Subtle Changes in Myocardial Work Indices Assessed by 2D-Speckle Tracking Echocardiography Are Linked with Pathological LV Remodeling and MACEs Following an Acute Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Data Extraction
2.3. Definition of Covariates
2.4. Blood Tests
2.5. PCI
2.6. Echocardiography
2.6.1. Conventional Echocardiography
2.6.2. D-STE
2.6.3. Myocardial Work Determinations
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of the STEMI Patients
3.2. Angiographic Results
3.3. Echocardiographic Data
3.4. Independent Predictors of 3-Months LV Remodeling
3.5. Independent Predictors of MACEs
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Opie, L.H.; Commerford, P.J.; Gersh, B.J.; Pfeffer, M.A. Controversies in ventricular remodeling. Lancet 2006, 367, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Kramer, D.G.; Patel, A.R.; Maron, M.S.; Udelson, J.E. Left ventricular remodeling in heart failure: Current concepts in clinical significance and assessment. JACC Cardiovasc. Imaging 2011, 4, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, L.; Neskovic, A.N.; Parodi, G.; Cerisano, G.; Buonamici, P.; Santoro, G.M.; Antoniucci, D. Left ventricular remodeling after primary coronary angioplasty: Patterns of left ventricular dilation and long-term prognostic implications. Circulation 2002, 106, 2351–2357. [Google Scholar] [CrossRef] [PubMed]
- Møller, J.E.; Hillis, G.S.; Oh, J.K.; Reeder, G.S.; Gersh, B.J.; Pellikka, P.A. Wall motion score index and ejection fraction for risk stratification after acute myocardial infarction. Am. Heart J. 2006, 151, 419–425. [Google Scholar] [CrossRef]
- Suzuki, S.; Yoshimura, M.; Nakayama, M.; Mizuno, Y.; Harada, E.; Ito, T.; Nakamura, S.; Abe, K.; Yamamuro, M.; Sakamoto, T.; et al. Plasma level of B-type natriuretic peptide as a prognostic marker after acute myocardial infarction: A long-term follow-up analysis. Circulation 2004, 110, 1387–1391. [Google Scholar] [CrossRef]
- Liu, Y.W.; Tsai, W.C.; Su, C.T.; Lin, C.C.; Chen, J.H. Evidence of left ventricular systolic dysfunction detected by auto-mated function imaging in patients with heart failure and preserved left ventricular ejection fraction. J. Card. Fail. 2009, 15, 782–789. [Google Scholar] [CrossRef]
- Chan, J.; Shiino, K.; Obonyo, N.G.; Hanna, J.; Chamberlain, R.; Small, A.; Scalia, I.; Scalia, W.; Yamada, A.; Hamilton-Craig, C.; et al. Left ventricular global strain analysis by two-dimensional speckle-tracking echocardiography: The learning curve. J. Am. Soc. Echocardiogr. 2017, 30, 1081–1090. [Google Scholar] [CrossRef]
