Molecular Characterization of Patients with Cryptorchidism: Preliminary Search for an Expression Profile Related to That of Testicular Germ-Cell Tumors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Histological and Immunohistochemical Analysis
2.3. RNA Isolation and RT-qPCR
2.4. Selection of Candidate Genes
2.5. Bioinformatic Analysis
2.6. Statistical Analysis
3. Results
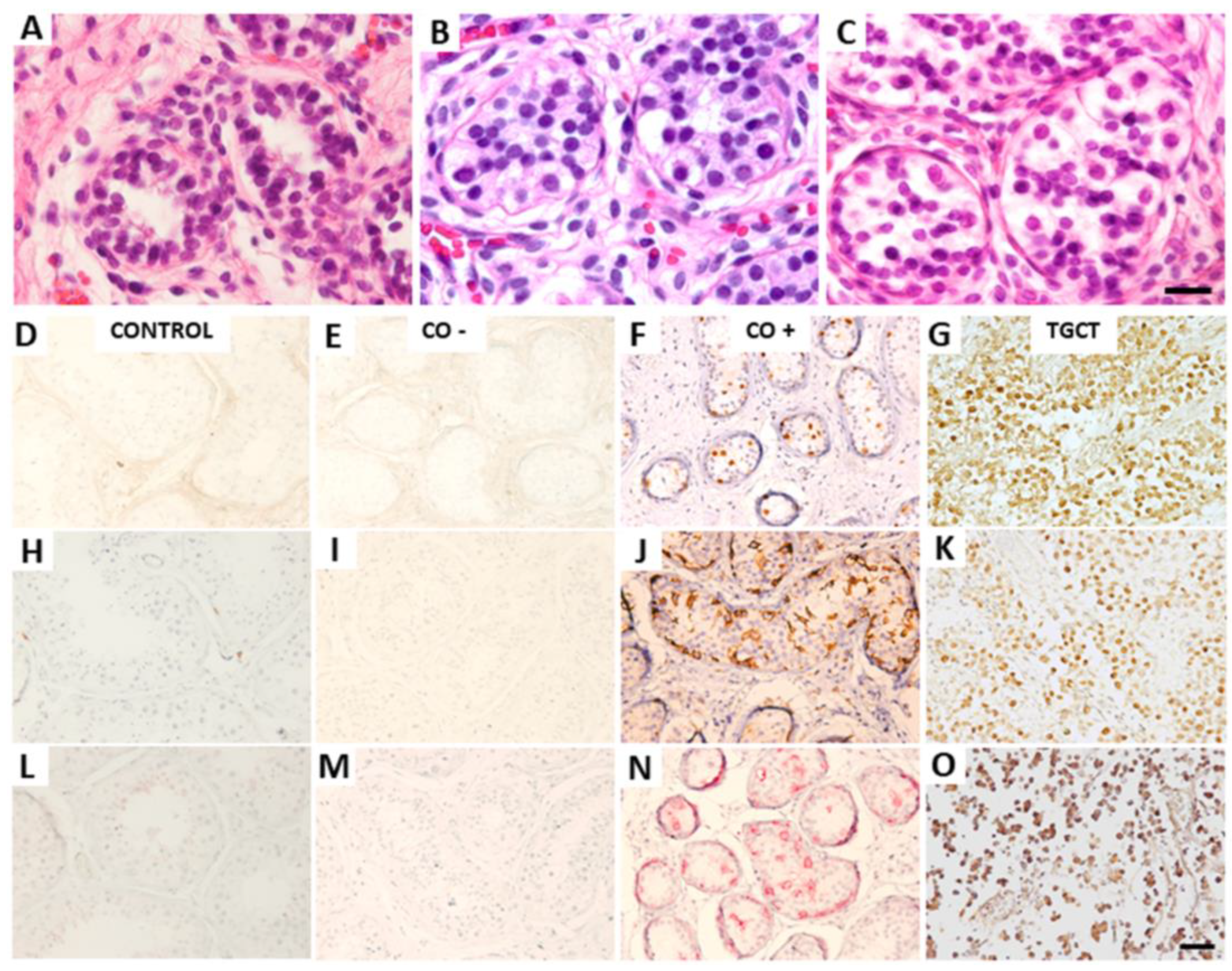
3.1. Histological and Immunohistochemical Analysis
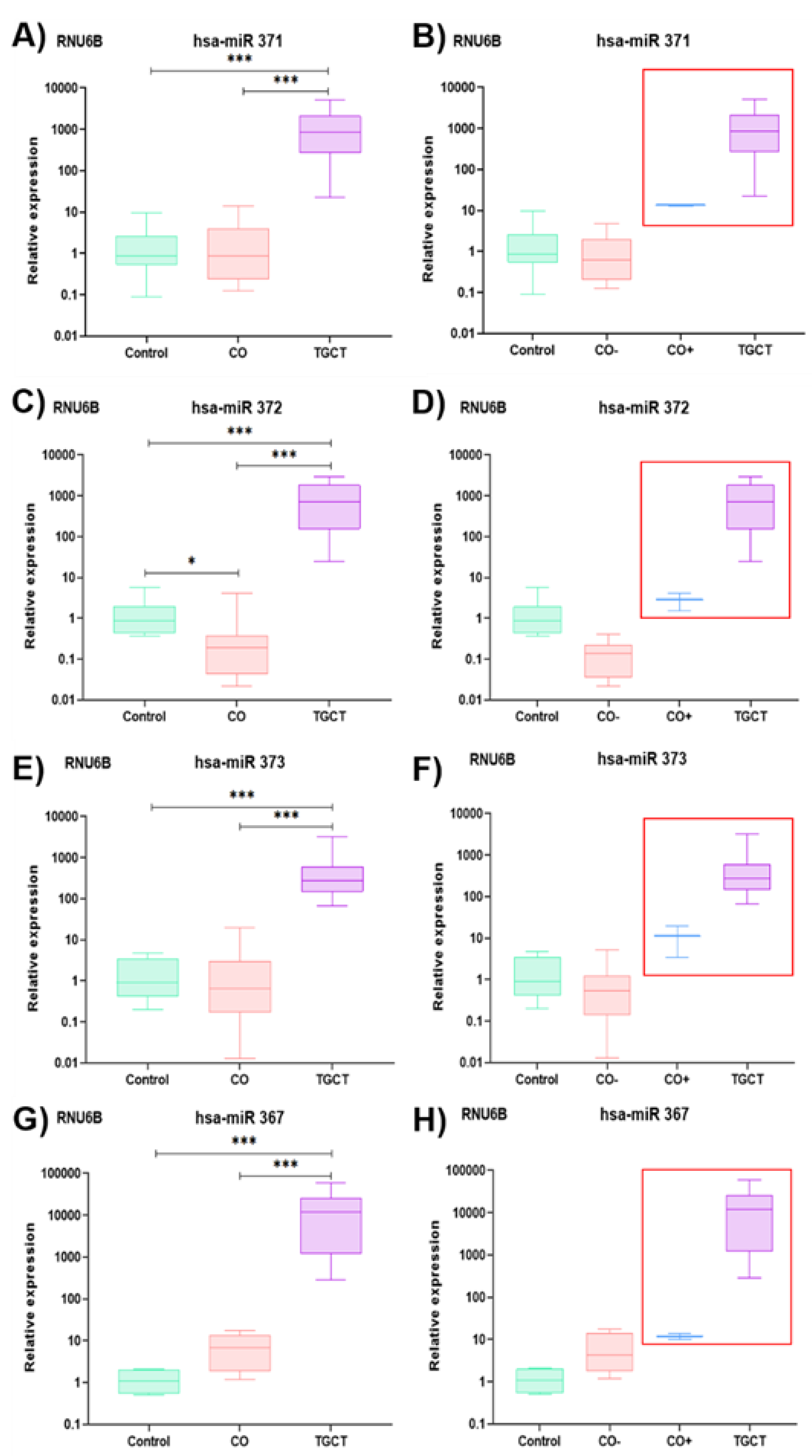
3.2. Relative Expression of the hsa-miR-371-373 Cluster and hsa-miR-367
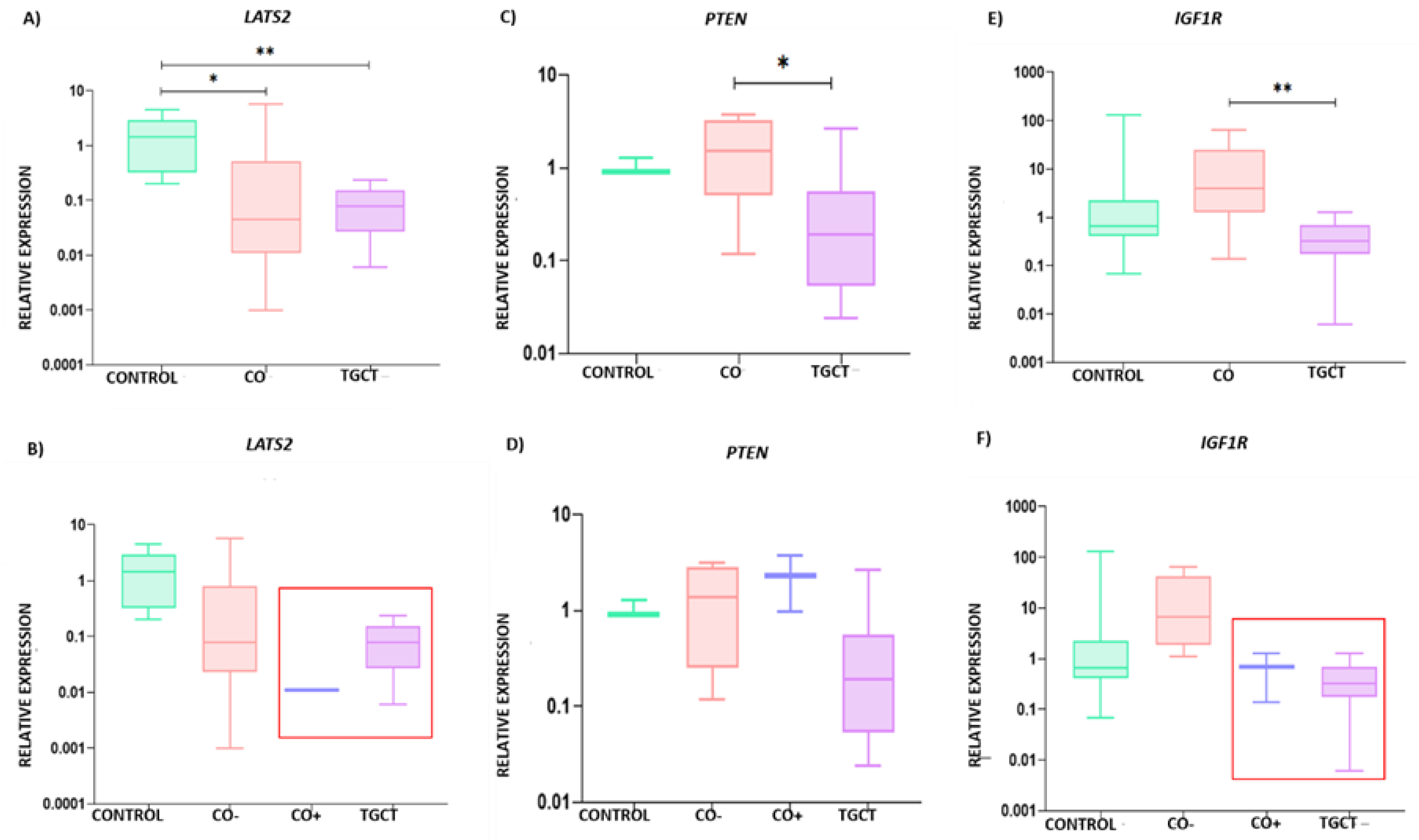
3.3. Relative Expression of LATS2, PTEN, and IGF1R
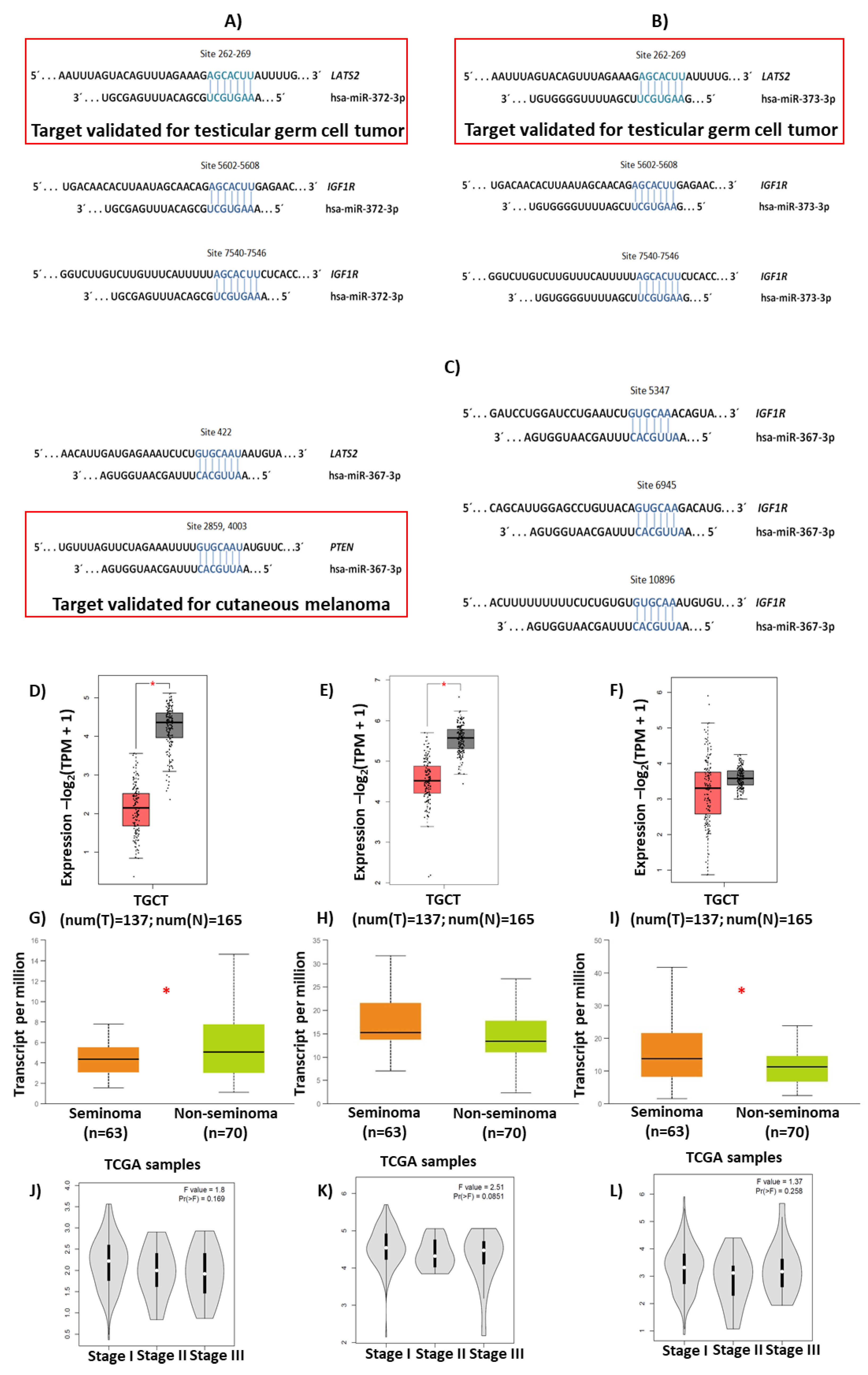
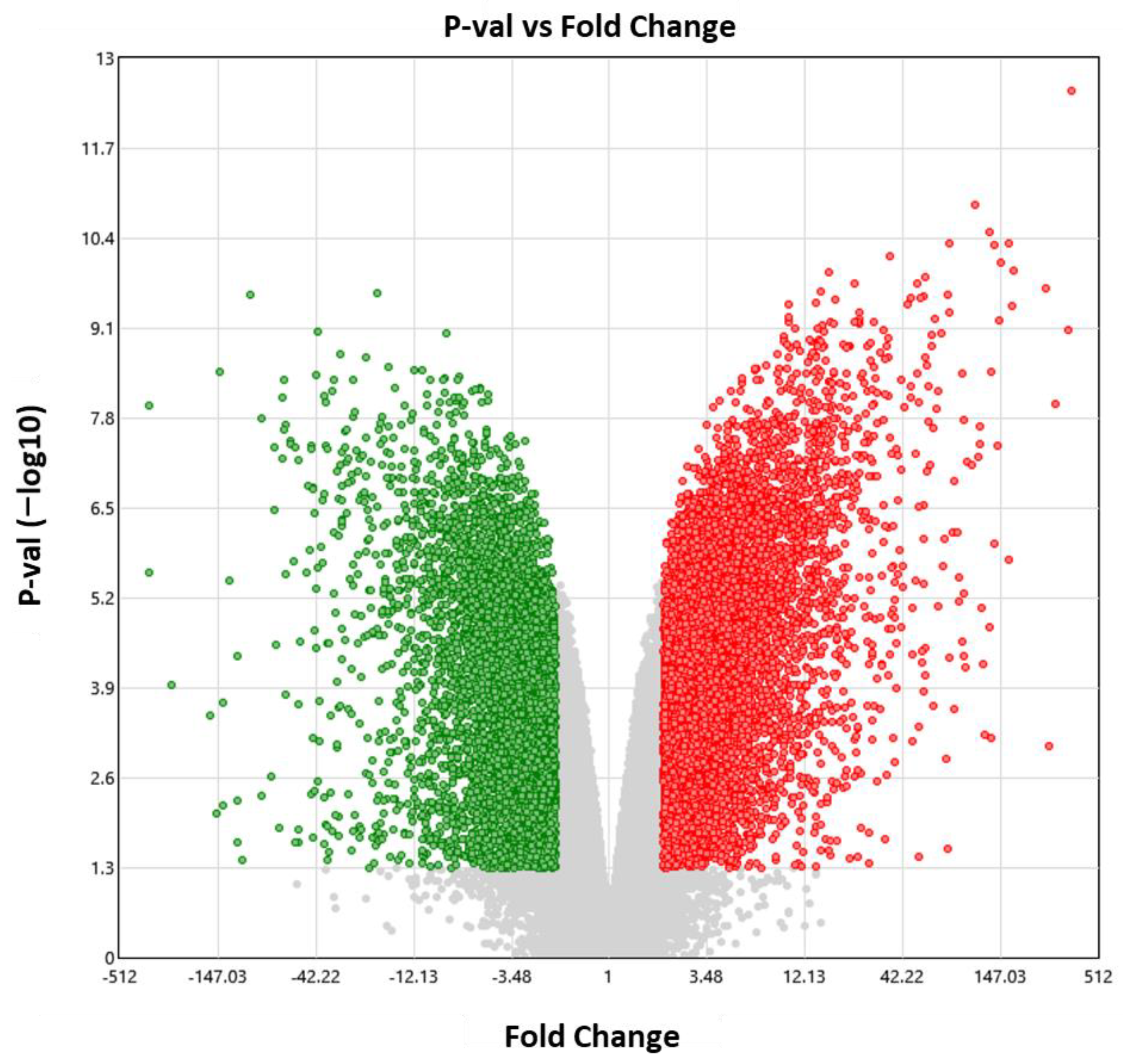
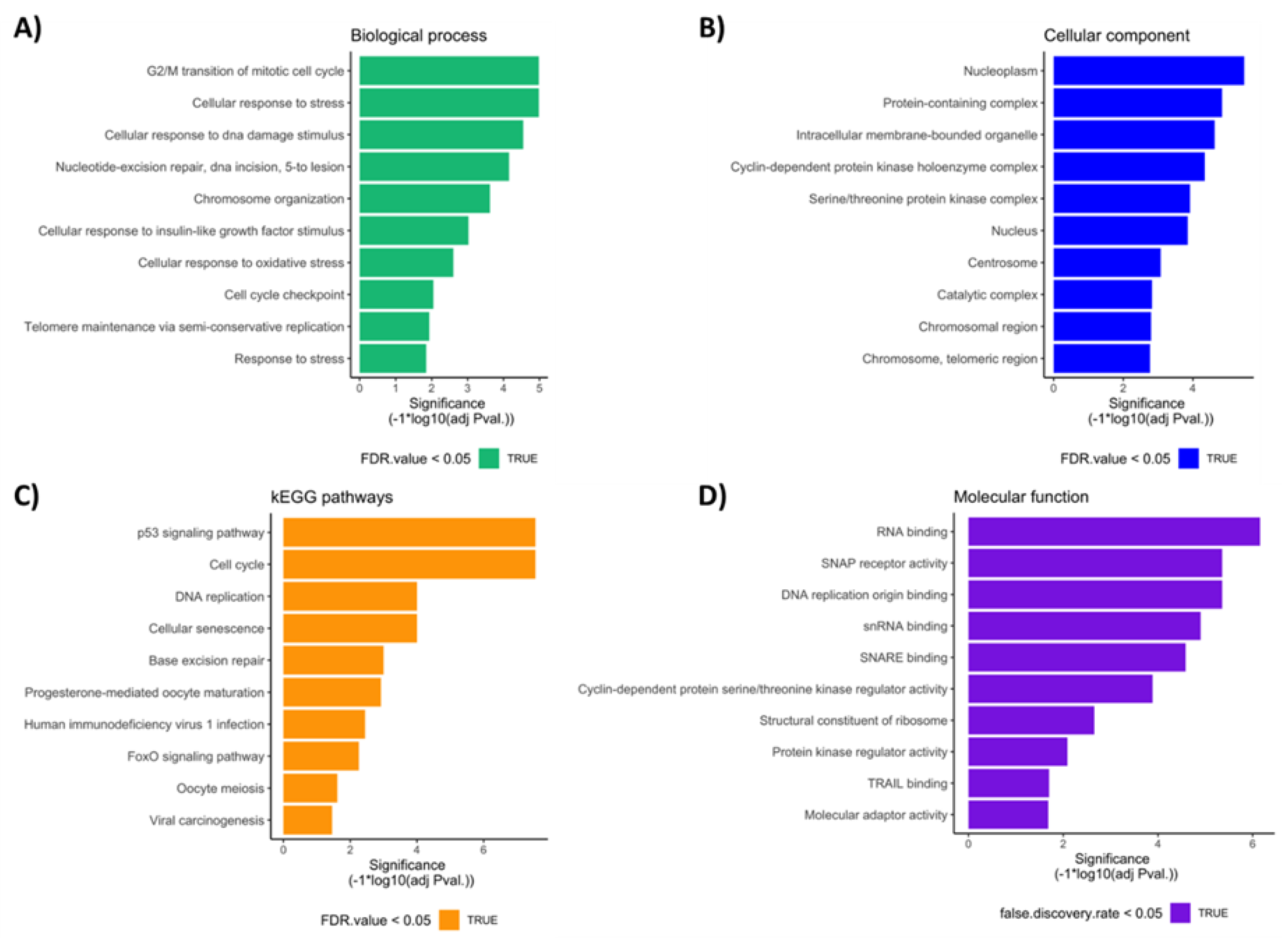
3.4. Bioinformatic Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elamo, H.P.; Virtanen, H.E.; Toppari, J. Genetics of cryptorchidism and testicular regression. Best Pr. Res. Clin. Endocrinol. Metab. 2022, 36, 101619. [Google Scholar] [CrossRef] [PubMed]
- Rodprasert, W.; Virtanen, H.E.; Mäkelä, J.-A.; Toppari, J. Hypogonadism and Cryptorchidism. Front. Endocrinol. 2020, 10, 906. [Google Scholar] [CrossRef] [PubMed]
- Skakkebæk, N.E.; Berthelsen, J.G.; Giwercman, A.; Müller, J. Carcinoma-in-situ of the testis: Possible origin from gonocytes and precursor of all types of germ cell tumours except spermatocytoma. Int. J. Androl. 1987, 10, 19–28. [Google Scholar] [CrossRef]
- Oosterhuis, J.W.; Looijenga, L.H.J. Testicular germ-cell tumours in a broader perspective. Nat. Rev. Cancer 2005, 5, 210–222. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.M.; Stoop, H.; Biermann, K.; van Gurp, R.J.H.L.M.; Swartzman, E.; Cribbes, S.; Ferlinz, A.; Shannon, M.; Oosterhuis, J.; Looijenga, L.H.J. Expression and interdependencies of pluripotency factors LIN28, OCT3/4, NANOG and SOX2 in human testicular germ cells and tumours of the testis. Int. J. Androl. 2011, 34, e160–e174. [Google Scholar] [CrossRef]
- Vigueras-Villaseñor, R.M.; Cortés-Trujillo, L.; Chávez-Saldaña, M.; Vázquez, F.G.; Carrasco-Daza, D.; Cuevas-Alpuche, O.; Rojas-Castañeda, J.C. Analysis of POU5F1, c-Kit, PLAP, AP2γ and SALL4 in gonocytes of patients with cryptorchidism. Acta Histochem. 2015, 117, 752–761. [Google Scholar] [CrossRef]
