Dermatofibromas with Aberrant Expression of CD34 Protein: A Systematic Review and a Reappraisal of Clinicopathological Features and Histogenesis
Abstract
:1. Background
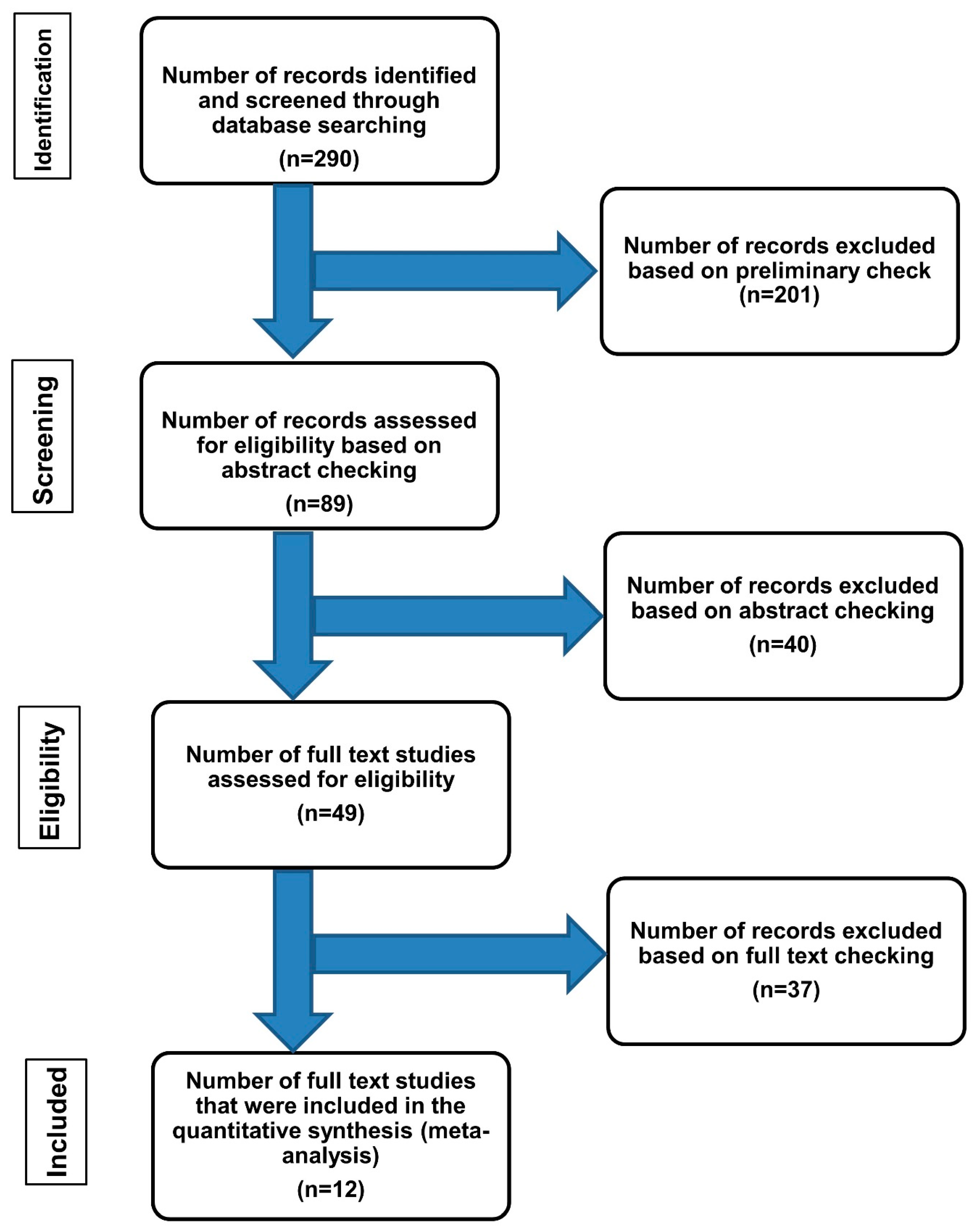
2. Methods
3. Results
4. Discussion
5. Conclusions
6. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DF | dermatofibroma |
| H & E | Hematoxylin and eosin |
| CD34 | Cluster of differentiation 34 |
| PRISMA | Preferred reporting items for systematic reviews and meta-analysis |
| IHC | Immunohistochemistry |
| IGFBP7 | Insulin-like growth factor-binding protein 7 |
| Matrix metalloproteinase family member | MMP-11 |
| HMGA1 and HMGA2 | High-Mobility Group Proteins |
| PDGFB gene | Platelet-derived growth factor-beta chain |
| COL1A1 gene | Collagen type 1 alpha 1 gene |
| FISH | Fluorescence in situ hybridization |
| RT-PCR | Multiplex reverse transcriptase-polymerase chain reaction |
References
- Myers, D.J.; Fillman, E.P. Dermatofibroma; StatPearls: Treasure Island, FL, USA, 2020. [Google Scholar]
- Katenkamp, D.; Stiller, D. Cellular composition of the so-called dermatofibroma (histiocytoma cutis). Virchows Arch. A Pathol. Anat. Histol. 1975, 367, 325–336. [Google Scholar] [CrossRef]
- Song, Y.; Sakamoto, F.; Ito, M. Characterization of factor XIIIa+ dendritic cells in dermatofibroma: Immunohistochemical, electron and immunoelectron microscopical observations. J. Dermatol. Sci. 2005, 39, 89–96. [Google Scholar] [CrossRef]
- Quintana, F.S.L.; Almeida, H.L., Jr.; Ruas, C.P.; Jorge, V.M. Scanning electron microscopy of dermatofibroma. An. Bras. Dermatol. 2019, 94, 358–360. [Google Scholar] [CrossRef] [Green Version]
- Cazzato, G.; Colagrande, A.; Cimmino, A.; Marrone, M.; Stellacci, A.; Arezzo, F.; Lettini, T.; Resta, L.; Ingravallo, G. Granular Cell Dermatofibroma: When Morphology Still Matters. Dermatopathology 2021, 8, 371–375. [Google Scholar] [CrossRef]
- Aloi, F.; Albertazzi, D.; Pippione, M. Dermatofibroma with granular cells: A report of two cases. Dermatology 1999, 199, 54–56. [Google Scholar] [CrossRef]
- LeBoit, P.E.; Barr, R.J.; Burall, S.; Metcalf, J.S.; Yen, T.S.; Wick, M.R. Primitive polypoid granular-cell tumor and other cutaneous granular-cell neoplasms of apparent nonneural origin. Am. J. Surg. Pathol. 1991, 15, 48–58. [Google Scholar] [CrossRef]
- Hussein, M.R. Evaluation of angiogenesis in normal and lichen planus skin by CD34 protein immunohistochemistry: Preliminary findings. Cell Biol. Int. 2007, 31, 1292–1295. [Google Scholar] [CrossRef]
