Signal Thresholding Segmentation of Ventricular Volumes in Young Patients with Various Diseases—Can We Trust the Numbers?
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Imaging Technique
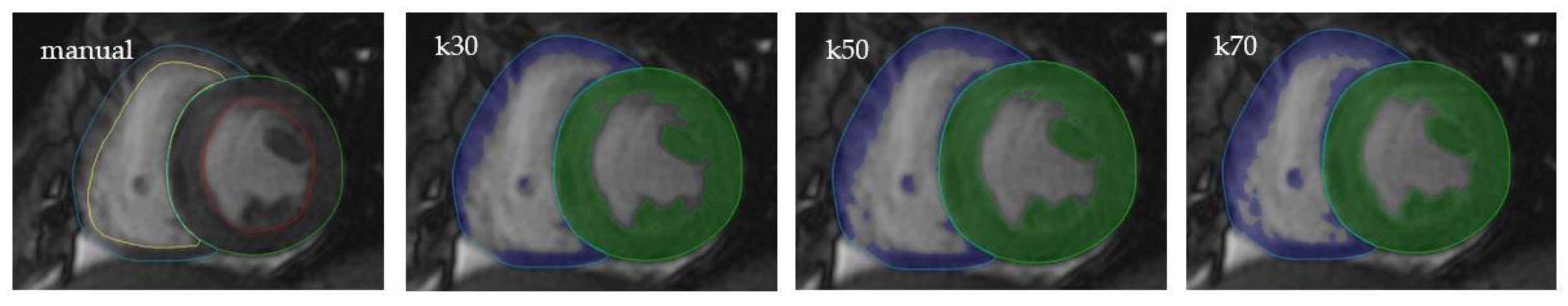
2.3. Image Analysis
2.4. Ventricular Volumes
2.5. Flow Volumes
2.6. Interobserver Variability
2.7. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Ventricular Volumes
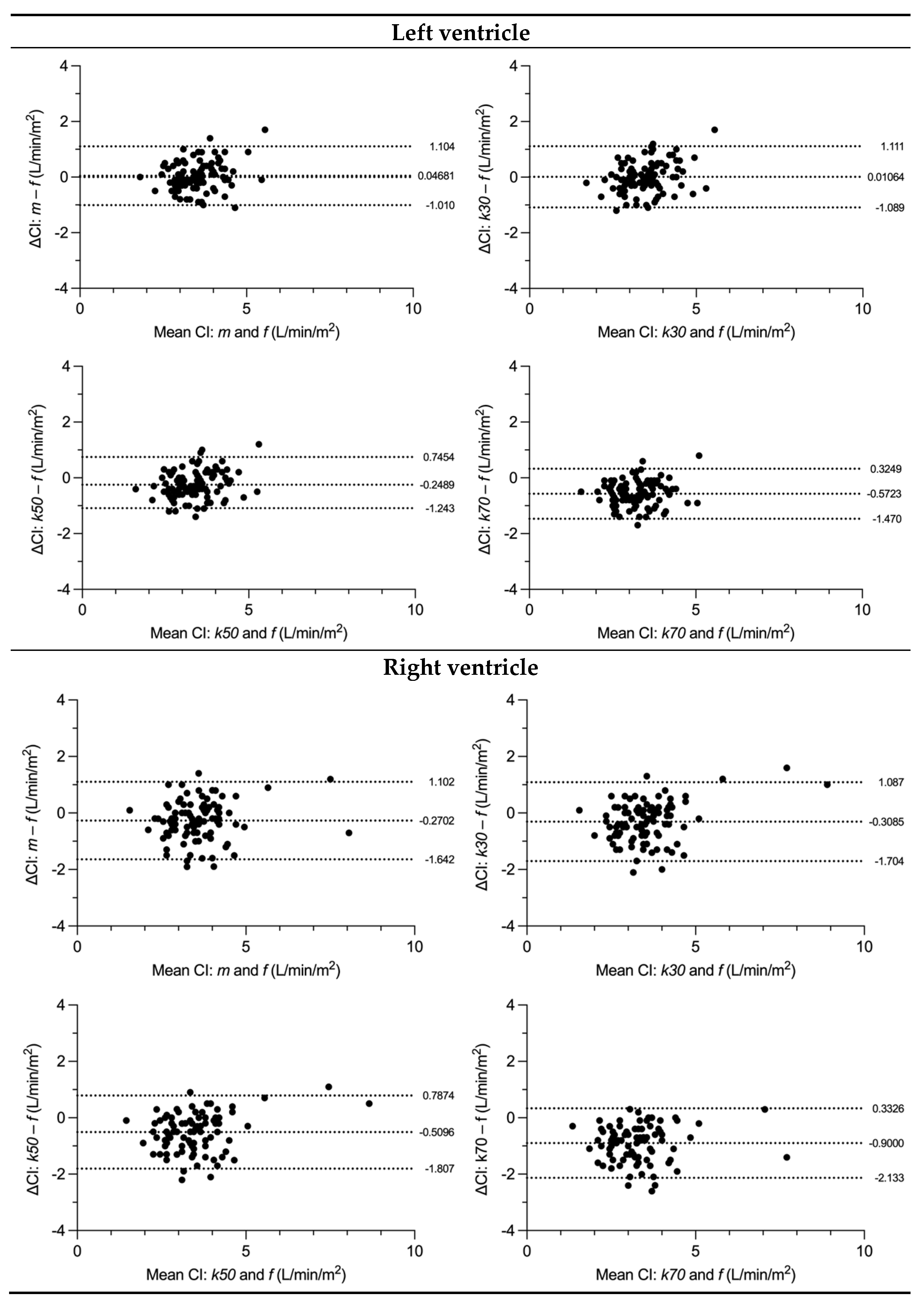
3.3. SV and CI Comparison
3.4. Variability
3.5. Reproducibility
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Koren, M.J.; Devereux, R.B.; Casale, P.N.; Savage, D.D.; Laragh, J.H. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann. Intern. Med. 1991, 114, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.V.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Candesartan in Heart Failure Reduction in Mortality (CHARM) Investigators Influence of ejection fraction on cardiovascular outcomes in a broad spectrum of heart failure patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef] [PubMed]
- Pattynama, P.M.; Lamb, H.J.; Van der Velde, E.A.; Van der Geest, R.J.; Van der Wall, E.E.; De Roos, A. Reproducibility of MRI-derived measurements of right ventricular volumes and myocardial mass. Magn. Reson. Imaging 1995, 13, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Buechel, E.R.V.; Dave, H.H.; Kellenberger, C.J.; Dodge-Khatami, A.; Pretre, R.; Berger, F.; Bauersfeld, U. Remodelling of the right ventricle after early pulmonary valve replacement in children with repaired tetralogy of Fallot: Assessment by cardiovascular magnetic resonance. Eur. Heart J. 2005, 26, 2721–2727. [Google Scholar] [CrossRef] [PubMed]
- van der Ven, J.P.G.; Sadighy, Z.; Valsangiacomo Buechel, E.R.; Sarikouch, S.; Robbers-Visser, D.; Kellenberger, C.J.; Kaiser, T.; Beerbaum, P.; Boersma, E.; Helbing, W.A. Multicentre reference values for cardiac magnetic resonance imaging derived ventricular size and function for children aged 0-18 years. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 102–113. [Google Scholar] [CrossRef]
- Buechel, E.V.; Kaiser, T.; Jackson, C.; Schmitz, A.; Kellenberger, C.J. Normal right- and left ventricular volumes and myocardial mass in children measured by steady state free precession cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2009, 11, 19. [Google Scholar] [CrossRef]
