmania Parasites
5.1. Polymerase Chain Reaction (PCR)
5.2. PCR–RFLP
5.3. LAMP
5.4. Recombinase Polymerase Amplification Assay (RPA)
5.5. Multi-Locus Enzyme Electrophoresis (MLEE)
5.6. Multi-Locus Microsatellite Typing (MLMT)
| Methods | Species Determination | Primers | References |
|---|---|---|---|
| PCR based on KDNA amplification | 1. kDNA Leishmania-genus specific 2. kDNA Leishmania donovani-species-specific PCR. | 1. JW11 (forward): 5′-CCTATTTTACACCAACCCCCAGT-3′ JW12 (reverse): 5′-GGGTAGGGGCGTTCTGCGAAA-3′ 2. LdF (forward): 5′-AAATCGGCTCCGAGGCGGGAAAC-3′ LdR (reverse): 5′-GGTACACTCTATCAGTAGCAC-3′ | [7,72] |
| Nested PCR | Leishmania genus-specific primers | outer primers P221: 5′-GGTTCCTTTCCTGATTTACG-3′ P332: 5′-GGCCGGTAAAGGCCGAATAG-3′ inner primers P223: | [73] |
| ITS1 PCR amplification followed by RFLP | Leishmania-genus-specific | Outer primers LITSR 5′-CTGGATCATTTTCCGATG-3′ L5.8S 5′-TGATACCACTTATCGCACTT-3′ Inner primers LITSR inner 5′-CATTTTCCGATGATTACACC-3′ L5.8S inner 5′-TACTGCGTTCTTCAACGA-3′ | [74,75,76] |
| LAMP for kinetoplast minicircle DNA | Leishmania-genus-specific | Primers for first round R 221: 5′GGTTCCTTTCCTGATTTACG3′ R332: 5′GGCCGGTAAAGGCCGAATA3′ Primers for the nested PCR R223: 5′TCCCATCGAACCTCGGTT3′ R333: 5′AAAGCGGGCGCGGTGCTG3′ | [80] |
| Recombinase Polymerase Amplification Assay (RPA) | Leishmania-genus-specific | RPA primers FP3: 5’-ATG GGC CAA AAA CCC AAA CTT TTC TGG TCC TC-3’ RP3: 5’-CTC CAC CCGACC CTA TTT TAC ACC AAC CCC CAG T-3’ Probe: CGC CTC GGA GCC GAT (BHQ1dT) (Tetrahydrofuran) (FAMdT) TGG CAT TTT TGG CTATTT TTT GAA CGG GAT-phosphate) | [81] |
| Multi-locus Enzyme Electrophoresis (MLEE) | Leishmania donovani MON-37 zymodeme | 6PGDH-Forward: AATCGAGCAGCTCAAGGAAG 6PGDH-Reverse: GAGCTTGGCGAGAATCTGAC) | [15] |
| Multi-locus Microsatellite Typing (MLMT) | Leishmania donovani MON-37 zymodeme | The 14 variable microsatellite markers Li 22-35, Li 23-41, Li 41-56, Li 45-24, Li 46-67, Li 71-5/2, Li 71-7, Li 71-33, Lm2TG, Lm4TA, TubCA, CS20, kLIST 7031 and kLIST 7039 were used | [83] |
6. Immunological Techniques
6.1. Enzyme-Linked Immunosorbent Assay (ELISA) Based Diagnostics
6.2. Immunochromatographic Strip Test (ICT)
6.3. Direct Agglutination Test (DAT)
| Methods | Target Antigen | Commercial Product/In-House Assay | References |
|---|---|---|---|
| Enzyme linked immunosorbent assay (ELISA) | rK39 | In-house assay | [26,86,87] |
| KRP42 | In-house assay | [88] | |
| Crude soluble Leishmania donovani antigen | In-house assay | [89,90,91,92] | |
| Immunochromatographic strip test (ICT) | rK39 | Kalazar DetectTM by In Bios International, Inc., Seattle, WA, USA | [93,94] |
| Peroxidoxin | CL DetectTM by In Bios International, Inc., Seattle, WA, USA | [50,95,97] | |
| Direct agglutination test (DAT) | Crude parasite antigen | In-house assay | [99] |
7. Challenges in Diagnosing Atypical Cutaneous Leishmaniasis Caused by Leishmania donovani
8. Innovative Approaches for Diagnosis of Cutaneous Leishmaniasis
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Factsheets. Leishmaniasis. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/leishmaniasis (accessed on 16 June 2023).
- Patel, T.A.; Scadding, G.K.; Phillips, D.E.; Lockwood, D.N. Case report: Old world mucosal leishmaniasis: Report of five imported cases to the hospital for tropical diseases, London, United Kingdom. Am. J. Trop. Med. Hyg. 2017, 97, 1116. [Google Scholar] [CrossRef]
- Thakur, L.; Singh, K.K.; Shanker, V.; Negi, A.; Jain, A.; Matlashewski, G.; Jain, M. Atypical leishmaniasis: A global perspective with emphasis on the Indian subcontinent. PLoS Neglected Trop. Dis. 2018, 12, e0006659. [Google Scholar] [CrossRef]
- World Health Organization Global Health Observatory, Leishmaniasis. 2017. Available online: http://www.who.int/gho/neglected_diseases/leishmaniasis/en/ (accessed on 12 June 2023).