- Tien, M.Y.; Abeydeera, S.A.; Cho, H.-J.; Sarila, G.; Catubig, A.; Burton, E.; Hutson, J.; Li, R. Does the apoptosis pathway play a critical role in gonocyte transformation? J. Pediatr. Surg. 2020, 55, 1947–1951. [Google Scholar] [CrossRef] [PubMed]
- Hutson, J.M.; Li, R.; Southwell, B.R.; Petersen, B.L.; Thorup, J.; Cortes, D. Germ cell development in the postnatal testis: The key to prevent malignancy in cryptorchidism? Front. Endocrinol. 2013, 3, 176. [Google Scholar] [CrossRef]
- Novotny, G.W.; Belling, K.C.; Bramsen, J.B.; E Nielsen, J.; Bork-Jensen, J.; Almstrup, K.; Sonne, S.B.; Kjems, J.; Meyts, E.R.-D.; Leffers, H. MicroRNA expression profiling of carcinoma in situ cells of the testis. Endocrine-Related Cancer 2012, 19, 365–379. [Google Scholar] [CrossRef]
- Radtke, A.; Cremers, J.-F.; Kliesch, S.; Riek, S.; Junker, K.; Mohamed, S.A.; Anheuser, P.; Belge, G.; Dieckmann, K.-P. Can germ cell neoplasia in situ be diagnosed by measuring serum levels of microRNA371a-3p? J. Cancer Res. Clin. Oncol. 2017, 143, 2383–2392. [Google Scholar] [CrossRef]
- García-Andrade, F.; Vigueras-Villaseñor, R.M.; Chávez-Saldaña, M.D.; Rojas-Castañeda, J.C.; Bahena-Ocampo, I.U.; Aréchaga-Ocampo, E.; Díaz-Chávez, J.; Landero-Huerta, D.A. The Role of microRNAs in the Gonocyte Theory as Target of Malignancy: Looking for Potential Diagnostic Biomarkers. Int. J. Mol. Sci. 2022, 23, 10526. [Google Scholar] [CrossRef] [PubMed]
- Gillis, A.J.; Rijlaarsdam, M.A.; Eini, R.; Dorssers, L.C.; Biermann, K.; Murray, M.J.; Nicholson, J.C.; Coleman, N.; Dieckmann, K.-P.; Belge, G.; et al. Targeted serum miRNA (TSmiR) test for diagnosis and follow-up of (testicular) germ cell cancer patients: A proof of principle. Mol. Oncol. 2013, 7, 1083–1092. [Google Scholar] [CrossRef]
- Regouc, M.; Belge, G.; Lorch, A.; Dieckmann, K.-P.; Pichler, M. Non-Coding microRNAs as Novel Potential Tumor Markers in Testicular Cancer. Cancers 2020, 12, 749. [Google Scholar] [CrossRef] [PubMed]
- van Agthoven, T.; Looijenga, L.H. Accurate primary germ cell cancer diagnosis using serum based microRNA detection (ampTSmiR test). Oncotarget 2016, 8, 58037–58049. [Google Scholar] [CrossRef]