- Pattynama, P.M.; Lamb, H.J.; van der Velde, E.A.; van der Wall, E.E.; de Roos, A. Left ventricular measurements with cine and spin-echo MR imaging: A study of reproducibility with variance component analysis. Radiology 1993, 187, 261–268. [Google Scholar] [CrossRef]
- Grothues, F.; Smith, G.C.; Moon, J.C.C.; Bellenger, N.G.; Collins, P.; Klein, H.U.; Pennell, D.J. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002, 90, 29–34. [Google Scholar] [CrossRef]
- Sievers, B.; Kirchberg, S.; Bakan, A.; Franken, U.; Trappe, H.-J. Impact of papillary muscles in ventricular volume and ejection fraction assessment by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2004, 6, 9–16. [Google Scholar] [CrossRef]
- Papavassiliu, T.; Kühl, H.P.; Schröder, M.; Süselbeck, T.; Bondarenko, O.; Böhm, C.K.; Beek, A.; Hofman, M.M.B.; van Rossum, A.C. Effect of endocardial trabeculae on left ventricular measurements and measurement reproducibility at cardiovascular MR imaging. Radiology 2005, 236, 57–64. [Google Scholar] [CrossRef]
- Petitjean, C.; Dacher, J.-N. A review of segmentation methods in short axis cardiac MR images. Med. Image Anal. 2011, 15, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Janik, M.; Cham, M.D.; Ross, M.I.; Wang, Y.; Codella, N.; Min, J.K.; Prince, M.R.; Manoushagian, S.; Okin, P.M.; Devereux, R.B.; et al. Effects of papillary muscles and trabeculae on left ventricular quantification: Increased impact of methodological variability in patients with left ventricular hypertrophy. J. Hypertens. 2008, 26, 1677–1685. [Google Scholar] [CrossRef] [PubMed]
- Chuang, M.L.; Gona, P.; Hautvast, G.L.T.F.; Salton, C.J.; Blease, S.J.; Yeon, S.B.; Breeuwer, M.; O’Donnell, C.J.; Manning, W.J. Correlation of trabeculae and papillary muscles with clinical and cardiac characteristics and impact on CMR measures of LV anatomy and function. JACC Cardiovasc. Imaging 2012, 5, 1115–1123. [Google Scholar] [CrossRef]
- Nassenstein, K.; de Greiff, A.; Hunold, P. MR evaluation of left ventricular volumes and function: Threshold-based 3D segmentation versus short-axis planimetry. Invest. Radiol. 2009, 44, 635–640. [Google Scholar] [CrossRef]
- Fernández-Golfín, C.; Pachón, M.; Corros, C.; Bustos, A.; Cabeza, B.; Ferreirós, J.; de Isla, L.P.; Macaya, C.; Zamorano, J. Left ventricular trabeculae: Quantification in different cardiac diseases and impact on left ventricular morphological and functional parameters assessed with cardiac magnetic resonance. J. Cardiovasc. Med. 2009, 10, 827–833. [Google Scholar] [CrossRef]