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.D.; WHO Leishmaniasis Control Team. Leishmaniasis worldwide and global estimates of its incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- Gelanew, T.; Hurissa, Z.; Diro, E.; Kassahun, A.; Kuhls, K.; Schönian, G.; Hailu, A. Case report: Disseminated cutaneous leishmaniasis resembling post-kala-azar dermal leishmaniasis caused by Leishmania donovani in three patients co-infected with visceral leishmaniasis and human immunodeficiency virus/acquired immunodeficiency syndrome in Ethiopia. Am. J. Trop. Med. Hyg. 2011, 84, 906. [Google Scholar]
- Lata, S.; Kumari, S.; Das, R.; Pasi, S.; Dhiman, R.C. Typical and atypical cutaneous leishmaniasis in Himachal Pradesh (India). Heliyon 2021, 7, e07282. [Google Scholar] [CrossRef]
- Krayter, L.; Bumb, R.A.; Azmi, K.; Wuttke, J.; Malik, M.D.; Schnur, L.F.; Salotra, P.; Schönian, G. Multilocus microsatellite typing reveals a genetic relationship but, also, genetic differences between Indian strains of Leishmania tropica causing cutaneous leishmaniasis and those causing visceral leishmaniasis. Parasites Vectors 2014, 7, 123. [Google Scholar] [CrossRef]
- Kumar, N.P.; Srinivasan, R.; Anish, T.S.; Nandakumar, G.; Jambulingam, P. Cutaneous leishmaniasis caused by Leishmania donovani in the tribal population of the Agasthyamala Biosphere Reserve forest, Western Ghats, Kerala, India. J. Med. Microbiol. 2015, 64, 157–163. [Google Scholar] [CrossRef]
- Sharma, N.L.; Mahajan, V.K.; Kanga, A.; Sood, A.; Katoch, V.M.; Mauricio, I.; Singh, C.D.; Parwan, U.C.; Sharma, V.K.; Sharma, R.C. Localized cutaneous leishmaniasis due to Leishmania donovani and Leishmania tropica: Preliminary findings of the study of 161 new cases from a new endemic focus in himachal pradesh, India. Am. J. Trop. Med. Hyg. 2005, 72, 819–824. [Google Scholar] [CrossRef]
- Mebrahtu, Y.; Lawyer, P.; Githure, J.; Were, J.B.; Muigai, R.; Hendricks, L.; Leeuwenburg, J.; Koech, D.; Roberts, C. Visceral leishmaniasis unresponsive to pentostam caused by Leishmania tropica in Kenya. Am. J. Trop. Med. Hyg. 1989, 41, 289–294. [Google Scholar] [CrossRef]
- Mebrahtu, Y.B.; Van Eys, G.; Guizani, I.; Lawyer, P.G.; Pamba, H.; Koech, D.; Roberts, C.; Perkins, P.V.; Were, J.B.; Hendricks, L.D. Human cutaneous leishmaniasis caused by Leishmania donovani sl in Kenya. Trans. R. Soc. Trop. Med. Hyg. 1993, 87, 598–601. [Google Scholar] [CrossRef]
- Karunaweera, N.D.; Pratlong, F.; Siriwardane, H.V.Y.D.; Ihalamulla, R.L.; Dedet, J.P. Sri Lankan cutaneous leishmaniasis is caused by Leishmania donovani zymodeme MON-37. Trans. R. Soc. Trop. Med. Hyg. 2003, 97, 380–381. [Google Scholar] [CrossRef]
- Karunaweera, N.D. Leishmania donovani causing cutaneous leishmaniasis in Sri Lanka: A wolf in sheep’s clothing? Trends Parasitol. 2009, 25, 458–463. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Zhang, W.W.; Wickremasinghe, R.; Abeygunasekera, P.; Chandrasekharan, V.; Athauda, S.; Mendis, S.; Hulangamuwa, S.; Matlashewski, G.; Pratlong, F. Leishmania donovani zymodeme MON-37 isolated from an autochthonous visceral leishmaniasis patient in Sri Lanka. Pathog. Glob. Health 2012, 106, 421–424. [Google Scholar] [CrossRef]
- Siriwardana, H.Y.; Noyes, H.A.; Beeching, N.J.; Chance, M.L.; Karunaweera, N.D.; Bates, P.A. Leishmania donovani and cutaneous leishmaniasis, Sri Lanka. Emerg. Infect. Dis. 2007, 13, 476. [Google Scholar] [CrossRef]
- Elamin, E.M.; Guizani, I.; Guerbouj, S.; Gramiccia, M.; El Hassan, A.M.; Di Muccio, T.; Taha, M.A.; Mukhtar, M.M. Identification of Leishmania donovani as a cause of cutaneous leishmaniasis in Sudan. Trans. R. Soc. Trop. Med. Hyg. 2008, 102, 54–57. [Google Scholar] [CrossRef]
- Koliou, M.G.; Antoniou, Y.; Antoniou, M.; Christodoulou, V.; Mazeris, A.; Soteriades, E.S. A cluster of four cases of cutaneous leishmaniasis by Leishmania donovani in Cyprus: A case series. J. Med. Case Rep. 2014, 8, 354. [Google Scholar] [CrossRef]
- Del Giudice, P.; Marty, P.; Lacour, J.P.; Perrin, C.; Pratlong, F.; Haas, H.; Dellamonica, P.; Le Fichoux, Y. Cutaneous leishmaniasis due to Leishmania infantum: Case reports and literature review. Arch. Dermatol. 1998, 134, 193–198. [Google Scholar] [CrossRef]
- Magill, A.J.; Grogl, M.; Gasser, R.A., Jr.; Sun, W.; Oster, C.N. Visceral infection caused by Leishmania tropica in veterans of Operation Desert Storm. N. Engl. J. Med. 1993, 328, 1383–1387. [Google Scholar] [CrossRef]
- Khatri, M.L.; Di Muccio, T.; Fiorentino, E.; Gramiccia, M. Ongoing outbreak of cutaneous leishmaniasis in northwestern Yemen: Clinicoepidemiologic, geographic, and taxonomic study. Int. J. Dermatol. 2016, 55, 1210–1218. [Google Scholar] [CrossRef]
- Khatri, M.L.; Di Muccio, T.; Gramiccia, M. Cutaneous leishmaniasis in North-Western Yemen: A clinicoepidemiologic study and Leishmania species identification by polymerase chain reaction–restriction fragment length polymorphism analysis. J. Am. Acad. Dermatol. 2009, 61, e15–e21. [Google Scholar] [CrossRef]
- Thakur, L.; Singh, K.K.; Kushwaha, H.R.; Sharma, S.K.; Shankar, V.; Negi, A.; Verma, G.; Kumari, S.; Jain, A.; Jain, M. Leishmania donovani infection with atypical cutaneous manifestations, Himachal Pradesh, India, 2014–2018. Emerg. Infect. Dis. 2020, 26, 1864. [Google Scholar] [CrossRef]
- De Vries, H.J.; Schallig, H.D. Cutaneous leishmaniasis: A 2022 updated narrative review into diagnosis and management developments. Am. J. Clin. Dermatol. 2022, 23, 823–840. [Google Scholar] [CrossRef]
- World Health Organization Factsheets. Diagnosis, Detection and Surveillance of Leishmaniasis. 2023. Available online: https://www.who.int/teams/control-of-neglected-tropical-diseases/leishmaniasis/diagnosis (accessed on 27 August 2023).