- Palmer, R.D.; Murray, M.J.; Saini, H.K.; van Dongen, S.; Abreu-Goodger, C.; Muralidhar, B.; Pett, M.R.; Thornton, C.M.; Nicholson, J.C.; Enright, A.J.; et al. Malignant Germ Cell Tumors Display Common MicroRNA Profiles Resulting in Global Changes in Expression of Messenger RNA Targets. Cancer Res 2010, 70, 2911–2923. [Google Scholar] [CrossRef]
- Chang, Y.; Wang, X.; Xu, Y.; Yang, L.; Qian, Q.; Ju, S.; Chen, Y.; Chen, S.; Qin, N.; Ma, Z.; et al. Comprehensive characterization of cancer-testis genes in testicular germ cell tumor. Cancer Med. 2019, 8, 3511–3519. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X. miRDB: An online database for prediction of functional microRNA targets. Nucleic Acids Res. 2019, 48, D127–D131. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.-W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4, e05005. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Tang, Z.; Zhang, W.; Ye, Z.; Liu, F. GEPIA2021: Integrating multiple deconvolution-based analysis into GEPIA. Nucleic Acids Res. 2021, 49, W242–W246. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Ponce-Rodriguez, I.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Mi, H.; Muruganujan, A.; Ebert, D.; Huang, X.; Thomas, P.D. PANTHER version 14: More genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucleic Acids Res. 2018, 47, D419–D426. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kvist, K.; Clasen-Linde, E.; Langballe, O.; Hansen, S.H.; Cortes, D.; Thorup, J. The Expression of Markers for Intratubular Germ Cell Neoplasia in Normal Infantile Testes. Front. Endocrinol. 2018, 9, 286. [Google Scholar] [CrossRef]
- Murray, M.J.; Bell, E.; Raby, K.L.; A Rijlaarsdam, M.; Gillis, A.J.M.; Looijenga, L.H.J.; Brown, H.; Destenaves, B.; Nicholson, J.C.; Coleman, N. A pipeline to quantify serum and cerebrospinal fluid microRNAs for diagnosis and detection of relapse in paediatric malignant germ-cell tumours. Br. J. Cancer 2015, 114, 151–162. [Google Scholar] [CrossRef]
- Rosa, A.; Papaioannou, M.D.; Krzyspiak, J.E.; Brivanlou, A.H. miR-373 is regulated by TGFβ signaling and promotes mesendoderm differentiation in human Embryonic Stem Cells. Dev. Biol. 2014, 391, 81–88. [Google Scholar] [CrossRef]