- Gutiérrez, R.; García, M.P.; Sáez, F.; Diaz-Flores, L.; Valladares, F.; Madrid, J.F. CD34+ stromal cells/fibroblasts/fibrocytes/telocytes as a tissue reserve and a principal source of mesenchymal cells. Location, morphology, function and role in pathology. Histol. Histopathol. 2014, 29, 831–870. [Google Scholar]
- Sidney, L.E.; Branch, M.J.; Dunphy, S.E.; Dua, H.S.; Hopkinson, A. Concise review: Evidence for CD34 as a common marker for diverse progenitors. Stem. Cells 2014, 32, 1380–1389. [Google Scholar] [CrossRef] [Green Version]
- Monge, M.; Chauveau, D.; Cordonnier, C.; Noël, L.H.; Presne, C.; Makdassi, R.; Jauréguy, M.; Lecaque, C.; Renou, M.; Grünfeld, J.P.; et al. Localized amyloidosis of the genitourinary tract: Report of 5 new cases and review of the literature. Medicine 2011, 90, 212–222. [Google Scholar] [CrossRef]
- Zelger, B.G.; Calonje, E.; Zelger, B. Myxoid dermatofibroma. Histopathology 1999, 34, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Zelger, B.W.; Zelger, B.G.; Rappersberger, K. Prominent myofibroblastic differentiation. A pitfall in the diagnosis of dermatofibroma. Am. J. Dermatopathol. 1997, 19, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Zelger, B.G.; Steiner, H.; Kutzner, H.; Rutten, A.; Zelger, B. Granular cell dermatofibroma. Histopathology 1997, 31, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Kiyohara, T.; Kumakiri, M.; Kobayashi, H.; Ohkawara, A.; Lao, L.M. Atrophic dermatofibroma. Elastophagocytosis by the tumor cells. J. Cutan. Pathol. 2000, 27, 312–315. [Google Scholar] [CrossRef]
- Erdag, G.; Qureshi, H.S.; Patterson, J.W.; Wick, M.R. CD34-positive dendritic cells disappear from scars but are increased in pericicatricial tissue. J. Cutan. Pathol. 2008, 35, 752–756. [Google Scholar] [CrossRef]
- Hussein, M.R.; Al-Badaiwy, Z.H.; Guirguis, M.N. Analysis of p53 and bcl-2 protein expression in the non-tumorigenic, pretumorigenic, and tumorigenic keratinocytic hyperproliferative lesions. J. Cutan. Pathol. 2004, 31, 643–651. [Google Scholar] [CrossRef]
- Hantschmann, P.; Sterzer, S.; Jeschke, U.; Friese, K. P53 expression in vulvar carcinoma, vulvar intraepithelial neoplasia, squamous cell hyperplasia and lichen sclerosus. Anticancer. Res. 2005, 25, 1739–1745. [Google Scholar]
- Hasby, E.A.; El Mashad, N.; Eltatawy, R. C-Kit, CD34 & alpha-SMA Immunohistochemical Features in Classic Kaposi Sarcoma and Kaposiform Hemangioendothelioma. J. Microsc. Ultrastruct. 2017, 5, 49–57. [Google Scholar]