- Hubert, A.; Le Rolle, V.; Leclercq, C.; Galli, E.; Samset, E.; Casset, C.; Mabo, P.; Hernandez, A.; Donal, E. Estimation of myocardial work from pressure-strain loops analysis: An experimental evaluation. Eur. Heart J. Cardiovasc. Imaging 2018, 19, 1372–1379. [Google Scholar] [CrossRef]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Remme, E.W.; Haugaa, K.H.; Opdahl, A.; Fjeld, J.G.; Gjesdal, O.; et al. A novel clinical method for quantification of regional left ventricular pressure-strain loop area: A non-invasive index of myocardial work. Eur. Heart J. 2012, 33, 724–733. [Google Scholar] [CrossRef]
- Chan, J.; Edwards, N.F.A.; Khandheria, B.J.; Shiino, K.; Sabapathy, S.; Anderson, B.A.; Chamberlain, R.; Scalia, G.M. A new approach to assess myocardial work by non-invasive left ventricular pressure-strain relations in hypertension and dilated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 31–39. [Google Scholar] [CrossRef]
- Boe, E.; Russell, K.; Eek, C.; Eriksen, M.; Remme, E.W.; Smiseth, O.A.; Skulstad, H. Noninvasive myocardial work index identifies acute coronary occlusion in patients with non-ST-segment elevation-acute coronary syndrome. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [PubMed]
- Kourosh, S.; Diana, S.; Hassan, J. Prediction of survival after myocardial infarction using Killip class. Int. J. Clin. Med. 2012, 3, 563–568. [Google Scholar]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- The TIMI Study Group. The thrombolysis in myocardial infarction (TIMI) trial. New Engl. J. Med. 1985, 312, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Lang, R.M.; Bierig, M.; Devereux, R.B.; Flachskampf, F.A.; Foster, E.; Pellikka, P.A.; Picard, M.H.; Roman, M.J.; Seward, J.; Shanewise, J.S.; et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J. Am. Soc. Echocardiogr. 2005, 18, 1440–1463. [Google Scholar]
- Quiñones, M.A.; Otto, C.M.; Stoddard, M.; Waggoner, A.; Zoghbi, W.A. Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. Recommendations for quantifica-tion of Doppler echocardiography: A report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2002, 15, 167–184. [Google Scholar]
- Ingul, C.B.; Malm, S.; Refsdal, E.; Hegbom, K.; Amundsen, B.H.; Støylen, A.A. Recovery of function after acute myocardial infarction evaluated by tissue Doppler strain and strain rate. J. Am. Soc. Echocardiogr. 2010, 23, 432–438. [Google Scholar] [CrossRef]