- Stadler, B.; Ivanovska, I.; Mehta, K.; Song, S.; Nelson, A.; Tan, Y.; Mathieu, J.; Darby, C.; Blau, C.A.; Ware, G.; et al. Characterization of microRNAs Involved in Embryonic Stem Cell States. Stem Cells Dev. 2010, 19, 935–950. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gan, L.; Si, J.; Zhang, J.; Liu, Z.; Zhao, J.; Gou, Z.; Zhang, H. Role of miR-302/367 cluster in human physiology and pathophysiology. Acta Biochim. Biophys. Sin. 2020, 52, 791–800. [Google Scholar] [CrossRef]
- Clasen-Linde, E.; Kvist, K.; Cortes, D.; Thorup, J. The value of positive Oct3/4 and D2-40 immunohistochemical expression in prediction of germ cell neoplasia in prepubertal boys with cryptorchidism. Scand. J. Urol. 2015, 50, 74–79. [Google Scholar] [CrossRef] [PubMed]
- Lobo, J.; van Zogchel, L.M.J.; Nuru, M.G.; Gillis, A.J.M.; van der Schoot, C.E.; Tytgat, G.A.M.; Looijenga, L.H.J. Combining Hypermethylated RASSF1A Detection Using ddPCR with miR-371a-3p Testing: An Improved Panel of Liquid Biopsy Biomarkers for Testicular Germ Cell Tumor Patients. Cancers 2021, 13, 5228. [Google Scholar] [CrossRef]
- Tang, D.; Huang, Z.; He, X.; Wu, H.; Peng, D.; Zhang, L.; Zhang, X. Altered miRNA profile in testis of post-cryptorchidopexy patients with non-obstructive azoospermia. Reprod. Biol. Endocrinol. 2018, 16, 78. [Google Scholar] [CrossRef]
- Woo, C.G.; Lee, O.-J.; Yang, Y.; Kim, Y.J.; Lee, J.; Son, S.-M. Collision tumor comprising metastatic cholangiocarcinoma and seminoma in an undescended testis: A case report. J. Int. Med Res. 2019, 47, 5809–5816. [Google Scholar] [CrossRef] [PubMed]
- Osterballe, L.; Clasen-Linde, E.; Cortes, D.; Engholm, G.; Hertzum-Larsen, R.; Reinhardt, S.; Thorup, J. The diagnostic impact of testicular biopsies for intratubular germ cell neoplasia in cryptorchid boys and the subsequent risk of testicular cancer in men with prepubertal surgery for syndromic or non-syndromic cryptorchidism. J. Pediatr. Surg. 2017, 52, 587–592. [Google Scholar] [CrossRef] [PubMed]
- Duale, N.; Lindeman, B.; Komada, M.; Olsen, A.-K.; Andreassen, A.; Soderlund, E.J.; Brunborg, G. Molecular portrait of cisplatin induced response in human testis cancer cell lines based on gene expression profiles. Mol. Cancer 2007, 6, 53. [Google Scholar] [CrossRef]
