Keystroke Biometrics as a Tool for the Early Diagnosis and Clinical Assessment of Parkinson’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Source and Subjects
2.2. Data Processing
2.3. Management of Outliers
2.4. Assessments of Fluctuation of the Time Intervals
2.5. Statistical Analyses
3. Results
3.1. Demographic Data of the Participants
3.2. Comparisons of Clinical Severity and Keystroke Parameters in Controls and Patients with De Novo and Early PD
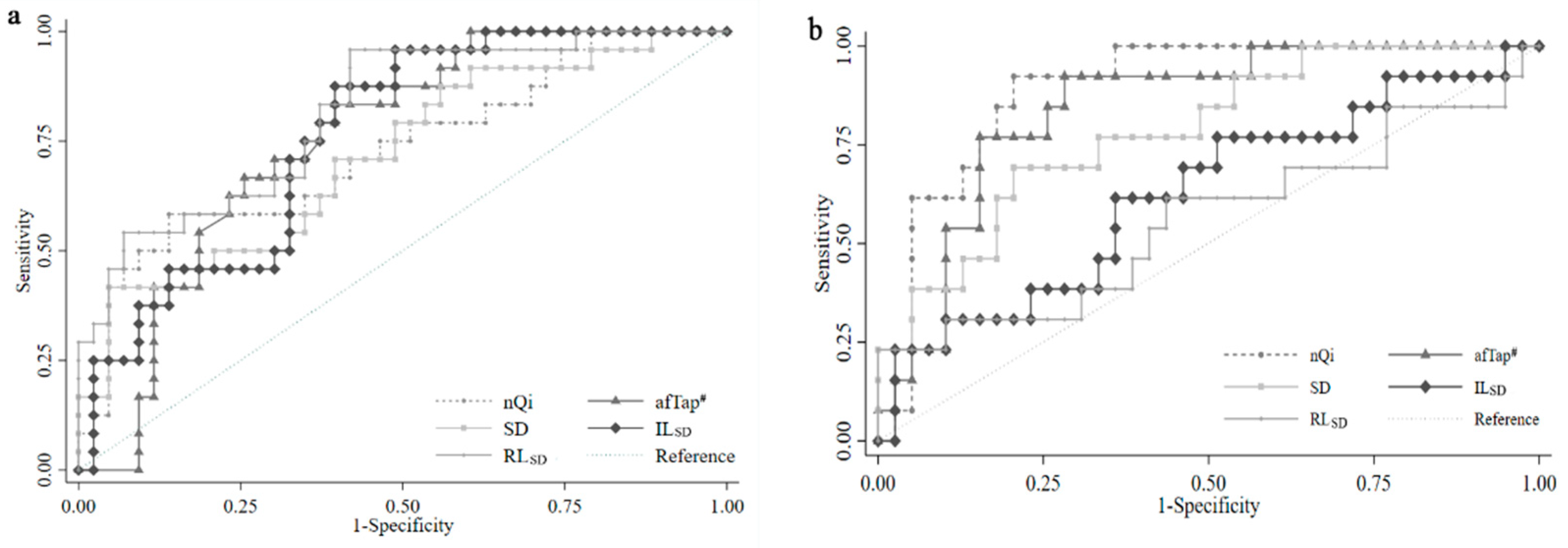
3.3. The Value of Keystroke Biometric Parameters for Early Diagnosis of PD
3.4. The Correlations between Clinical Severity and Keystroke Biometric Parameters in Patients with PD
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Kalia, L.V.; Lang, A.E. Parkinson’s disease. Lancet 2015, 386, 896–912. [Google Scholar] [CrossRef] [PubMed]
- Connolly, B.S.; Lang, A.E. Pharmacological treatment of Parkinson disease: A review. JAMA 2014, 311, 1670–1683. [Google Scholar] [CrossRef] [PubMed]
- Thanvi, B.; Lo, N.; Robinson, T. Levodopa-induced dyskinesia in Parkinson’s disease: Clinical features, pathogenesis, prevention and treatment. Postgrad. Med. J. 2007, 83, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Mason, S.; Foltynie, T.; Winder-Rhodes, S.; Barker, R.A.; Williams-Gray, C.H. Motor complications in Parkinson’s disease: 13-year follow-up of the CamPaIGN cohort. Mov. Disord. 2020, 35, 185–190. [Google Scholar] [CrossRef]
- Stoker, T.B.; Barker, R.A. Recent developments in the treatment of Parkinson’s Disease. F1000Research 2020, 9, 862. [Google Scholar] [CrossRef]
- Postuma, R.B.; Aarsland, D.; Barone, P.; Burn, D.J.; Hawkes, C.H.; Oertel, W.; Ziemssen, T. Identifying prodromal Parkinson’s disease: Pre-motor disorders in Parkinson’s disease. Mov. Disord. 2012, 27, 617–626. [Google Scholar] [CrossRef]
- Akhtar, R.S.; Stern, M.B. New concepts in the early and preclinical detection of Parkinson’s disease: Therapeutic implications. Expert. Rev. Neurother. 2012, 12, 1429–1438. [Google Scholar] [CrossRef]
- Lawrence, A.D.; Evans, A.H.; Lees, A.J. Compulsive use of dopamine replacement therapy in Parkinson’s disease: Reward systems gone awry? Lancet Neurol. 2003, 2, 595–604. [Google Scholar] [CrossRef]
- Farzanehfar, P.; Woodrow, H.; Braybrook, M.; Osborn, S.; Evans, A.; Nicklason, F.; Horne, M. Objective measurement in routine care of people with Parkinson’s disease improves outcomes. NPJ Park. Dis. 2018, 4, 10. [Google Scholar] [CrossRef]