- Ranawaka, R.R.; Abeygunasekara, P.H.; Weerakoon, H.S. Correlation of clinical, parasitological and histopathological diagnosis of cutaneous leishmaniasis in an endemic region in Sri Lanka. Ceylon Med. J. 2013, 57, 149. [Google Scholar] [CrossRef]
- Siriwardana, H.V.Y.D.; Thalagala, N.; Karunaweera, N.D. Clinical and epidemiological studies on the cutaneous leishmaniasis caused by Leishmania (Leishmania) donovani in Sri Lanka. Ann. Trop. Med. Parasitol. 2010, 104, 213–223. [Google Scholar] [CrossRef]
- Samaranayake, T.N.; Dissanayake, V.H.; Fernando, S.D. Clinical manifestations of cutaneous leishmaniasis in Sri Lanka—Possible evidence for genetic susceptibility among the Sinhalese. Ann. Trop. Med. Parasitol. 2008, 102, 383–390. [Google Scholar] [CrossRef]
- Rajapaksa, U.S.; Ihalamulla, R.L.; Udagedera, C.; Karunaweera, N.D. Cutaneous leishmaniasis in southern Sri Lanka. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 799–803. [Google Scholar] [CrossRef]
- Nawaratna, S.S.; Weilgama, D.J.; Wijekoon, C.J.; Dissanayake, M.; Rajapaksha, K. Cutaneous leishmaniasis, Sri Lanka. Emerg. Infect. Dis. 2007, 13, 1068. [Google Scholar] [CrossRef]
- Siriwardana, H.V.Y.D.; Udagedara, C.U.; Karunaweera, N.D. Clinical features, risk factors and efficacy of cryotherapy in cutaneous leishmaniasis in Sri Lanka. Ceylon Med. J. 2003, 48, 10–12. [Google Scholar] [CrossRef]
- Yadav, P.; Azam, M.; Ramesh, V.; Singh, R. Unusual Observations in Leishmaniasis—An Overview. Pathogens 2023, 12, 297. [Google Scholar] [CrossRef]
- Torres-Guerrero, E.; Quintanilla-Cedillo, M.R.; Ruiz-Esmenjaud, J.; Arenas, R. Leishmaniasis: A review. F1000Research 2017, 6, 750. [Google Scholar] [CrossRef]
- Remadi, L.; Haouas, N.; Chaara, D.; Slama, D.; Chargui, N.; Dabghi, R.; Jbeniani, H.; Mezhoud, H.; Babba, H. Clinical presentation of cutaneous leishmaniasis caused by Leishmania major. Dermatology 2017, 232, 752–759. [Google Scholar] [CrossRef]
- Van Thiel, P.P.; Leenstra, T.; de Vries, H.J.; van der Sluis, A.; van Gool, T.; Krull, A.C.; van Vugt, M.; de Vries, P.J.; Zeegelaar, J.E.; Bart, A.; et al. Cutaneous leishmaniasis (Leishmania major infection) in Dutch troops deployed in northern Afghanistan: Epidemiology, clinical aspects, and treatment. Am. J. Trop. Med. Hyg. 2010, 83, 1295. [Google Scholar] [CrossRef]
- Alraey, Y. Distribution and epidemiological features of cutaneous leishmaniasis in Asir Province, Saudi Arabia, from 2011 to 2020. J. Infect. Public Health 2022, 15, 757–765. [Google Scholar] [CrossRef]
- Bousslimi, N.; Aoun, K.; Ben-Abda, I.; Ben-Alaya-Bouafif, N.; Raouane, M.; Bouratbine, A. Epidemiologic and clinical features of cutaneous leishmaniasis in southeastern Tunisia. Am. J. Trop. Med. Hyg. 2010, 83, 1034. [Google Scholar] [CrossRef]
- Suprien, C.; Rocha, P.N.; Teixeira, M.; Carvalho, L.P.; Guimarães, L.H.; Bonvoisin, T.; Machado, P.R.; Carvalho, E.M. Clinical presentation and response to therapy in children with cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2020, 102, 777. [Google Scholar] [CrossRef]
- Vieira-Gonçalves, R.; Pirmez, C.; Jorge, M.E.; Souza, W.J.; Oliveira, M.P.; Rutowitsch, M.S.; Da-Cruz, A.M. Clinical features of cutaneous and disseminated cutaneous leishmaniasis caused by Leishmania (Viannia) braziliensis in Paraty, Rio de Janeiro. Int. J. Dermatol. 2008, 47, 926–932. [Google Scholar] [CrossRef]
- Guimaraes, L.H.; Queiroz, A.; Silva, J.A.; Silva, S.C.; Magalhaes, V.; Lago, E.L.; Machado, P.R.; Bacellar, O.; Wilson, M.E.; Beverley, S.M.; et al. Atypical manifestations of cutaneous leishmaniasis in a region endemic for Leishmania braziliensis: Clinical, immunological and parasitological aspects. PLoS Neglected Trop. Dis. 2016, 10, e0005100. [Google Scholar] [CrossRef]
- Silveira, F.T.; Lainson, R.; De Castro Gomes, C.M.; Laurenti, M.D.; Corbett, C.E. Immunopathogenic competences of Leishmania (V.) braziliensis and L.(L.) amazonensis in American cutaneous leishmaniasis. Parasite Immunol. 2009, 31, 423–431. [Google Scholar] [CrossRef]
- Andrade-Narvaez, F.J.; Medina-Peralta, S.; Vargas-Gonzalez, A.; Canto-Lara, S.B.; Estrada-Parra, S. The histopathology of cutaneous leishmaniasis due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Rev. Inst. Med. Trop. Sao Paulo 2005, 47, 191–194. [Google Scholar] [CrossRef]