- Yang, N.-Q.; Luo, X.-J.; Zhang, J.; Wang, G.-M.; Guo, J.-M. Crosstalk between Meg3 and miR-1297 regulates growth of testicular germ cell tumor through PTEN/PI3K/AKT pathway. Am. J. Transl. Res. 2016, 8, 1091–1099. [Google Scholar] [PubMed]
- Selfe, J.; Shipley, J.M. IGFsignalling in germ cells and testicular germ cell tumours: Roles and therapeutic approaches. Andrology 2019, 7, 536–544. [Google Scholar] [CrossRef] [PubMed]
- Voorhoeve, P.M.; le Sage, C.; Schrier, M.; Gillis, A.J.; Stoop, H.; Nagel, R.; Liu, Y.-P.; van Duijse, J.; Drost, J.; Griekspoor, A.; et al. A Genetic Screen Implicates miRNA-372 and miRNA-373 As Oncogenes in Testicular Germ Cell Tumors. Cell 2006, 124, 1169–1181. [Google Scholar] [CrossRef] [PubMed]
- Long, J.; Luo, J.; Yin, X. miR-367 enhances the proliferation and invasion of cutaneous malignant melanoma by regulating phosphatase and tensin homolog expression. Mol. Med. Rep. 2018, 17, 6526–6532. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Chen, T.; Liu, G.; He, N.; Hu, H. MiR-371 promotes proliferation and metastasis in hepatocellular carcinoma by targeting PTEN. BMB Rep. 2019, 52, 312–317. [Google Scholar] [CrossRef]
- Shin, J.W.; Kim, S.-H.; Yoon, J.Y. PTEN downregulation induces apoptosis and cell cycle arrest in uterine cervical cancer cells. Exp. Ther. Med. 2021, 22, 1–7. [Google Scholar] [CrossRef]
- Neuvians, T.P.; Gashaw, I.; Hasenfus, A.; Häcker, A.; Winterhager, E.; Grobholz, R. Differential Expression of IGF Components and Insulin Receptor Isoforms in Human Seminoma Versus Normal Testicular Tissue. Neoplasia 2005, 7, 446–456. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; La Vignera, S.; Calogero, A.E. Effects of the insulin-like growth factor system on testicular differentiation and function: A review of the literature. Andrology 2017, 6, 3–9. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Ji, P.; Jin, X. Application of the microRNA-302/367 cluster in cancer therapy. Cancer Sci. 2020, 111, 1065–1075. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.-C.; Jiang, M.; Beaudet, A.L.; Wu, M.-Y. ARID4A and ARID4B regulate male fertility, a functional link to the AR and RB pathways. Proc. Natl. Acad. Sci. USA 2013, 110, 4616–4621. [Google Scholar] [CrossRef]