- Petersen, S.E.; Selvanayagam, J.B.; Wiesmann, F.; Robson, M.D.; Francis, J.M.; Anderson, R.H.; Watkins, H.; Neubauer, S. Left ventricular non-compaction: Insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 2005, 46, 101–105. [Google Scholar] [CrossRef]
- Varga-Szemes, A.; Muscogiuri, G.; Schoepf, U.J.; Wichmann, J.L.; Suranyi, P.; De Cecco, C.N.; Cannaò, P.M.; Renker, M.; Mangold, S.; Fox, M.A.; et al. Clinical feasibility of a myocardial signal intensity threshold-based semi-automated cardiac magnetic resonance segmentation method. Eur. Radiol. 2016, 26, 1503–1511. [Google Scholar] [CrossRef]
- Jaspers, K.; Freling, H.G.; van Wijk, K.; Romijn, E.I.; Greuter, M.J.W.; Willems, T.P. Improving the reproducibility of MR-derived left ventricular volume and function measurements with a semi-automatic threshold-based segmentation algorithm. Int. J. Cardiovasc. Imaging 2013, 29, 617–623. [Google Scholar] [CrossRef]
- Greil, G.; Geva, T.; Maier, S.E.; Powell, A.J. Effect of acquisition parameters on the accuracy of velocity encoded cine magnetic resonance imaging blood flow measurements. J. Magn. Reson. Imaging 2002, 15, 47–54. [Google Scholar] [CrossRef]
- Schulz-Menger, J.; Bluemke, D.A.; Bremerich, J.; Flamm, S.D.; Fogel, M.A.; Friedrich, M.G.; Kim, R.J.; von Knobelsdorff-Brenkenhoff, F.; Kramer, C.M.; Pennell, D.J.; et al. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J. Cardiovasc. Magn. Reson. 2013, 15, 35. [Google Scholar] [CrossRef]
- Gatehouse, P.D.; Rolf, M.P.; Graves, M.J.; Hofman, M.B.; Totman, J.; Werner, B.; Quest, R.A.; Liu, Y.; von Spiczak, J.; Dieringer, M.; et al. Flow measurement by cardiovascular magnetic resonance: A multi-centre multi-vendor study of background phase offset errors that can compromise the accuracy of derived regurgitant or shunt flow measurements. J. Cardiovasc. Magn. Reson. 2010, 12, 5. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 1, 307–310. [Google Scholar] [CrossRef] [PubMed]
- Holland, B.J.; Printz, B.F.; Lai, W.W. Baseline correction of phase-contrast images in congenital cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Csecs, I.; Czimbalmos, C.; Suhai, F.I.; Mikle, R.; Mirzahosseini, A.; Dohy, Z.; Szűcs, A.; Kiss, A.R.; Simor, T.; Tóth, A.; et al. Left and right ventricular parameters corrected with threshold-based quantification method in a normal cohort analyzed by three independent observers with various training-degree. Int. J. Cardiovasc. Imaging 2018, 34, 1127–1133. [Google Scholar] [CrossRef]