- Andrade-Narváez, F.J.; Vargas-González, A.; Canto-Lara, S.B.; Damián-Centeno, A.G. Clinical picture of cutaneous leishmaniases due to Leishmania (Leishmania) mexicana in the Yucatan peninsula, Mexico. Mem. Inst. Oswaldo Cruz 2001, 96, 163–167. [Google Scholar] [CrossRef]
- Siriwardana, H.V.Y.D.; Deepachandi, B.; Gunasekara, C.; Warnasooriya, W.; Karunaweera, N.D. Leishmania donovani induced cutaneous leishmaniasis: An insight into atypical clinical variants in Sri Lanka. J. Trop. Med. 2019, 2019, 4538597. [Google Scholar] [CrossRef]
- Gurel, M.S.; Tekin, B.; Uzun, S. Cutaneous leishmaniasis: A great imitator. Clin. Dermatol. 2020, 38, 140–151. [Google Scholar] [CrossRef]
- Özbilgin, A.; Harman, M.; Karakuş, M.; Bart, A.; Töz, S.; Kurt, Ö.; Çavuş, İ.; Polat, E.; Gündüz, C.; Van Gool, T.; et al. Leishmaniasis in Turkey: Visceral and cutaneous leishmaniasis caused by Leishmania donovani in Turkey. Acta Trop. 2017, 173, 90–96. [Google Scholar] [CrossRef]
- Shirian, S.; Oryan, A.; Hatam, G.R.; Panahi, S.; Daneshbod, Y. Comparison of conventional, molecular, and immunohistochemical methods in diagnosis of typical and atypical cutaneous leishmaniasis. Arch. Pathol. Lab. Med. 2014, 138, 235–240. [Google Scholar] [CrossRef]
- Ponce, C.; Ponce, E.; Cruz, A.; Kreutzer, R.; Pratt, D.M.; Neva, F. Leishmania donovani chagasi: New clinical variant of cutaneous leishmaniasis in Honduras. Lancet 1991, 337, 67–70. [Google Scholar] [CrossRef]
- Siriwardana, H.V.Y.D.; Senarath, U.; Chandrawansa, P.H.; Karunaweera, N.D. Use of a clinical tool for screening and diagnosis of cutaneous leishmaniasis in Sri Lanka. Pathog. Glob. Health 2015, 109, 174–183. [Google Scholar] [CrossRef]
- Kariyawasam, K.K.; Selvapandiyan, A.; Siriwardana, H.V.; Dube, A.; Karunanayake, P.; Senanayake, S.A.; Dey, R.; Gannavaram, S.; Nakhasi, H.L.; Karunaweera, N.D. Dermotropic Leishmania donovani in Sri Lanka: Visceralizing potential in clinical and preclinical studies. Parasitology 2018, 145, 443–452. [Google Scholar] [CrossRef]
- Rajapaksa, U.S.; Ihalamulla, R.L.; Karunaweera, N.D. First report of mucosal tissue localisation of leishmaniasis in Sri Lanka. Ceylon Med. J. 2005, 50, 90–91. [Google Scholar]
- Siriwardana, H.V.Y.D.; Chandrawansa, P.H.; Sirimanna, G.; Karunaweera, N.D. Leishmaniasis in Sri Lanka: A decade old story. Sri Lankan J. Infect. Dis. 2012, 2, 2. [Google Scholar] [CrossRef]
- Manamperi, N.H.; Oghumu, S.; Pathirana, N.; de Silva, M.V.; Abeyewickreme, W.; Satoskar, A.R.; Karunaweera, N.D. In situ immunopathological changes in cutaneous leishmaniasis due to Leishmania donovani. Parasite Immunol. 2017, 39, e12413. [Google Scholar] [CrossRef]
- Wijesinghe, H.; Gunathilaka, N.; Semege, S.; Pathirana, N.; Manamperi, N.; de Silva, C.; Fernando, D. Histopathology of cutaneous leishmaniasis caused by Leishmania donovani in Sri Lanka. BioMed Res. Int. 2020, 2020, 4926819. [Google Scholar] [CrossRef]
- Reimão, J.Q.; Coser, E.M.; Lee, M.R.; Coelho, A.C. Laboratory diagnosis of cutaneous and visceral leishmaniasis: Current and future methods. Microorganisms 2020, 8, 1632. [Google Scholar] [CrossRef]
- Pulimood, S.A.; Rupali, P.; Ajjampur, S.S.; Thomas, M.; Mehrotra, S.; Sundar, S. Atypical mucocutaneous involvement with Leishmania donovani. Natl. Med. J. India 2012, 25, 148–150. [Google Scholar]
- Eroglu, F.; Uzun, S.; Koltas, I.S. Comparison of clinical samples and methods in chronic cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2014, 91, 895. [Google Scholar] [CrossRef]
- Kariyawasam, K.K.; Edirisuriya, C.S.; Senerath, U.; Hensmen, D.; Siriwardana, H.V.; Karunaweera, N.D. Characterisation of cutaneous leishmaniasis in Matara district, southern Sri Lanka: Evidence for case clustering. Pathog. Glob. Health 2015, 109, 336–343. [Google Scholar] [CrossRef]
- Wijesinghe, H.D.; Wijesinghe, G.K.; Fernando, D.; Silva, C.D. Immunopathology of Cutaneous Leishmaniasis in a Cohort of Sri Lankan Patients. Clin. Pathol. 2022, 15, 2632010X221134804. [Google Scholar] [CrossRef]
- Manamperi, N.H.; de Silva, M.C.; Pathirana, N.; Abeyewickreme, W.; Karunaweera, N.D. Tissue impression smears as a supplementary diagnostic method for histopathology in cutaneous leishmaniasis in Sri Lanka. Am. J. Trop. Med. Hyg. 2018, 98, 759. [Google Scholar] [CrossRef]