- Lisovsky, M.; Hoang, M.P.; Dresser, K.A.; Kapur, P.; Bhawan, J.; Mahalingam, M. Apolipoprotein D in CD34-positive and CD34-negative cutaneous neoplasms: A useful marker in differentiating superficial acral fibromyxoma from dermatofibrosarcoma protuberans. Mod. Pathol. 2008, 21, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Sadullahoglu, C.; Dere, Y.; Atasever, T.R.; Oztop, M.T.; Karaaslan, O. The Role of CD34 and D2-40 in the Differentiation of Dermatofibroma and Dermatofibrosarcoma Protuberans. Turk. Patoloji Derg. 2017, 1, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Yu, Y.; Yang, Y.; Wang, L.; Cao, J.; Liang, X.; Xiao, X.; Tu, Y.; Chen, H. IGFBP7, a novel immunohistochemical marker in differentiating dermatofibroma from dermatofibrosarcoma protuberans. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.T.; Chen, W.T.; Huang, W.T.; Wu, C.C.; Chai, C.Y. Expression of MMP-2, MMP-9 and MMP-11 in dermatofibroma and dermatofibrosarcoma protuberans. Kaohsiung J. Med. Sci. 2012, 28, 545–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Guo, H.; Zhang, S.; Zhang, B.; Li, T. Differences of the molecular phenotypes and the histogenesis between dermatofibroma and dermatofibrosarcoma protuberans. Beijing Da Xue Xue Bao Yi Xue Ban 2008, 40, 395–400. [Google Scholar] [PubMed]
- Kim, H.; Lee, J.; Kim, S.; Seo, Y.-J.; Park, J.; Kim, M.; Cinn, Y.; Cho, K.; Yoon, T. Stromelysin-3 expression in the differential diagnosis of dermatofibroma and dermatofibrosarcoma protuberans: Comparison with factor XIIIa and CD34. Br. J. Dermatol. 2007, 157, 319–324. [Google Scholar] [CrossRef]
- Sachdev, R.; Sundram, U. Expression of CD163 in dermatofibroma, cellular fibrous histiocytoma, and dermatofibrosarcoma protuberans: Comparison with CD68, CD34, and Factor XIIIa. J. Cutan. Pathol. 2006, 33, 353–360. [Google Scholar] [CrossRef]
- Li, N.; McNiff, J.; Hui, P.; Manfioletti, G.; Tallini, G. Differential expression of HMGA1 and HMGA2 in dermatofibroma and dermatofibrosarcoma protuberans: Potential diagnostic applications, and comparison with histologic findings, CD34, and factor XIIIa immunoreactivity. Am. J. Dermatopathol. 2004, 26, 267–272. [Google Scholar] [CrossRef]
- Kahn, H.J.; Fekete, E.; From, L. Tenascin differentiates dermatofibroma from dermatofibrosarcoma protuberans: Comparison with CD34 and factor XIIIa. Hum. Pathol. 2001, 32, 50–56. [Google Scholar] [CrossRef]
- Goldblum, J.R.; Tuthill, R.J. CD34 and factor-XIIIa immunoreactivity in dermatofibrosarcoma protuberans and dermatofibroma. Am. J. Dermatopathol. 1997, 19, 147–153. [Google Scholar] [CrossRef]
