Impact of Phantom Size on Low-Energy Virtual Monoenergetic Images of Three Dual-Energy CT Platforms
Abstract
:1. Introduction
2. Materials and Methods
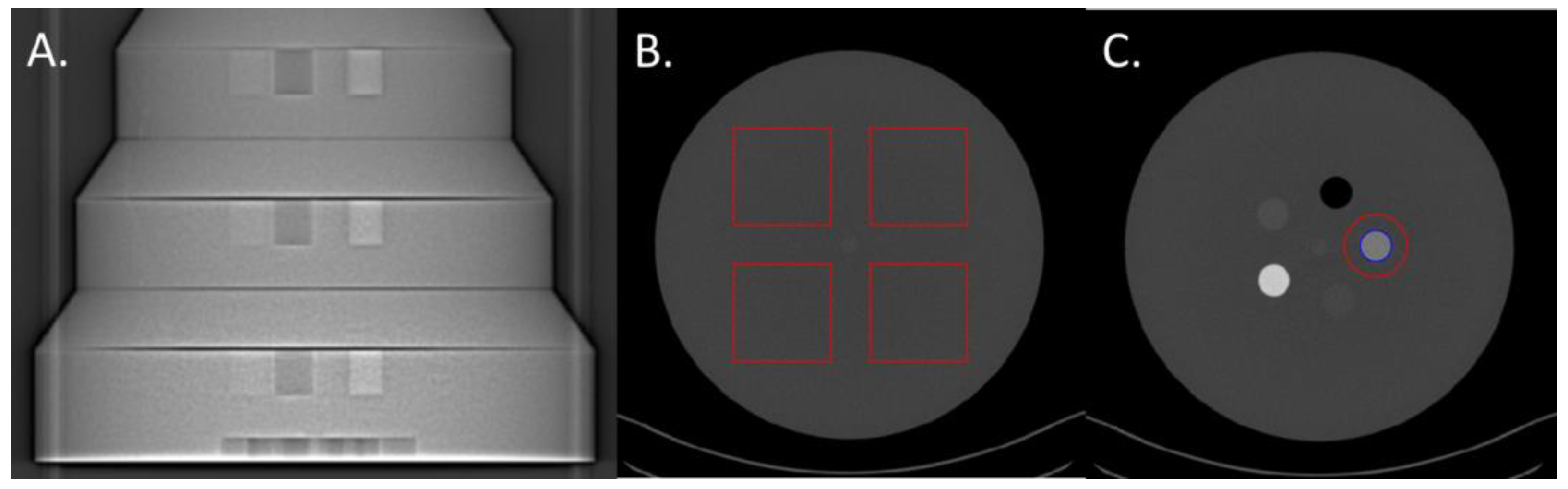
2.1. Phantom
2.2. CT Scanners and Scanning Protocol
2.3. Task-Based Image Quality Assessment on VMIs
2.3.1. Noise Power Spectrum
2.3.2. Task-Based Transfer Function
2.3.3. Detectability Index
3. Results
3.1. Noise Power Spectrum
3.1.1. Noise Magnitude
3.1.2. Noise Texture
3.2. Task-Based Transfer Function
3.3. Detectability Indexes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

Appendix B

References
- Greffier, J.; Villani, N.; Defez, D.; Dabli, D.; Si-Mohamed, S. Spectral CT imaging: Technical principles of dual-energy CT and multi-energy photon-counting CT. Diagn. Interv. Imaging 2023, 104, 167–177. [Google Scholar] [CrossRef] [PubMed]
- McCollough, C.H.; Leng, S.; Yu, L.; Fletcher, J.G. Dual- and Multi-Energy CT: Principles, Technical Approaches, and Clinical Applications. Radiology 2015, 276, 637–653. [Google Scholar] [CrossRef] [PubMed]
- Willemink, M.J.; Noel, P.B. The evolution of image reconstruction for CT-from filtered back projection to artificial intelligence. Eur. Radiol. 2019, 29, 2185–2195. [Google Scholar] [CrossRef] [PubMed]
- Goo, H.W.; Goo, J.M. Dual-Energy CT: New Horizon in Medical Imaging. Korean J. Radiol. 2017, 18, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Franck, C.; Zhang, G.; Deak, P.; Zanca, F. Preserving image texture while reducing radiation dose with a deep learning image reconstruction algorithm in chest CT: A phantom study. Phys. Med. 2021, 81, 86–93. [Google Scholar] [CrossRef]
- Racine, D.; Becce, F.; Viry, A.; Monnin, P.; Thomsen, B.; Verdun, F.R.; Rotzinger, D.C. Task-based characterization of a deep learning image reconstruction and comparison with filtered back-projection and a partial model-based iterative reconstruction in abdominal CT: A phantom study. Phys. Med. 2020, 76, 28–37. [Google Scholar] [CrossRef]
- Solomon, J.; Lyu, P.; Marin, D.; Samei, E. Noise and spatial resolution properties of a commercially available deep learning-based CT reconstruction algorithm. Med. Phys. 2020, 47, 3961–3971. [Google Scholar] [CrossRef]
- Jungblut, L.; Abel, F.; Nakhostin, D.; Mergen, V.; Sartoretti, T.; Euler, A.; Frauenfelder, T.; Martini, K. Impact of photon counting detector CT derived virtual monoenergetic images and iodine maps on the diagnosis of pleural empyema. Diagn. Interv. Imaging 2023, 104, 84–90. [Google Scholar] [CrossRef]
- Boccalini, S.; Dessouky, R.; Rodesch, P.A.; Lacombe, H.; Yagil, Y.; Lahoud, E.; Erhard, K.; Brendel, B.; Coulon, P.; Langlois, J.B.; et al. Gadolinium K-edge angiography with a spectral photon counting CT in atherosclerotic rabbits. Diagn. Interv. Imaging 2023. [Google Scholar] [CrossRef]