- Riyal, H.; Samaranayake, N.; Amarathunga, P.; Munidasa, D.; Karunaweera, N.D. Histological findings associated with treatment response in cutaneous leishmaniasis: A clinicopathological correlation study. Int. J. Dermatol. 2023, 62, 1237–1247. [Google Scholar]
- Herath, C.H.; Ratnatunga, N.V.; Waduge, R.; Ratnayake, P.; Ratnatunga, C.N.; Ramadasa, S. A histopathological study of cutaneous leishmaniasis in Sri Lanka. Ceylon Med. J. 2010, 55, 106. [Google Scholar] [CrossRef]
- Tasew, G.; Kebede, A.; Wolday, D.; Gadisa, E.; Britton, S.; Eidsmo, L.; Akuffo, H. Low-cost liquid medium for in vitro cultivation of Leishmania parasites in low-income countries. Glob. Health Action 2009, 2, 2046. [Google Scholar] [CrossRef]
- Ihalamulla, R.L.; Siriwardana, H.V.; Gamage, S.; Perera, A.J.; Karunaweera, N.D. First successful in vitro culture of autochthonous Leishmania sp. in Sri Lanka. Ceylon Med. J. 2002, 47, 58. [Google Scholar] [CrossRef]
- Ranasinghe, P.H.; Abeygunasekera, P.H.; Athauda, S.B.; Chandrasekharan, N.V.; Mendis, A.S.; Hulangamuwa, C.S.; Wickremasinghe, D.R. First successful in vitro culture of Leishmania sp. causing autochthonous visceral leishmaniasis in Sri Lanka. Ceylon Med. J. 2011, 56, 179–180. [Google Scholar] [CrossRef]
- Ihalamulla, R.L.; Rajapaksa, U.S.; Siriwardena, H.V.; Chance, M.L.; Karunaweera, N.D. A simple, cost effective method for isolation and transportation of Leishmania parasites. Ceylon Med. J. 2009, 54, 46–47. [Google Scholar] [CrossRef]
- Allahverdiyev, A.; Uzun, S.; Bagirova, M.E.; Durdu, M.; Memisoglu, H.R. A sensitive new microculture method for diagnosis of cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2004, 70, 294–297. [Google Scholar] [CrossRef]
- Ihalamulla, R.L.; Rajapaksa, U.S.; Karunaweera, N.D. Microculture for the isolation of Leishmania parasites from cutaneous lesions—Sri Lankan experience. Ann. Trop. Med. Parasitol. 2005, 99, 571–575. [Google Scholar] [CrossRef]
- Sundharkrishnan, L.; North, J.P. Histopathologic features of cutaneous leishmaniasis and use of CD1a staining for amastigotes in Old World and New World leishmaniasis. J. Cutan. Pathol. 2017, 44, 1005–1011. [Google Scholar] [CrossRef]
- Jayasena Kaluarachchi, T.; Wickremasinghe, R.; Weerasekera, M.; Yasawardene, S.; McBain, A.J.; Yapa, B.; De Silva, H.; Menike, C.; Jayathilake, S.; Munasinghe, A.; et al. Diagnosing human cutaneous leishmaniasis using fluorescence in situ hybridization. Pathog. Glob. Health 2021, 115, 307–314. [Google Scholar] [CrossRef]
- Kocher, A.; Valiere, S.; Banuls, A.L.; Murienne, J. High-throughput sequencing of kDNA amplicons for the analysis of Leishmania minicircles and identification of Neotropical species. Parasitology 2018, 145, 585–594. [Google Scholar] [CrossRef]
- Ranasinghe, S.; Wickremasinghe, R.; Hulangamuwa, S.; Sirimanna, G.; Opathella, N.; Maingon, R.D.; Chandrasekharan, V. Polymerase chain reaction detection of LeishmaniaDNA in skin biopsy samples in Sri Lanka where the causative agent of cutaneous leishmaniasis is Leishmania donovani. Mem. Inst. Oswaldo Cruz 2015, 110, 1017–1023. [Google Scholar] [CrossRef]
- Deepachandi, B.; Weerasinghe, S.; Soysa, P.; Karunaweera, N.; Siriwardana, Y. A highly sensitive modified nested PCR to enhance case detection in leishmaniasis. BMC Infect. Dis. 2019, 19, 623. [Google Scholar] [CrossRef]
- Graça, G.C.; Volpini, A.C.; Romero, G.A.; Oliveira Neto, M.P.; Hueb, M.; Porrozzi, R.; Boité, M.C.; Cupolillo, E. Development and validation of PCR-based assays for diagnosis of American cutaneous leishmaniasis and identificatio nof the parasite species. Mem. Inst. Oswaldo Cruz 2012, 107, 664–674. [Google Scholar] [CrossRef]
- De Silva, N.L.; De Silva, V.N.; Deerasinghe, A.T.; Rathnapala, U.L.; Itoh, M.; Takagi, H.; Weerasooriya, M.V.; Kato, H.; Yahathugoda, T.C. Development of a highly sensitive nested PCR and its application for the diagnosis of cutaneous leishmaniasis in Sri Lanka. Microorganisms 2022, 10, 990. [Google Scholar] [CrossRef]
- Schönian, G.; Schnur, L.; El Fari, M.; Oskam, L.; Kolesnikov, A.A.; Sokolowska-Köhler, W.; Presber, W. Genetic heterogeneity in the species Leishmania tropica revealed by different PCR-based methods. Trans. R. Soc. Trop. Med. Hyg. 2001, 95, 217–224. [Google Scholar] [CrossRef]