- Freling, H.G.; van Wijk, K.; Jaspers, K.; Pieper, P.G.; Vermeulen, K.M.; van Swieten, J.M.; Willems, T.P. Impact of right ventricular endocardial trabeculae on volumes and function assessed by CMR in patients with tetralogy of Fallot. Int. J. Cardiovasc. Imaging 2013, 29, 625–631. [Google Scholar] [CrossRef]
- Rompel, O.; Janka, R.; May, M.S.; Glöckler, M.; Cesnjevar, R.; Dittrich, S.; Lell, M.M.; Uder, M.; Hammon, M. Cardiac MRI in Children and Adolescents Who Have Undergone Surgical Repair of Right-Sided Congenital Heart Disease: Automated Left Ventricular Volumes and Function Analysis and Effects of Different Manual Adjustments. Rofo 2015, 187, 1099–1107. [Google Scholar] [CrossRef]
- Tóth, A.; Vágó, H.; Temesvári, A.; Bálint, H.O.; Juhász, C.; Jánosa, B.C.; Suhai, F.I.; Balázs, G.; Hüttl, K.; Szatmári, A.; et al. Detecting trabecules of the systemic right ventricle during quantification yields better correlation with flow measurement derived data. J. Cardiovasc. Magn. Reson. 2015, 17 (Suppl. 1), Q95. [Google Scholar] [CrossRef]
- Powell, A.J.; Geva, T. Blood flow measurement by magnetic resonance imaging in congenital heart disease. Pediatr. Cardiol. 2000, 21, 47–58. [Google Scholar] [CrossRef]
- Gregor, Z.; Kiss, A.R.; Szabó, L.E.; Tóth, A.; Grebur, K.; Horváth, M.; Dohy, Z.; Merkely, B.; Vágó, H.; Szűcs, A. Sex- and age- specific normal values of left ventricular functional and myocardial mass parameters using threshold-based trabeculae quantification. PLoS ONE 2021, 16, e0258362. [Google Scholar] [CrossRef]
- Codella, N.C.F.; Weinsaft, J.W.; Cham, M.D.; Janik, M.; Prince, M.R.; Wang, Y. Left ventricle: Automated segmentation by using myocardial effusion threshold reduction and intravoxel computation at MR imaging. Radiology 2008, 248, 1004–1012. [Google Scholar] [CrossRef]

| Age (years) | 15 ± 9 |
| Weight (kg) | 49.9 ± 20.5 |
| Height (cm) | 154 ± 22 |
| Body surface area (m2) | 1.4 ± 0.4 |
| Heart rate (bpm) | 77 ± 14 |
| Diagnosis | |
| Cardiomyopathy | 30 (32%) |
| Congenital heart disease | 26 (28%) |
| Aortic pathology | 23 (24%) |
| Healthy subject | 13 (14%) |
| Other | 2 (2%) |
| Manual | k30 | k50 | k70 | Flow | |
|---|---|---|---|---|---|
| Left ventricle | |||||