- Edwards, N.F.A.; Scalia, G.M.; Shiino, K.; Sabapathy, S.; Anderson, B.; Chamberlain, R.; Khandheria, B.K.; Chan, J. Global Myocardial Work Is Superior to Global Longitudinal Strain to Predict Significant Coronary Artery Disease in Patients With Normal Left Ventricular Function and Wall Motion. J. Am. Soc. Echocardiogr. 2019, 32, 947–957. [Google Scholar] [CrossRef]
- Gheorghiu, A.; Arnautu, S.-F.; Slovenski, M.; Malița, C.D.; Tomescu, M.C.; Arnautu, D.A. Myocardial Work Evaluation—A Useful Non-Invasive Method to Predict Coronary Artery Sub-Occlusion in a Patient with Unstable Angina and Multiple Myocardial Revascularization Interventions. Diagnostics 2023, 13, 1459. [Google Scholar] [CrossRef]
- Leancă, S.A.; Crișu, D.; Petriș, A.O.; Afrăsânie, I.; Genes, A.; Costache, A.D.; Tesloianu, D.N.; Costache, I.I. Left Ventricular Remodeling after Myocardial Infarction: From Physiopathology to Treatment. Life 2022, 12, 1111. [Google Scholar] [CrossRef] [PubMed]
- Skoric, B.; Milicic, D.; Lovric, D.; Gornik, I.; Narancic Skoric, C.; Sertic, J. Initial patency of the infarct-related artery in patients with acute ST elevation myocardial infarction is related to platelet response to aspirin. Int. J. Cardiol. 2010, 140, 356–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russell, K.; Eriksen, M.; Aaberge, L.; Wilhelmsen, N.; Skulstad, H.; Gjesdal, O.; Edvardsen, H.; Smiseth, O.A. Assessment of wasted myocardial work: A novel method to quantify energy loss due to uncoordinated left ventricular contractions. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H996–H1003. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, K.; Özden Tok, Ö.; Mitrousi, K.; Ikonomidis, I. Myocardial Work: Methodology and Clinical Applications. Diagnostics 2021, 11, 573. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, R.P.; van der Bijl, P.; El Mahdiui, M.; Montero-Cabezas, J.M.; Kostyukevich, M.V.; Marsan, N.A.; Bax, J.J.; Delgado, V. Noninvasive Myocardial Work Indices 3 Months after ST-Segment Elevation Myocardial Infarction: Prevalence and Characteristics of Patients with Postinfarction Cardiac Remodeling. J. Am. Soc. Echocardiogr. 2020, 33, 1172–1179. [Google Scholar] [CrossRef] [PubMed]
- Lustosa, R.P.; Fortuni, F.; van der Bijl, P.; Goedemans, L.; El Mahdiui, M.; Montero-Cabezas, J.M.; Kostyukevich, M.V.; Marsan, N.A.; Bax, J.J.; Delgado, V.; et al. Left ventricular myocardial work in the culprit vessel territory and impact on left ventricular remodelling in patients with ST-segment elevation myocardial infarction after primary percutaneous coronary intervention. Eur. Heart J. Cardiovasc. Imaging 2021, 22, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Coisne, A.; Fourdinier, V.; Lemesle, G.; Delsart, P.; Aghezzaf, S.; Lamblin, N.; Schurtz, G.; Verdier, B.; Ninni, S.; Delobelle, A.; et al. Clinical significance of myocardial work parameters after acute myocardial infarction. Eur. Heart J. Open 2022, 2, oeac037. [Google Scholar] [CrossRef] [PubMed]