- Rai, T.; Shrestha, S.; Prajapati, S.; Bastola, A.; Parajuli, N.; Ghimire, P.G.; Bhandari, P.; Pandey, K.; Jain, M.; Matlashewski, G.; et al. Leishmania donovani persistence and transmission causing cutaneous leishmaniasis in unusual-foci of Nepal. Sci. Rep. 2023, 13, 12329. [Google Scholar] [CrossRef]
- Moulik, S.; Sengupta, R.; Dighal, A.; Sardar, B.; Saha, B.; Das, N.K.; Chatterjee, M. Identification of atypical dermal leishmaniasis resolved by restriction fragment length polymorphism. Indian J. Dermatol. Venereol. Leprol. 2020, 86, 45. [Google Scholar]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, e63. [Google Scholar] [CrossRef]
- Kothalawala, H.S.; Karunaweera, N.D. Loop-mediated isothermal amplification assay as a sensitive diagnostic tool for Leishmania donovani infections in Sri Lanka. Ceylon Med. J. 2016, 61, 68. [Google Scholar] [CrossRef]
- Gunaratna, G.; Manamperi, A.; Böhlken-Fascher, S.; Wickremasinge, R.; Gunawardena, K.; Yapa, B.; Pathirana, N.; Pathirana, H.; de Silva, N.; Sooriyaarachchi, M.; et al. Evaluation of rapid extraction and isothermal amplification techniques for the detection of Leishmania donovani DNA from skin lesions of suspected cases at the point of need in Sri Lanka. Parasites Vectors 2018, 11, 665. [Google Scholar] [CrossRef]
- Ashton, F.E. Multilocus enzyme electrophoresis–application to the study of meningococcal meningitis and listeriosis. Can. J. Infect. Dis. Med. Microbiol. 1990, 1, 146–148. [Google Scholar] [CrossRef]
- Alam, M.Z.; Haralambous, C.; Kuhls, K.; Gouzelou, E.; Sgouras, D.; Soteriadou, K.; Schnur, L.; Pratlong, F.; Schönian, G. The paraphyletic composition of Leishmania donovani zymodeme MON-37 revealed by multilocus microsatellite typing. Microbes Infect. 2009, 11, 707–715. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Ali, N. Developments in diagnosis and treatment of visceral leishmaniasis during the last decade and future prospects. Expert Rev. Anti Infect. Ther. 2013, 11, 79–98. [Google Scholar] [CrossRef]
- Aronson, N.; Herwaldt, B.L.; Libman, M.; Pearson, R.; Lopez-Velez, R.; Weina, P.; Carvalho, E.; Ephros, M.; Jeronimo, S.; Magill, A. Diagnosis and treatment of leishmaniasis: Clinical practice guidelines by the Infectious Diseases Society of America (IDSA) and the American Society of Tropical Medicine and Hygiene (ASTMH). Am. J. Trop. Med. Hyg. 2017, 96, 24–45. [Google Scholar] [CrossRef]
- Hartzell, J.D.; Aronson, N.E.; Weina, P.J.; Howard, R.S.; Yadava, A.; Wortmann, G.W. Positive rK39 serologic assay results in US servicemen with cutaneous leishmaniasis. Am. J. Trop. Med. Hyg. 2008, 79, 843–846. [Google Scholar] [CrossRef]
- Svobodová, M.; Alten, B.; Zídková, L.; Dvořák, V.; Hlavačková, J.; Myšková, J.; Šeblová, V.; Kasap, O.E.; Belen, A.; Votýpka, J.; et al. Cutaneous leishmaniasis caused by Leishmania infantum transmitted by Phlebotomus tobbi. Int. J. Parasitol. 2009, 39, 251–256. [Google Scholar] [CrossRef]
- De Silva, N.L.; De Silva, V.N.; Deerasinghe, A.T.; Rathnapala, U.L.; Kato, H.; Itoh, M.; Takagi, H.; Weerasooriya, M.V.; Yahathugoda, T.C. Validation of an In-House ELISA Method in the Diagnosis of Cutaneous Leishmaniasis Caused by Leishmania donovani in Hambantota District, Sri Lanka. Microorganisms 2022, 10, 921. [Google Scholar] [CrossRef]
- Bracamonte, M.E.; Álvarez, A.M.; Sosa, A.M.; Hoyos, C.L.; Lauthier, J.J.; Cajal, S.P.; Juarez, M.; Uncos, R.E.; Sánchez-Valdéz, F.J.; Acuña, L.; et al. High performance of an enzyme linked immunosorbent assay for American tegumentary leishmaniasis diagnosis with Leishmania (Viannia) braziliensis amastigotes membrane crude antigens. PLoS ONE 2020, 15, e0232829. [Google Scholar] [CrossRef]
- Siriwardana, Y.D.; Deepachandi, B.; Ranasinghe, S.; Soysa, P.; Karunaweera, N. Evidence for seroprevalence in human localized cutaneous Leishmaniasis caused by Leishmania donovani in Sri Lanka. BioMed Res. Int. 2018, 2018, 9320367. [Google Scholar] [CrossRef]
- Deepachandi, B.; Weerasinghe, S.; Gunathilake, H.; Andrahennadi, T.P.; Wickramanayake, M.N.; Siri, S.; Chandrasekharan, V.; Soysa, P.; Siriwardana, Y. Prevalidation of an ELISA for Detection of a New Clinical Entity: Leishmania donovani-Induced Cutaneous Leishmaniasis. Int. J. Anal. Chem. 2020, 2020, 9289651. [Google Scholar] [CrossRef]