| EDVi (mL/m2) | 82.3 ± 16.1 | 75.4 ± 16.0 | 67.2 ± 14.4 | 59.6 ± 13.0 | N/A |
| ESVi (mL/m2) | 35.1 ± 11.0 | 28.8 ± 10.4 | 24.1 ± 8.9 | 20.7 ± 7.9 | N/A |
| EF (%) | 60 ± 10 | 60 ± 10 | 60 ± 10 | 70 ± 10 | N/A |
| SV (mL) | 68.8 ± 24.9 | 67.8 ± 25.0 | 62.8 ± 23.1 | 56.5 ± 20.6 | 66.8 ± 22.7 |
| CI (l/min/m2) | 3.6 ± 0.8 | 3.5 ± 0.8 | 3.3 ± 0.8 | 2.9 ± 0.7 | 3.5 ± 0.7 |
| Mass (g/m2) | 54.4 ± 11.0 | 60.8 ± 12.5 | 69.1 ± 13.8 | 76.3 ± 16.6 | N/A |
| Right ventricle | |||||
| EDVi (mL/m2) | 86.6 ± 22.2 | 80.1 ± 22.1 | 72.4 ± 20.9 | 62.7 ± 18.8 | N/A |
| ESVi (mL/m2) | 41.0 ± 13.9 | 35.0 ± 12.3 | 29.9 ± 11.1 | 25.3 ± 9.7 | N/A |
| EF (%) | 50 ± 10 | 60 ± 10 | 60 ± 10 | 60 ± 10 | N/A |
| SV (mL) | 66.6 ± 28.6 | 65.8 ± 29.6 | 62.1 ± 28.5 | 54.9 ± 26.2 | 70.3 ± 26.6 |
| CI (l/min/m2) | 3.4 ± 1.0 | 3.5 ± 1.2 | 3.2 ± 1.1 | 2.8 ± 1.0 | 3.7 ± 0.9 |
| Mass (g/m2) | 21.8 ± 5.2 | 28.6 ± 6.5 | 36.6 ± 8.3 | 46.8 ± 10.6 | N/A |
| Bland–Altman of | Manual | k30 | k50 | k70 |
|---|---|---|---|---|
| Left ventricle SV (mL) | 2.1 ± 9.4 | 1.1 ± 9.7 | −4.0 ± 8.8 | −10.2 ± 8.3 |
| Right ventricle SV (mL) | −3.7 ± 12.5 | −4.5 ± 12.4 | −8.3 ± 11.7 | −15.4 ± 11.0 |
| Left ventricle CI (l/min/m2) | 0.1 ± 0.5 | 0.0 ± 0.6 | −0.3 ± 0.5 | −0.6 ± 0.5 |
| Right ventricle CI (l/min/m2) | −0.3 ± 0.7 | −0.3 ± 0.7 | −0.5 ± 0.7 | −0.9 ± 0.6 |
| Manual | k30 | k50 | k70 | Flow | |
|---|---|---|---|---|---|
| Left ventricle | |||||
| EDVi (mL/m2) | 19.5% | 21.2% | 21.4% | 21.8% | N/A |
| ESVi (mL/m2) | 31.4% | 36.1% | 37.1% | 38.1% | N/A |
| EF (%) | 13.6% | 13.7% | 13% | 12.7% | N/A |
| SV (mL) | 36.1% | 36.9% | 37.8% | 36.4% | 34% |
| CI (l/min/m2) | 21.2% | 23.6% | 25% | 24.1% | 18.9% |
| Mass (g/m2) | 20.5% | 20.5% | 23.7% | 21.8% | N/A |
| Right ventricle | |||||
| EDVi (mL/m2) | 25.7% | 27.6% | 28.9% | 29.9% | N/A |
| ESVi (mL/m2) | 34% | 35.2% | 37% | 38.2% | N/A |
| EF (%) | 16.1% | 15.5% | 15% | 15% | N/A |
| SV (mL) | 42.9% | 44.9% | 45.9% | 47.6% | 37.8% |
| CI (l/min/m2) | 29.6% | 34.2% | 34.9% | 35% | 25.4% |
| Mass (g/m2) | 24% | 22.9% | 22.6% | 22.7% | N/A |
| Manual | k30 | k50 | k70 | Flow | |
|---|---|---|---|---|---|
| B.-A. | |||||
| Left ventricular parameters | |||||
| EDVi (mL/m2) | −1.0 ± 3.1 | 1.1 ± 2.8 | 1.3 ± 2.5 | 1.4 ± 2.1 | N/A |
| ESVi (mL/m2) | −0.6 ± 4.1 | −1 ± 3.5 | −0.6 ± 3.1 | −0.4 ± 2.8 | N/A |
| SV (mL) | −0.5 ± 7.2 | 3.3 ± 6.8 | 2.9 ± 6.3 | 2.7 ± 5.3 | −0.1 ± 0.2 |