- Adler, C.H.; Beach, T.G.; Hentz, J.G.; Shill, H.A.; Caviness, J.N.; Driver-Dunckley, E.; Sabbagh, M.N.; Sue, L.I.; Jacobson, S.A.; Belden, C.M.; et al. Low clinical diagnostic accuracy of early vs. advanced Parkinson disease: Clinicopathologic study. Neurology 2014, 83, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Adams, W.R. High-accuracy detection of early Parkinson’s Disease using multiple characteristics of finger movement while typing. PLoS ONE 2017, 12, e0188226. [Google Scholar] [CrossRef] [PubMed]
- Miyasaki, J.M.; Martin, W.; Suchowersky, O.; Weiner, W.J.; Lang, A.E. Practice parameter: Initiation of treatment for Parkinson’s disease: An evidence-based review: Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2002, 58, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Gwinn, K.; David, K.K.; Swanson-Fischer, C.; Albin, R.; Hillaire-Clarke, C.S.; Sieber, B.A.; Lungu, C.; Bowman, F.D.; Alcalay, R.N.; Babcock, D.; et al. Parkinson’s disease biomarkers: Perspective from the NINDS Parkinson’s Disease Biomarkers Program. Biomark. Med. 2017, 11, 451–473. [Google Scholar] [CrossRef]
- Movement Disorder Society Task Force on Rating Scales for Parkinson’s Disease. The unified Parkinson’s disease rating scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar]
- Grill, S.; Weuve, J.; Weisskopf, M.G. Predicting outcomes in Parkinson’s disease: Comparison of simple motor performance measures and The Unified Parkinson’s Disease Rating Scale-III. J. Park. Dis. 2011, 1, 287–298. [Google Scholar] [CrossRef]
- Lo, C.; Arora, S.; Lawton, M.; Barber, T.; Quinnell, T.; Dennis, G.J.; Ben-Shlomo, Y.; Hu, M.T.-M. A composite clinical motor score as a comprehensive and sensitive outcome measure for Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 2022, 93, 617. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Xiong, W.X.; Liu, F.T.; Sun, Y.M.; Luo, S.; Ding, Z.T.; Wu, J.J.; Wang, J. Objective and quantitative assessment of motor function in Parkinson’s disease-from the perspective of practical applications. Ann. Transl. Med. 2016, 4, 90. [Google Scholar] [CrossRef]
- Deb, R.; An, S.; Bhat, G.; Shill, H.; Ogras, U.Y.J.S. A systematic survey of research trends in technology usage for Parkinson’s disease. Sensors 2022, 22, 5491. [Google Scholar] [CrossRef]
- Arora, S.; Venkataraman, V.; Zhan, A.; Donohue, S.; Biglan, K.M.; Dorsey, E.R.; Little, M.A. Detecting and monitoring the symptoms of Parkinson’s disease using smartphones: A pilot study. Park. Relat. Disord. 2015, 21, 650–653. [Google Scholar] [CrossRef]