- Piyasiri, S.B.; Samaranayake, T.N.; Silva, H.; Manamperi, N.H.; Karunaweera, N.D. ELISA-based evaluation of antibody response to Leishmania in a region endemic for cutaneous leishmaniasis. Parasite Immunol. 2022, 44, e12940. [Google Scholar] [CrossRef]
- Molinet, F.J.; Ampuero, J.S.; Costa, R.D.; Noronha, E.F.; Romero, G.A. Specificity of the rapid rK39 antigen-based immunochromatographic test Kalazar Detect (r) in patients with cutaneous leishmaniasis in Brazil. Mem. Inst. Oswaldo Cruz 2013, 108, 293–296. [Google Scholar] [CrossRef]
- Ejazi, S.A.; Ghosh, S.; Saha, S.; Choudhury, S.T.; Bhattacharyya, A.; Chatterjee, M.; Pandey, K.; Das, V.N.; Das, P.; Rahaman, M.; et al. A multicentric evaluation of dipstick test for serodiagnosis of visceral leishmaniasis in India, Nepal, Sri Lanka, Brazil, Ethiopia and Spain. Sci. Rep. 2019, 9, 9932. [Google Scholar] [CrossRef]
- Van Henten, S.; Fikre, H.; Melkamu, R.; Dessie, D.; Mekonnen, T.; Kassa, M.; Bogale, T.; Mohammed, R.; Cnops, L.; Vogt, F.; et al. Evaluation of the CL detect rapid test in Ethiopian patients suspected for cutaneous leishmaniasis. PLoS Neglected Trop. Dis. 2022, 16, e0010143. [Google Scholar] [CrossRef]
- Bennis, I.; Verdonck, K.; El Khalfaoui, N.; Riyad, M.; Fellah, H.; Dujardin, J.C.; Sahibi, H.; Bouhout, S.; Van der Auwera, G.; Boelaert, M. Accuracy of a rapid diagnostic test based on antigen detection for the diagnosis of cutaneous leishmaniasis in patients with suggestive skin lesions in Morocco. Am. J. Trop. Med. Hyg. 2018, 99, 716. [Google Scholar] [CrossRef]
- De Silva, G.; Somaratne, V.; Senaratne, S.; Vipuladasa, M.; Wickremasinghe, R.; Wickremasinghe, R.; Ranasinghe, S. Efficacy of a new rapid diagnostic test kit to diagnose Sri Lankan cutaneous leishmaniasis caused by Leishmania donovani. PLoS ONE 2017, 12, e0187024. [Google Scholar] [CrossRef]
- Adams, E.R.; Jacquet, D.; Schoone, G.; Gidwani, K.; Boelaert, M.; Cunningham, J. Leishmaniasis direct agglutination test: Using pictorials as training materials to reduce inter-reader variability and improve accuracy. PLoS Neglected Trop. Dis. 2012, 6, e1946. [Google Scholar] [CrossRef]
- Ahmed, Z.; Chowdhury, S.A.; Bhuiyan, S.I. Cutaneous leishmaniasis. Mymensingh Med. J. MMJ 2009, 18, 260–263. [Google Scholar]
- Saavedra, A.C.; Valencia, B.M.; Tueros, P.; Wortsman, X.; Llanos-Cuentas, A.; Lavarello, R.J. Ultrasonographic characteristics of cutaneous leishmaniasis. J. Eur. Acad. Dermatol. Venereol. JEADV 2020, 34, e193. [Google Scholar] [CrossRef]
- Zare, M.; Akbarialiabad, H.; Parsaei, H.; Asgari, Q.; Alinejad, A.; Bahreini, M.S.; Hosseini, S.H.; Ghofrani-Jahromi, M.; Shahriarirad, R.; Amirmoezzi, Y.; et al. A machine learning-based system for detecting leishmaniasis in microscopic images. BMC Infect. Dis. 2022, 22, 48. [Google Scholar] [CrossRef]
- Khatami, A.; Yazdanparast, T.; Ahmad Nasrollahi, S.; Miramin Mohammadi, A.; Yadangi, S.; Khamesipour, A.; Kassir, M.; Firooz, A. Biophysical and ultrasonographic changes of acute Old World cutaneous leishmaniasis skin lesions in comparison with uninvolved skin: A possible tool for non-invasive early detection and treatment outcome assessment. Dermatol. Ther. 2022, 35, e15699. [Google Scholar] [CrossRef]
- Silva, H.; Chellappan, K.; Karunaweera, N. Image Processing for mHealth-Based Approach to Detect the Local Tissue Inflammation in Cutaneous Leishmaniasis: A Proof of Concept Study. Comput. Math. Methods Med. 2021, 2021, 4208254. [Google Scholar] [CrossRef]
- Dueñas, E.; Nakamoto, J.A.; Cabrera-Sosa, L.; Huaihua, P.; Cruz, M.; Arévalo, J.; Milón, P.; Adaui, V. Novel CRISPR-based detection of Leishmania species. Front. Microbiol. 2022, 13, 958693. [Google Scholar] [CrossRef]
- Castillo-Castañeda, A.; Patiño, L.H.; Muñoz, M.; Ayala, M.S.; Segura, M.; Bautista, J.; Shaban, M.V.; Paniz-Mondolfi, A.; Ramírez, J.D. Amplicon-based next-generation sequencing reveals the co-existence of multiple Leishmania species in patients with visceral leishmaniasis. Int. J. Infect. Dis. 2022, 115, 35–38. [Google Scholar] [CrossRef]
- Patiño, L.H.; Castillo-Castañeda, A.C.; Muñoz, M.; Jaimes, J.E.; Luna-Niño, N.; Hernández, C.; Ayala, M.S.; Fuya, P.; Mendez, C.; Hernández-Pereira, C.E.; et al. Development of an amplicon-based next-generation sequencing protocol to identify Leishmania species and other trypanosomatids in leishmaniasis endemic areas. Microbiol. Spectr. 2021, 9, e00652-21. [Google Scholar] [CrossRef]