- Wu, R.-C.; Zeng, Y.; Pan, I.-W.; Wu, M.-Y. Androgen Receptor Coactivator ARID4B Is Required for the Function of Sertoli Cells in Spermatogenesis. Mol. Endocrinol. 2015, 29, 1334–1346. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Mori, M.; Yoshida, C.A.; Ito, C.; Yamatoya, K.; Moriishi, T.; Kawai, Y.; Komori, H.; Kawane, T.; Izumi, S.-I.; et al. Galnt3 deficiency disrupts acrosome formation and leads to oligoasthenoteratozoospermia. Histochem. 2012, 139, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Campagnoli, M.F. Familial tumoral calcinosis and testicular microlithiasis associated with a new mutation of GALNT3 in a white family. J. Clin. Pathol. 2006, 59, 440–442. [Google Scholar] [CrossRef]
- Richenberg, J.; Belfield, J.; Ramchandani, P.; Rocher, L.; Freeman, S.; Tsili, A.C.; Cuthbert, F.; Studniarek, M.; Bertolotto, M.; Turgut, A.T.; et al. Testicular microlithiasis imaging and follow-up: Guidelines of the ESUR scrotal imaging subcommittee. Eur. Radiol. 2014, 25, 323–330. [Google Scholar] [CrossRef]
- Liu, N.; Qadri, F.; Busch, H.; Huegel, S.; Sihn, G.; Chuykin, I.; Hartmann, E.; Bader, M.; Rother, F. Kpna6 deficiency causes infertility in male mice by disrupting spermatogenesis. Development 2021, 148, dev198374. [Google Scholar] [CrossRef]
- Ying, W. Phenomic Studies on Diseases: Potential and Challenges. Phenomics 2023, 3, 285–299. [Google Scholar] [CrossRef]





| (A) Control group | ||||||||
| Case | Age | Histology | ||||||
| Control 1 | 17 years | Normal spermatogenesis | ||||||
| Control 2 | 8 years | Normal for age | ||||||
| Control 3 | 39 years | Normal spermatogenesis | ||||||
| Control 4 | 17 years | Normal spermatogenesis | ||||||
| Control 5 | 17 years | Hypospermatogenesis | ||||||
| Control 6 | 18 years | Normal spermatogenesis | ||||||
| Control 7 | 18 years | Normal spermatogenesis | ||||||
| Control 8 | 18 years | Normal spermatogenesis | ||||||
| Control 9 | 22 years | Normal spermatogenesis | ||||||
| Control 10 | 4 years | Normal for age | ||||||
| Control 11 | 1 year | Normal for age | ||||||
| Control 12 | 14 years | Normal spermatogenesis | ||||||
| (B) CO group | ||||||||
| Case | Age | Personal history of CO | CO laterality | Histology | ||||