- Jin, W.; Wang, L.; Zhu, T.; Ma, Y.; Yu, C.; Zhang, F. Usefulness of echocardiographic myocardial work in evaluating the microvascular perfusion in STEMI patients after revascularization. BMC Cardiovasc. Disord. 2022, 22, 218–227. [Google Scholar] [CrossRef]
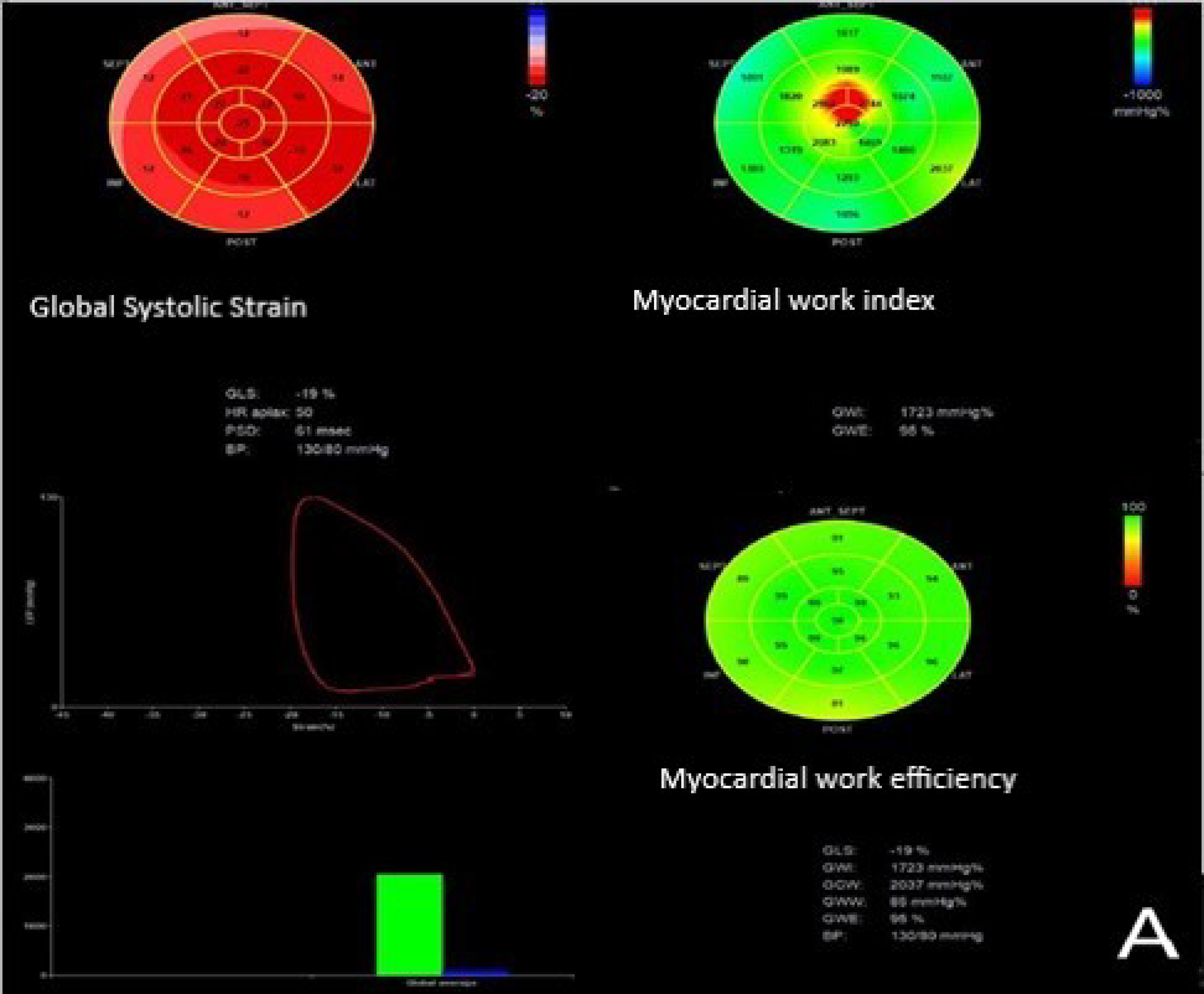





| Parameter | Group A No LV Remodeling (n = 188) | Group B LV Remodeling (n = 58) | All STEMI Patients with LVEF ≥ 50% Following PCI (n = 246) | p Value |
|---|---|---|---|---|
| At baseline | ||||
| Age (years) | 64.8 ± 12 | 72.0 ± 13 | 66 ± 13 | <0.0001 |
| Male sex (n, %) | 130 (70) | 48 (82) | 178 (73) | 0.06 |
| Diabetes mellitus | 104 (56) | 34 (58) | 138 (56) | 0.78 |
| Systemic hypertension | 117 (63) | 54 (94) | 171 (70) | <0.0001 |
| Hypercholesterolemia | 140 (74) | 49 (85) | 189 (76) | 0.05 |
| Smoking history | 66 (35) | 29 (51) | 95 (39) | 0.03 |
| Obesity | 51 (27) | 11 (19) | 62 (25) | 0.22 |
| SBP (mmHg) | 129.2 ± 13.2 | 144.6 ± 13.5 | 130.8 ± 12.5 | <0.0001 |
| DBP (mmHg) | 78.5 ± 6.2 | 86.5 ± 18.1 | 73.7 ± 12.7 | <0.0001 |
| Heart rate (beats/min) | 81 ± 19 | 80 ± 21 | 80 ± 20 | 0.73 |
| Chronic renal failure | 49 (26) | 16 (28) | 65 (26) | 0.11 |
Killip class
| 51 (27) 109 (58) 18 (10) 10 (5) | 1 (2) 11 (19) 29 (50) 17 (29) | 52 (21) 120 (48) 47 (19) 27 (12) | <0.0001 <0.0001 <0.0001 <0.0001 |
| Peak CPK-MB (IU/L), median (25th, 75th percentile) | 220 (87.2, 449.7) | 317 (200.0, 620.0) | 251 (107.7, 449.2) | <0.01 |
| NT-proBNP (ng/L) median (25th, 75th percentile) | 227 (58, 310) | 921 (214, 2550) | 228 (83, 520) | 0.68 |
| eGFR (mL/min/1.73m2) | 75.8 ± 21.7 | 73.8 ± 23.0 | 79.4± | |
Culprit vessel
| 95 (51) 22 (12) 69 (37) 37 (20) | 22 (38) 8 (14) 24 (42%) 7 (12) | 97 (40) 30 (12) 93 (41) 44 (18) | 0.07 0.68 0.12 0.16 |
Coronary artery disease
| 126 (68) 32 (17) 16 (15) | 13 (22) 19 (33) 26 (45) | 139 (62) 51 (23) 42 (19) | <0.0001 <0.01 <0.0001 |
Medication at discharge
| 151 (81) 147 (79) 41 (22) 138 (74) | 45 (78) 44 (76) 14 (24) 42 (72) | 196 (80) 191 (78) 55 (23) 180 (74) | 0.60 0.62 0.74 0.75 |
| Major cardiac events during the 4-year follow-up | ||||
| Hospitalizations for HF n (%) | 17 (9) | 11 (19) | 29 (12) | 0.03 |
| Repeated PCI n (%) | 8 (4) | 7 (12) | 15 (6) | 0.02 |