- Cosset, B.; Sigovan, M.; Boccalini, S.; Farhat, F.; Douek, P.; Boussel, L.; Si-Mohamed, S.A. Bicolor K-edge spectral photon-counting CT imaging for the diagnosis of thoracic endoleaks: A dynamic phantom study. Diagn. Interv. Imaging 2023, 104, 235–242. [Google Scholar] [CrossRef]
- Boccalini, S.; Si-Mohamed, S. Spectral photon counting CT: Not just a pimped-up new version of dual-energy CT. Diagn. Interv. Imaging 2023, 104, 51–52. [Google Scholar] [CrossRef]
- Marin, D.; Boll, D.T.; Mileto, A.; Nelson, R.C. State of the art: Dual-energy CT of the abdomen. Radiology 2014, 271, 327–342. [Google Scholar] [CrossRef] [PubMed]
- Chandarana, H.; Megibow, A.J.; Cohen, B.A.; Srinivasan, R.; Kim, D.; Leidecker, C.; Macari, M. Iodine quantification with dual-energy CT: Phantom study and preliminary experience with renal masses. AJR Am. J. Roentgenol. 2011, 196, W693–W700. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, M.D.; Pinho, D.F.; Kulkarni, N.M.; Hahn, P.F.; Guimaraes, A.R.; Sahani, D.V. Oncologic applications of dual-energy CT in the abdomen. Radiographics 2014, 34, 589–612. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shi, G.; Qi, X.; Fan, X.; Wang, L. Quantitative analysis of the dual-energy CT virtual spectral curve for focal liver lesions characterization. Eur. J. Radiol. 2014, 83, 1759–1764. [Google Scholar] [CrossRef] [PubMed]
- Greffier, J.; Si-Mohamed, S.; Dabli, D.; de Forges, H.; Hamard, A.; Douek, P.; Beregi, J.P.; Frandon, J. Performance of four dual-energy CT platforms for abdominal imaging: A task-based image quality assessment based on phantom data. Eur. Radiol. 2021, 31, 5324–5334. [Google Scholar] [CrossRef]
- Greffier, J.; Si-Mohamed, S.; Guiu, B.; Frandon, J.; Loisy, M.; de Oliveira, F.; Douek, P.; Beregi, J.P.; Dabli, D. Comparison of virtual monoenergetic imaging between a rapid kilovoltage switching dual-energy computed tomography with deep-learning and four dual-energy CTs with iterative reconstruction. Quant. Imaging Med. Surg. 2022, 12, 1149–1162. [Google Scholar] [CrossRef]
- Ren, T.; Zhang, W.; Li, S.; Deng, L.; Xue, C.; Li, Z.; Liu, S.; Sun, J.; Zhou, J. Combination of clinical and spectral-CT parameters for predicting lymphovascular and perineural invasion in gastric cancer. Diagn. Interv. Imaging 2022, 103, 584–593. [Google Scholar] [CrossRef]
- Greffier, J.; Van Ngoc Ty, C.; Fitton, I.; Frandon, J.; Beregi, J.P.; Dabli, D. Spectral performance of two split-filter dual-energy CT systems: A phantom study. Med. Phys. 2023. [Google Scholar] [CrossRef]
- Cester, D.; Eberhard, M.; Alkadhi, H.; Euler, A. Virtual monoenergetic images from dual-energy CT: Systematic assessment of task-based image quality performance. Quant. Imaging Med. Surg. 2022, 12, 726–741. [Google Scholar] [CrossRef]
- Szczykutowicz, T.P.; Michaelson, B.S. Using the Gammex Mercury 4.0™ Phantom for Common Clinical Tasks in CT. White-Paper Mercury 4.0. 2018. Available online: https://sunnuclear.com/uploads/documents/whitepapers/White-Paper_Mercury4.0_26Jun2018_Final_CoverPage.pdf (accessed on 30 July 2023).