- Espay, A.J.; Bonato, P.; Nahab, F.B.; Maetzler, W.; Dean, J.M.; Klucken, J.; Eskofier, B.M.; Merola, A.; Horak, F.; Lang, A.E.; et al. Technology in Parkinson’s disease: Challenges and opportunities. Mov. Disord. 2016, 31, 1272–1282. [Google Scholar] [CrossRef] [PubMed]
- Douhou, S.; Magnus, J.R. The reliability of user authentication through keystroke dynamics. Stat. Neerl. 2009, 63, 432–449. [Google Scholar] [CrossRef]
- Thomas, P.A.; Preetha Mathew, K. A broad review on non-intrusive active user authentication in biometrics. J. Ambient Intell. Humaniz. Comput. 2023, 14, 339–360. [Google Scholar] [CrossRef] [PubMed]
- Acien, A.; Morales, A.; Vera-Rodriguez, R.; Fierrez, J.; Mondesire-Crump, I.; Arroyo-Gallego, T. Detection of mental fatigue in the general population: Feasibility study of keystroke dynamics as a real-world biomarker. JMIR Biomed. Eng. 2022, 7, e41003. [Google Scholar] [CrossRef]
- Demir, B.; Ulukaya, S.; Erdem, O. Detection of Parkinson’s disease with keystroke data. Comput. Methods Biomech. Biomed. 2023, 26, 1653–1667. [Google Scholar] [CrossRef]
- Bernardo, L.S.; Damaševičius, R.; Ling, S.H.; de Albuquerque, V.H.C.; Tavares, J. Modified SqueezeNet architecture for Parkinson’s disease detection based on keypress data. Biomedicines 2022, 10, 2746. [Google Scholar] [CrossRef]
- Tripathi, S.; Arroyo-Gallego, T.; Giancardo, L. Keystroke-Dynamics for Parkinson’s disease signs detection in an at-home uncontrolled population: A new benchmark and method. IEEE. Trans. Biomed. Eng 2023, 70, 182–192. [Google Scholar] [CrossRef]
- Roy, S.; Roy, U.; Sinha, D.; Pal, R.K. Imbalanced ensemble learning in determining Parkinson’s disease using Keystroke dynamics. Expert Syst. Appl. 2023, 217, 119522. [Google Scholar] [CrossRef]
- Giancardo, L.; Sánchez-Ferro, A.; Arroyo-Gallego, T.; Butterworth, I.; Mendoza, C.S.; Montero, P.; Matarazzo, M.; Obeso, J.A.; Gray, M.L.; Estépar, R.S. Computer keyboard interaction as an indicator of early Parkinson’s disease. Sci. Rep. 2016, 6, 34468. [Google Scholar] [CrossRef]
- Goldberger, A.L.; Amaral, L.A.N.; Glass, L.; Hausdorff, J.M.; Ivanov PCh Mark, R.G.; Mietus, J.E.; Moody, G.B.; Peng, C.-K.; Stanley, H.E. PhysioBank, PhysioToolkit, and PhysioNet: Components of a New Research Resource for Complex Physiologic Signals. Circulation 2000, 101, e215–e220. [Google Scholar] [CrossRef]
- Acien, A.; Morales, A.; Monaco, J.V.; Vera-Rodriguez, R.; Fierrez, J. TypeNet: Deep learning keystroke biometrics. IEEE Trans. Biom. Behav. 2022, 4, 57–70. [Google Scholar] [CrossRef]
- Behrens, J.T. Principles and procedures of exploratory data analysis. Psychol. Methods 1997, 2, 131–160. [Google Scholar] [CrossRef]