| CO 1 | 1 year | Non-syndromic | Bilateral | Normal for age | ||||
| CO 2 | 2 years | Non-syndromic | Right | Tubular atrophy | ||||
| CO 3 | 1 years | Non-syndromic | Left | Normal for age | ||||
| CO 4 | 12 years | Non-syndromic | Bilateral | Maturation arrest | ||||
| CO 5 | 1 years | Non-syndromic | Bilateral | Tubular atrophy | ||||
| CO 6 | 15 years | Non-syndromic | Right | Tubular atrophy | ||||
| CO 7 | 1 year | Non-syndromic | Left | Normal for age | ||||
| CO 8 | 4 years | Non-syndromic | Left | Normal for age | ||||
| CO 9 | 10 years | Non-syndromic | Bilateral | Tubular atrophy | ||||
| CO 10 | 5 years | Non-syndromic | Left | Tubular atrophy | ||||
| CO 11 | 1 year | Non-syndromic | Left | Tubular atrophy | ||||
| CO 12 | 8 years | Non-syndromic | Bilateral | Tubular atrophy | ||||
| (C) TGCT group | ||||||||
| Case | Age | Diagnosis | Histology | TGCT laterality | Clinical stage | Metastasis | Personal history of CO | CO laterality |
| TGCT 1 | 49 years | nsTGCT | T | Right | ll | No | Non-syndromic | Bilateral |
| TGCT 2 | 27 years | sTGCT | S | Right | I | No | Non-syndromic | Bilateral |
| TGCT 3 | 17 years | nsTGCT | T | Left | II | Pelvis | Non-syndromic | Bilateral |
| TGCT 4 | 19 years | nsTGCT | YS + CH | Left | ll | Retroperitoneum | Non-syndromic | Left |
| TGCT 5 | 16 years | nsTGCT | T | Right | I | No | Non-syndromic | Bilateral |
| TGCT 6 | 22 years | sTGCT | S | Left | II | Retroperitoneum | Non-syndromic | Left |
| TGCT 7 | 22 years | nsTGCT | S + T | Bilateral | II | No | Non-syndromic | Bilateral |
| TGCT 8 | 16 years | nsTGCT | S + T + CH | Right | II | Retroperitoneum | Non-syndromic | Bilateral |
| TGCT 9 | 23 years | nsTGCT | T | Right | IV | Lung | Non-syndromic | Right |
| TGCT 10 | 27 years | nsTGCT | T | Left | ll | Retroperitoneum | Non-syndromic | Left |
| TGCT 11 | 18 years | sTGCT | S | Right | l | No | Non-syndromic | Right |
| TGCT 12 | 24 years | nsTGCT | S +T | Right | l | No | Non-syndromic | Right |
| Sample | Control | CO | TGCT | ||||||
|---|---|---|---|---|---|---|---|---|---|
| POU5F1 | PLAP | KIT | POU5F1 | PLAP | KIT | POU5F1 | PLAP | KIT | |
| 1 | − | − | − | − | − | − | + | − | − |
| 2 | − | − | − | − | − | − | + | + | + |
| 3 | − | − | − | − | − | − | − | − | − |