- Greffier, J.; Barbotteau, Y.; Gardavaud, F. iQMetrix-CT: New software for task-based image quality assessment of phantom CT images. Diagn. Interv. Imaging 2022, 103, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Richard, S.; Husarik, D.B.; Yadava, G.; Murphy, S.N.; Samei, E. Towards task-based assessment of CT performance: System and object MTF across different reconstruction algorithms. Med. Phys. 2012, 39, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Samei, E.; Bakalyar, D.; Boedeker, K.L.; Brady, S.; Fan, J.; Leng, S.; Myers, K.J.; Popescu, L.M.; Ramirez Giraldo, J.C.; Ranallo, F.; et al. Performance evaluation of computed tomography systems: Summary of AAPM Task Group 233. Med. Phys. 2019, 46, e735–e756. [Google Scholar] [CrossRef]
- Eckstein, M.; Bartroff, J.; Abbey, C.; Whiting, J.; Bochud, F. Automated computer evaluation and optimization of image compression of X-ray coronary angiograms for signal known exactly detection tasks. Opt. Express 2003, 11, 460–475. [Google Scholar] [CrossRef] [PubMed]




| CT Scan Model | SOMATOM Edge | SOMATOM X.Cite | SOMATOM Force | |
|---|---|---|---|---|
| SFCT system generation | 1st generation | 2nd generation | - | |
| AuSn filter thickness | Au (mm) | 0.05 | 0.07 | - |
| Sn (mm) | 0.60 | 0.70 | - | |
| Tube voltage (kVp) | AuSn120 | AuSn120 | 100/Sn150 | |
| Pitch factor | 0.3 | 0.3 | 0.6 | |
| Rotation time (rot/s) | 0.33 | 0.3 | 0.5 | |
| Beam collimation (mm) | 38.4 | 38.4 | 38.4 | |
| CTDIvol (mGy) | 26 cm diameter 31 cm diameter 36 cm diameter | 6.89 9.10 11.99 | 6.95 9.18 12.15 | 6.86 9.10 12.04 |
| NPS Peak (HU2·mm2) | fpeak (mm−1) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Diameter | 40 keV | 50 keV | 60 keV | 70 keV | 40 keV | 50 keV | 60 keV | 70 keV | |
| SFCT-1st | 26 cm | 3514/1369 | 1157/696 | 414/407 | 288 | 0.03/0.16 | 0.03/0.16 | 0.05/0.16 | 0.16 |
| 31 cm | 8675/2862 | 2805/1439 | 933/808 | 561 | 0.03/0.16 | 0.03/0.16 | 0.05/0.16 | 0.16 | |
| 36 cm | 80824/6322 | 24714/3036 | 6606/1574 | 1350/1057 | 0.02/0.15 | 0.02/0.15 | 0.02/0.16 | 0.02/0.16 | |
| SFCT-2nd | 26 cm | 2427/922 | 781/486 | 261/285 | 199 | 0.04/0.16 | 0.04/0.17 | 0.05/0.17 | 0.17 |
| 31 cm | 5370/1845 | 1753/936 | 566/531 | 370 | 0.04/0.15 | 0.04/0.15 | 0.04/0.16 | 0.17 | |
| 36 cm | 12320/3755 | 3978/1853 | 1198/1026 | 719 | 0.02/0.14 | 0.02/0.14 | 0.02/0.14 | 0.15 | |
| DSCT | 26 cm | 1131/1015 | 536 | 320 | 214 | 0.07/0.21 | 0.21 | 0.21 | 0.21 |
| 31 cm | 2232/1873 | 981 | 579 | 384 | 0.07/0.17 | 0.17 | 0.17 | 0.17 | |
| 36 cm | 4541/4248 | 1757/1729 | 826/962 | 632 | 0.02/0.07 | 0.01/0.07 | 0.01/0.15 | 0.15 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Greffier, J.; Van Ngoc Ty, C.; Fitton, I.; Frandon, J.; Beregi, J.-P.; Dabli, D. Impact of Phantom Size on Low-Energy Virtual Monoenergetic Images of Three Dual-Energy CT Platforms. Diagnostics 2023, 13, 3039. https://doi.org/10.3390/diagnostics13193039
Greffier J, Van Ngoc Ty C, Fitton I, Frandon J, Beregi J-P, Dabli D. Impact of Phantom Size on Low-Energy Virtual Monoenergetic Images of Three Dual-Energy CT Platforms. Diagnostics. 2023; 13(19):3039. https://doi.org/10.3390/diagnostics13193039
Chicago/Turabian StyleGreffier, Joël, Claire Van Ngoc Ty, Isabelle Fitton, Julien Frandon, Jean-Paul Beregi, and Djamel Dabli. 2023. "Impact of Phantom Size on Low-Energy Virtual Monoenergetic Images of Three Dual-Energy CT Platforms" Diagnostics 13, no. 19: 3039. https://doi.org/10.3390/diagnostics13193039