- Buffi, G.; Ceccarelli, M.; Diotallevi, A.; Abruzzese, M.; Bruno, F.; Castelli, G.; Vitale, F.; Andreoni, F.; Bencardino, D.; Magnani, M.; et al. High-resolution melting (HRM)-based detection of polymorphisms in the malic enzyme and glucose-6-phosphate isomerase genes for Leishmania infantum genotyping. Parasites Vectors 2023, 16, 282. [Google Scholar] [CrossRef]

| Country/Region | Typical CL Caused by | Atypical CL Caused by | Reference |
|---|---|---|---|
| Cyprus | L. major L. tropica | L. donovani L. infantum | [5,18,19] |
| Ethiopia | L. aethiopica, L. major, L. tropica | L. donovani | [4,6] |
|
India (Himachal Pradesh and Kerala) | L. major, L. tropica | L. donovani | [7,8,9,10,23] |
| Israel | L. major, L. tropica | L. donovani, L. infantum, | [4,5,20] |
| Kenya | L. tropica, L. aethiopica, L. major | L. donovani zymodeme z6 | [11,12] |
| Sri Lanka | No typical form of CL is existing | L. donovani Mon 37 zymodeme | [5,13,14,15,16] |
| Sudan | L. major | L. donovani Mon-82 zymodeme | [17] |
| Uganda | No typical form of CL is existing | L. donovani | [4,5] |
| Yemen | L. major L. tropica | L. donovani | [21,22] |
| Species Causing Typical CL | Clinical Features of CL Lesions | References | |
|---|---|---|---|
| Similarities to Lesions Caused by L. donovani | Differences to Atypical Lesions Caused by L. donovani | ||
| L. major | Dry, crusted, and scaly lesions. Localized to the site of the sand fly bite. Lesions have a central depression and a raised border. Painless, with minimal inflammation. | Wet lesions are typically larger, more inflamed. Lesions have an ulcerative appearance with moist, weeping, or pustular areas. Painful. | [32,33,34,35] |
| L. tropica | Typically, dry, ulcerative sores with a scaly border. Localized to the site of the sand fly bite. | Wet lesions appear larger, more inflamed, and exudative. Painful and more uncomfortable. | [32,33,36,37] |
| L. braziliensis | No similarities shown. | Typically ulcerative in nature, with a central area of ulceration and raised, inflamed borders. Vary in size and shape, from small ulcers to larger sores. | [33,38,39,40,41] |
| L. amazonensis | Non-ulcerative nodules or plaques on the skin. Lesions can be dry, scaly, and may have a raised border. Painless. | Present as multiple lesions. | [33,41] |
| L. mexicana | Localized lesions. Lesions often start as a single nodule or ulcer at the site of the sand fly bite. The lesions may be painless or only mildly painful. | A distinctive form of localized cutaneous lesion involving the ear known as a “chiclero ulcer”. | [33,42,43] |
| Method | Tissue/Sample Used | Microscopy | Reference |
|---|---|---|---|
| Giemsa staining | lesion aspirates scrapings/slit-skin smears | Direct microscopy—light microscope | [28,31,33] |
| Hematoxylin-Eosin staining | lesion aspirates scrapings/slit-skin smears | Direct microscopy—light microscope | [29,31] |
| Fluorescence in situ Hybridization method (FISH) | scrapings/slit-skin smears tissue biopsies | Fluorescent microscope | [45] |
| Histopathological methods | tissue biopsies or isoleted parasites | Direct microscopy—light microscope | [23,28,29,34,35,37] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piyasiri, S.B.; Dewasurendra, R.; Samaranayake, N.; Karunaweera, N. Diagnostic Tools for Cutaneous Leishmaniasis Caused by Leishmania donovani: A Narrative Review. Diagnostics 2023, 13, 2989. https://doi.org/10.3390/diagnostics13182989
Piyasiri SB, Dewasurendra R, Samaranayake N, Karunaweera N. Diagnostic Tools for Cutaneous Leishmaniasis Caused by Leishmania donovani: A Narrative Review. Diagnostics. 2023; 13(18):2989. https://doi.org/10.3390/diagnostics13182989
Chicago/Turabian StylePiyasiri, Sachee Bhanu, Rajika Dewasurendra, Nilakshi Samaranayake, and Nadira Karunaweera. 2023. "Diagnostic Tools for Cutaneous Leishmaniasis Caused by Leishmania donovani: A Narrative Review" Diagnostics 13, no. 18: 2989. https://doi.org/10.3390/diagnostics13182989
APA StylePiyasiri, S. B., Dewasurendra, R., Samaranayake, N., & Karunaweera, N. (2023). Diagnostic Tools for Cutaneous Leishmaniasis Caused by Leishmania donovani: A Narrative Review. Diagnostics, 13(18), 2989. https://doi.org/10.3390/diagnostics13182989