| CABG n (%) | 3 (2) | 2 (3) | 5 (2) | 0.27 |
| Sudden cardiac deaths n (%) | 0 (0) | 0 (0) | 0 (0) | 1.00 |
| Total cardiac events n (%) | 28 (15%) | 20 (34%) | 48 (19%) | 0.01 |
| Parameter | Group A without LV Remodeling n = 188 | Group B with LV Remodeling n = 58 | All STEMI Patients n = 246 | p Value |
|---|---|---|---|---|
| Baseline | ||||
| LVEF (%) | 57.5 ± 7.1 | 56.3 ± 4.5 | 57.1 ± 6.1 | 0.22 |
| LVEDV (mL) | 106 ± 15 | 104 ± 12 | 105 ± 15 | 0.35 |
| LVESV (mL) | 44 ± 7.5 | 42 ± 8.6 | 43.5 ± 9.1 | 0.08 |
| Stroke volume index (mL/m2) | 41 ± 10.4 | 39.6 ± 11.4 | 40.3 ± 10.8 | 0.37 |
| E/A ratio | 1.09 ± 0.32 | 1.02 ± 0.28 | 1.07 ± 0.30 | 0.13 |
| WMSI | 2.23 ± 0.19 | 2.25 ± 0.23 | 2.23 ± 0.21 | 0.09 |
| GLS (%) | −18.3 ± 3.5 | −17.6 ± 3.9 | −18.0 ± 3.6 | 0.19 |
| GWI (mmHg%) | 10,886 + 465 | 1529 ± 142 | 1802 ± 439 | <0.0001 |
| GWE (%) | 89.6 ± 6.6 | 77.7 ± 4.3 | 87.2 ± 7.1 | <0.0001 |
| GWW (mmHg%) | 198.3 ± 47 | 224 ± 21 | 204.3 ± 52.8 | 0.001 |
| GCW (mmHg%) | 2110 ± 195 | 1622 ± 148 | 1994.40 ± 278 | <0.0001 |
| After 3 months | ||||
| LVEF (%) | 61.3 ± 7.2 | 56.7 ± 8.2 | 59.1 ± 7.6 | <0.0001 |
| LVEDV (mL) | 123 ± 27 | 132 ± 30 | 127.5 ± 28 | 0.03 |
| LVESV (mL) | 47 ± 14 | 52 ± 12 | 50 ± 13 | 0.01 |
| Stroke volume index (mL/m2) | 46.2 ± 5.5 | 43.5 ± 7.5 | 45.85 ± 6.5 | <0.01 |
| E/A ratio | 1.08 ± 0.30 | 1.01 ± 0.24 | 1.06 ± 0.31 | 0.10 |
| WMSI | 1.95 ± 0.3 | 2.14 ± 0.5 | 2.03 ± 0.3 | <0.001 |
| GLS (%) | −20.1 ± 2.8 | −19.2 ± 3.1 | −19.8 ± 2.9 | 0.03 |
| GWI (mmHg%) | 1938 ± 151 | 1868 ± 236 | 1921 ± 177 | <0.001 |
| GWE (%) | 91.3 ± 4.3 | 83.6 + 5.8 | 88.9 ± 5.7 | <0.001 |
| GWW (mmHg%) | 179.9 ± 55 | 209.0 ± 64 | 95.5 ± 68 | <0.001 |
| GCW (mmHg%) | 2206 ± 235 | 2080 ± 302 | 1656 ± 288 | 0.001 |
| Univariate Logistic Regression | Odds Ratio | 95% CI | p Value |
|---|---|---|---|
| Age (years) | 1.05 | 1.03–1.07 | <0.01 |
| Systemic hypertension | 3.72 | 1.35–9.62 | <0.01 |
| Hypercholesterolemia | 3.4 | 1.50–9.09 | <0.01 |
| Smoking | 0.5 | 0.28–0.83 | <0.01 |
| Killip class | 3.98 | 1.65–9.32 | <0.001 |
| Peak CPK-MB (IU/L) | 1.24 | 1.07–1.85 | <0.0001 |
| 2-vessel CAD | 2.4 | 1.16–4.28 | <0.02 |
| 3-vessel CAD | 3.8 | 1.80–7.34 | <0.0001 |
| Baseline GWI (mmHg%) | 3.68 | 2.53–3.35 | <0.0001 |
| Baseline GCW (mmHg%) | 2.94 | 3.17–4.18 | <0.0001 |
| Baseline GWE (%) | 0.72 | 0.66–0.79 | 0.01 |
| Baseline GWW (mmHg%) | 1.01 | 1.00–1.02 | 0.0001 |
| Multivariate logistic regression | Odds Ratio | 95% CI | p-value |
| Killip class | 2.44 | 1.18–5.01 | <0.001 |
| Baseline GWI (mmHg%) | 0.96 | 0.94–0.98 | <0.001 |