| 4 | − | − | − | − | − | − | − | + | + |
| 5 | − | − | − | + | + | + | − | − | − |
| 6 | − | − | − | + | + | + | + | + | + |
| 7 | − | − | − | − | − | − | + | + | + |
| 8 | − | − | − | − | − | − | + | + | + |
| 9 | − | − | − | − | − | − | − | − | − |
| 10 | − | − | − | − | − | − | + | − | − |
| 11 | − | − | − | − | − | − | + | + | + |
| 12 | − | − | − | − | − | − | + | + | + |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Andrade, F.; Vigueras-Villaseñor, R.M.; Chávez-Saldaña, M.D.; Rojas-Castañeda, J.C.; Bahena-Ocampo, I.U.; Aréchaga-Ocampo, E.; Flores-Fortis, M.; Díaz-Chávez, J.; Herrera, L.A.; Landero-Huerta, D.A. Molecular Characterization of Patients with Cryptorchidism: Preliminary Search for an Expression Profile Related to That of Testicular Germ-Cell Tumors. Diagnostics 2023, 13, 3020. https://doi.org/10.3390/diagnostics13183020
García-Andrade F, Vigueras-Villaseñor RM, Chávez-Saldaña MD, Rojas-Castañeda JC, Bahena-Ocampo IU, Aréchaga-Ocampo E, Flores-Fortis M, Díaz-Chávez J, Herrera LA, Landero-Huerta DA. Molecular Characterization of Patients with Cryptorchidism: Preliminary Search for an Expression Profile Related to That of Testicular Germ-Cell Tumors. Diagnostics. 2023; 13(18):3020. https://doi.org/10.3390/diagnostics13183020
Chicago/Turabian StyleGarcía-Andrade, Fabiola, Rosa María Vigueras-Villaseñor, Margarita Dolores Chávez-Saldaña, Julio César Rojas-Castañeda, Ivan Uriel Bahena-Ocampo, Elena Aréchaga-Ocampo, Mauricio Flores-Fortis, José Díaz-Chávez, Luis Alonso Herrera, and Daniel Adrian Landero-Huerta. 2023. "Molecular Characterization of Patients with Cryptorchidism: Preliminary Search for an Expression Profile Related to That of Testicular Germ-Cell Tumors" Diagnostics 13, no. 18: 3020. https://doi.org/10.3390/diagnostics13183020
APA StyleGarcía-Andrade, F., Vigueras-Villaseñor, R. M., Chávez-Saldaña, M. D., Rojas-Castañeda, J. C., Bahena-Ocampo, I. U., Aréchaga-Ocampo, E., Flores-Fortis, M., Díaz-Chávez, J., Herrera, L. A., & Landero-Huerta, D. A. (2023). Molecular Characterization of Patients with Cryptorchidism: Preliminary Search for an Expression Profile Related to That of Testicular Germ-Cell Tumors. Diagnostics, 13(18), 3020. https://doi.org/10.3390/diagnostics13183020