| CI (l/min/m2) | 0 ± 0.3 | 0.2 ± 0.4 | 0.2 ± 0.3 | 0.1 ± 0.3 | 0 ± 0 |
| VMi (g/m2) | −2.2 ± 3.9 | −4.5 ± 2.8 | −4.5 ± 3 | −4.4 ± 3.3 | N/A |
| Right ventricular parameters | |||||
| EDVi (mL/m2) | −2.4 ± 3.7 | −0.7 ± 3.3 | −0.6 ± 3.0 | −0.6 ± 3.0 | N/A |
| ESVi (mL/m2) | −4.79 ± 5.41 | −3.34 ± 4.19 | −2.48 ± 3.77 | −2.0 ± 3.4 | N/A |
| SV (mL) | 3.40 ± 7.42 | 3.75 ± 6.42 | 2.65 ± 6.02 | 2.0 ± 5.4 | −0.15 ± 0.67 |
| CI (l/min/m2) | 0.17 ± 0.39 | 0.22 ± 0.34 | 0.14 ± 0.30 | 0.1 ± 0.3 | −0.02 ± 0.07 |
| VMi (g/m2) | −1.87 ± 2.23 | −3.48 ± 2.25 | −3.68 ± 2.50 | −3.6 ± 2.5 | N/A |
| ICC | |||||
| Left ventricular parameters | |||||
| EDVi | 0.99 | 0.99 | 0.99 | 0.99 | N/A |
| ESVi | 0.96 | 0.96 | 0.96 | 0.96 | N/A |
| SV | 0.94 | 0.96 | 0.96 | 0.96 | 1.00 |
| CI | 0.87 | 0.90 | 0.91 | 0.92 | 1.00 |
| VMi | 0.91 | 0.97 | 0.97 | 0.97 | N/A |
| Right ventricular parameters | |||||
| EDVi | 0.99 | 0.99 | 0.99 | 0.99 | N/A |
| ESVi | 0.91 | 0.93 | 0.93 | 0.93 | N/A |
| SV | 0.96 | 0.974 | 0.98 | 0.98 | 1.00 |
| CI | 0.88 | 0.922 | 0.94 | 0.94 | 1.00 |
| VMi | 0.88 | 0.95 | 0.96 | 0.97 | N/A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thut, T.; Valsangiacomo Büchel, E.; Geiger, J.; Kellenberger, C.J.; Rücker, B.; Burkhardt, B.E.U. Signal Thresholding Segmentation of Ventricular Volumes in Young Patients with Various Diseases—Can We Trust the Numbers? Diagnostics 2023, 13, 180. https://doi.org/10.3390/diagnostics13020180
Thut T, Valsangiacomo Büchel E, Geiger J, Kellenberger CJ, Rücker B, Burkhardt BEU. Signal Thresholding Segmentation of Ventricular Volumes in Young Patients with Various Diseases—Can We Trust the Numbers? Diagnostics. 2023; 13(2):180. https://doi.org/10.3390/diagnostics13020180
Chicago/Turabian StyleThut, Titus, Emanuela Valsangiacomo Büchel, Julia Geiger, Christian Johannes Kellenberger, Beate Rücker, and Barbara Elisabeth Ursula Burkhardt. 2023. "Signal Thresholding Segmentation of Ventricular Volumes in Young Patients with Various Diseases—Can We Trust the Numbers?" Diagnostics 13, no. 2: 180. https://doi.org/10.3390/diagnostics13020180
APA StyleThut, T., Valsangiacomo Büchel, E., Geiger, J., Kellenberger, C. J., Rücker, B., & Burkhardt, B. E. U. (2023). Signal Thresholding Segmentation of Ventricular Volumes in Young Patients with Various Diseases—Can We Trust the Numbers? Diagnostics, 13(2), 180. https://doi.org/10.3390/diagnostics13020180