| Baseline GWE (%) | 0.56 | 0.40–0.78 | <0.001 |
| Univariate Logistic Regression | Multivariate Logistic Regression | |||||
|---|---|---|---|---|---|---|
| Odds Ratio | 095% CI | p Value | Odds Ratio | 95% CI | p Value | |
| Killip class | 2.75 | 1.89–3.98 | <0.0001 | 1.89 | 1.05–3.3 | <0.001 |
| 3-vessel CAD | 2.4 | 1.22–4.80 | <0.012 | - | - | - |
| NT-pro BNP (ng/L) | 1.00 | 1.00–1.00 | <0.01 | - | - | - |
| CK-MB (IU/L) | 1.00 | 1.00–1.00 | <0.01 | 1.00 | 1.00–1.00 | <0.01 |
| GWI | 0.99 | 0.99–0.99 | <0.0001 | 0.99 | 0.98–0.99 | <0.0001 |
| GWE | 0.81 | 0.76–0.86 | <0.0001 | 0.85 | 0.79–0.92 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arnautu, D.-A.; Gheorghiu, A.; Arnautu, S.-F.; Tomescu, M.-C.; Malita, C.-D.; Banciu, C.; Vacarescu, C.; Ionac, I.; Luca, S.; Cozma, D.; et al. Subtle Changes in Myocardial Work Indices Assessed by 2D-Speckle Tracking Echocardiography Are Linked with Pathological LV Remodeling and MACEs Following an Acute Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Diagnostics 2023, 13, 3108. https://doi.org/10.3390/diagnostics13193108
Arnautu D-A, Gheorghiu A, Arnautu S-F, Tomescu M-C, Malita C-D, Banciu C, Vacarescu C, Ionac I, Luca S, Cozma D, et al. Subtle Changes in Myocardial Work Indices Assessed by 2D-Speckle Tracking Echocardiography Are Linked with Pathological LV Remodeling and MACEs Following an Acute Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Diagnostics. 2023; 13(19):3108. https://doi.org/10.3390/diagnostics13193108
Chicago/Turabian StyleArnautu, Diana-Aurora, Alexandru Gheorghiu, Sergiu-Florin Arnautu, Mirela-Cleopatra Tomescu, Claudiu-Daniel Malita, Christian Banciu, Cristina Vacarescu, Ioana Ionac, Silvia Luca, Dragos Cozma, and et al. 2023. "Subtle Changes in Myocardial Work Indices Assessed by 2D-Speckle Tracking Echocardiography Are Linked with Pathological LV Remodeling and MACEs Following an Acute Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention" Diagnostics 13, no. 19: 3108. https://doi.org/10.3390/diagnostics13193108
APA StyleArnautu, D.-A., Gheorghiu, A., Arnautu, S.-F., Tomescu, M.-C., Malita, C.-D., Banciu, C., Vacarescu, C., Ionac, I., Luca, S., Cozma, D., Mornos, C., Gaita, D., & Luca, C.-T. (2023). Subtle Changes in Myocardial Work Indices Assessed by 2D-Speckle Tracking Echocardiography Are Linked with Pathological LV Remodeling and MACEs Following an Acute Myocardial Infarction Treated by Primary Percutaneous Coronary Intervention. Diagnostics, 13(19), 3108. https://doi.org/10.3390/diagnostics13193108