- Lan, B.L.; Yeo, J.H.W. Comparison of computer-key-hold-time and alternating-finger-tapping tests for early-stage Parkinson’s disease. PLoS ONE 2019, 14, e0219114. [Google Scholar] [CrossRef] [PubMed]
- DeLong, E.R.; DeLong, D.M.; Clarke-Pearson, D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988, 44, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Youden, W.J. Index for rating diagnostic tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, D.M. Identification of Outliers; Monographs on Statistics and Applied Probability; Springer: Berlin/Heidelberg, Germany, 1980. [Google Scholar] [CrossRef]
- Maxim, L.D.; Niebo, R.; Utell, M.J. Screening tests: A review with examples. Inhal. Toxicol. 2014, 26, 811–828. [Google Scholar] [CrossRef]
- Ganguly, J.; Kulshreshtha, D.; Almotiri, M.; Jog, M. Muscle tone physiology and abnormalities. Toxins 2021, 13, 282. [Google Scholar] [CrossRef]
- di Biase, L.; Di Santo, A.; Caminiti, M.L.; De Liso, A.; Shah, S.A.; Ricci, L.; Di Lazzaro, V. Gait analysis in Parkinson’s disease: An overview of the most accurate markers for diagnosis and symptoms monitoring. Sensors 2020, 20, 3529. [Google Scholar] [CrossRef]
- Meigal, A.Y.; Rissanen, S.M.; Tarvainen, M.P.; Airaksinen, O.; Kankaanpää, M.; Karjalainen, P.A. Non-linear EMG parameters for differential and early diagnostics of Parkinson’s disease. Front. Neurol. 2013, 4, 135. [Google Scholar] [CrossRef]
- Amato, F.; Borzì, L.; Olmo, G.; Orozco-Arroyave, J.R. An algorithm for Parkinson’s disease speech classification based on isolated words analysis. Health Inf. Sci. 2021, 9, 32. [Google Scholar] [CrossRef]
- Holden, S.K.; Finseth, T.; Sillau, S.H.; Berman, B.D. Progression of MDS-UPDRS Scores over five years in de novo Parkinson Disease from the Parkinson’s Progression Markers Initiative Cohort. Mov. Disord. Clin. Pract. 2018, 5, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Evers, L.J.W.; Krijthe, J.H.; Meinders, M.J.; Bloem, B.R.; Heskes, T.M. Measuring Parkinson’s disease over time: The real-world within-subject reliability of the MDS-UPDRS. Mov. Disord. 2019, 34, 1480–1487. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.H.; Twose, J.; Lissenberg-Witte, B.; Licitra, G.; Meijer, K.; Uitdehaag, B.; De Groot, V.; Killestein, J. The Use of Smartphone keystroke dynamics to passively monitor upper limb and cognitive function in multiple sclerosis: Longitudinal analysis. J. Med. Internet Res. 2022, 24, e37614. [Google Scholar] [CrossRef] [PubMed]
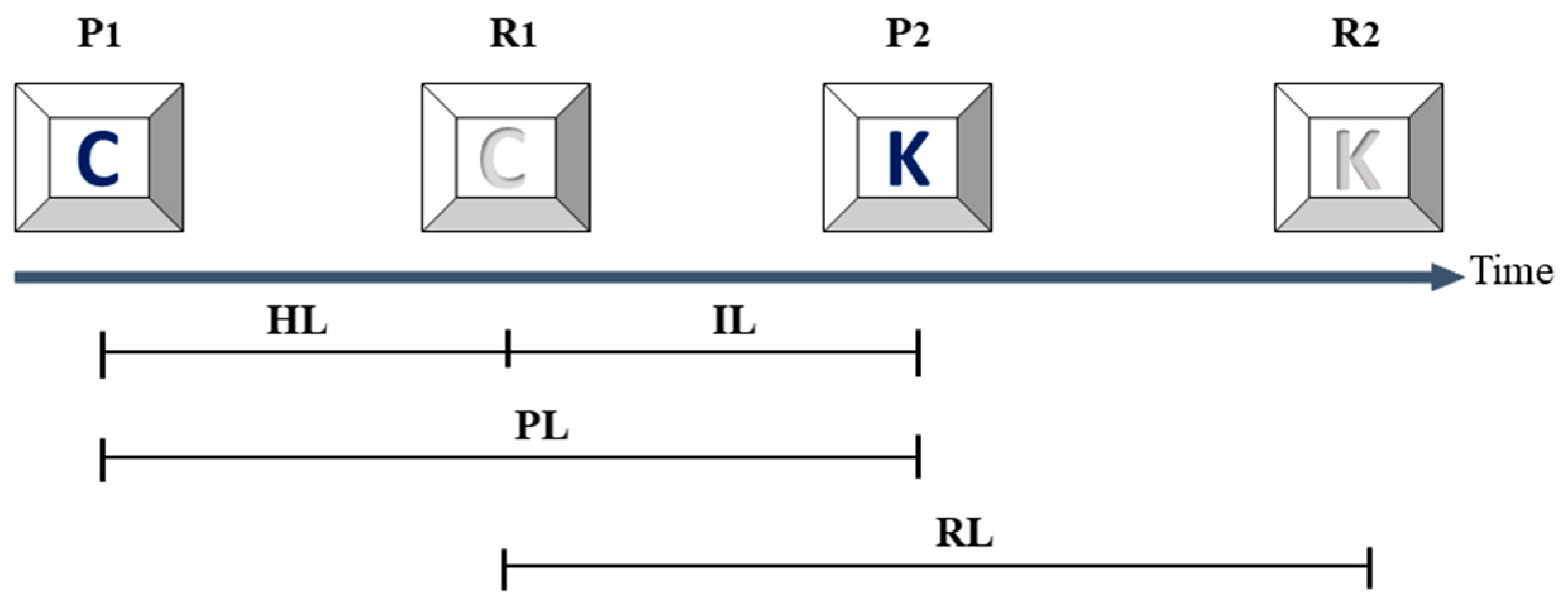
| Parameter | Healthy Controls (n = 43) | De Novo PD (n = 24) | Early PD (n = 18) |
|---|---|---|---|
| Age | 60.10 ± 10.20 | 61.4 ± 10.50 | 55.90 ± 8.00 |
| Sex (male) | 17 (40%) | 14 (58%) | 10 (56%) |
| Average duration after diagnosis (years) | 0 | 1.60 ± 1.22 | 3.89 ± 1.23 * |
| Average education (years) | 15.30 ± 5.20 | 15.50 ± 3.80 | 14.83 ± 4.60 |
| No. of outliers (%) | 0.56 ± 0.64 | 0.40 ± 0.53 | 0.36 ± 0.42 |
| Parameter | Healthy Controls (n = 43) | De Novo PD (n = 24) | Early PD (n = 18) |
|---|---|---|---|
| UPDRS-III (range) | 1.92 ± 1.79 (0~6) | 19.33 ± 6.70 * (7~36) | 22.32 ± 8.69 † (11~40) |
| Typing speed (words/min) | 112.34 ± 58.75 | 97.20 ± 42.53 | 98.86 ± 45.94 |
| No. samples | 1634.33 ± 793.04 | 1454.21 ± 497.72 | 1320.56 ± 581.98 |
| nQi | 0.06 ± 0.06 | 0.12 ± 0.10 * | 0.14 ± 0.06 † |
| sTap (msec) | 170.85 ± 16.45 | 165.48 ± 24.24 | 159.42 ± 24.13 |
| afTap # (msec) | 128.99 ± 27.85 | 94.85 ± 23.54 * | 96.33 ± 19.75 † |
| SD | 0.34 ± 0.08 | 0.41 ± 0.11 * | 0.46 ± 0.14 † |
| HLSD | 0.50 ± 0.16 | 0.57 ± 0.15 | 0.53 ± 0.15 |
| ILSD | 1.15 ± 0.15 | 1.28 ± 0.11 * | 1.27 ± 0.16 † |
| PLSD | 0.95 ± 0.13 | 1.01 ± 0.16 | 1.02 ± 0.22 |
| RLSD | 1.07 ± 0.12 | 1.23 ± 0.14 * | 1.20 ± 0.19 † |
| Parameter | De Novo PD | Early PD | ||
|---|---|---|---|---|
| Sensitivity | Specificity | Sensitivity | Specificity | |
| nQi | 58% | 86% | 94% | 79% |
| afTap # | 83% | 60% | 92% | 72% |
| SD | 42% | 95% | 72% | 77% |
| ILSD | 88% | 60% | 44% | 91% |
| RLSD | 96% | 58% | 44% | 100% |
| Parameter | De Novo PD | Early PD |
|---|---|---|
| (n = 24) | (n = 18) | |
| Typing speed | −0.371 | −0.757 † |
| No. samples | −0.452 * | −0.733 † |
| nQi | 0.243 | 0.353 |
| sTap | −0.112 | 0.077 |
| afTap | −0.484 * | −0.095 |
| SD | 0.089 | 0.212 |
| HLSD | −0.005 | −0.019 |
| ILSD | −0.09 | −0.283 |
| PLSD | 0.274 | 0.347 |
| RLSD | −0.085 | −0.144 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.-M.; Yeh, C.-L.; Chen, P.-W.; Lin, C.-W.; Liu, A.-B. Keystroke Biometrics as a Tool for the Early Diagnosis and Clinical Assessment of Parkinson’s Disease. Diagnostics 2023, 13, 3061. https://doi.org/10.3390/diagnostics13193061
Liu W-M, Yeh C-L, Chen P-W, Lin C-W, Liu A-B. Keystroke Biometrics as a Tool for the Early Diagnosis and Clinical Assessment of Parkinson’s Disease. Diagnostics. 2023; 13(19):3061. https://doi.org/10.3390/diagnostics13193061
Chicago/Turabian StyleLiu, Wei-Min, Che-Lun Yeh, Po-Wei Chen, Che-Wei Lin, and An-Bang Liu. 2023. "Keystroke Biometrics as a Tool for the Early Diagnosis and Clinical Assessment of Parkinson’s Disease" Diagnostics 13, no. 19: 3061. https://doi.org/10.3390/diagnostics13193061