- Hsi, E.D.; Nickoloff, B.J. Dermatofibroma and dermatofibrosarcoma protuberans: An immunohistochemical study reveals distinctive antigenic profiles. J. Dermatol. Sci. 1996, 11, 1–9. [Google Scholar] [CrossRef]
- Zelger, B.W.; Ofner, D.; Zelger, B.G. Atrophic variants of dermatofibroma and dermatofibrosarcoma protuberans. Histopathology 1995, 26, 519–527. [Google Scholar] [CrossRef]
- Zelger, B.; Sidoroff, A.; Stanzl, U.; Fritsch, P.O.; Öfner, D.; Zelger, B.; Jasani, B.; Schmid, K.W. Deep penetrating dermatofibroma versus dermatofibrosarcoma protuberans. A clinicopathologic comparison. Am. J. Surg. Pathol. 1994, 18, 677–686. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Malagon, H.; Valdez-Carrillo Mdel, C.; Cano-Valdez, A.M. Dermatofibroma and dermatofibrosarcoma protuberans: A comparative ultrastructural study. Ultrastruct. Pathol. 2006, 30, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Desmouliere, A.; Geinoz, A.; Gabbiani, F.; Gabbiani, G. Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. J. Cell Biol. 1993, 122, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Cohen, P.R.; Erickson, C.P.; Calame, A. Atrophic Dermatofibroma: A Comprehensive Literature Review. Dermatol. Ther. 2019, 9, 449–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prieto, V.G.; Reed, J.A.; Shea, C.R. Immunohistochemistry of dermatofibromas and benign fibrous histiocytomas. J. Cutan. Pathol. 1995, 22, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Aiba, S.; Tagami, H. Inverse correlation between CD34 expression and proline-4-hydroxylase immunoreactivity on spindle cells noted in hypertrophic scars and keloids. J. Cutan. Pathol. 1997, 24, 65–69. [Google Scholar]
- Hui, P.; Glusac, E.J.; Sinard, J.H.; Perkins, A.S. Clonal analysis of cutaneous fibrous histiocytoma (dermatofibroma). J. Cutan. Pathol. 2002, 29, 385–389. [Google Scholar] [CrossRef]
- Mentzel, T.; Wiesner, T.; Cerroni, L.; Hantschke, M.; Kutzner, H.; Rütten, A.; Häberle, M.; Bisceglia, M.; Chibon, F.; Coindre, J.-M. Malignant dermatofibroma: Clinicopathological, immunohistochemical, and molecular analysis of seven cases. Mod. Pathol. 2013, 26, 256–267. [Google Scholar] [CrossRef] [Green Version]
- Taniuchi, K.; Yamada, Y.; Nonomura, A.; Takehara, K. Immunohistochemical analysis of platelet-derived growth factor and its receptors in fibrohistiocytic tumors. J. Cutan. Pathol. 1997, 24, 393–397. [Google Scholar] [CrossRef]
- Wang, W.-L.; Patel, K.U.; Coleman, N.M.; Smith-Zagone, M.J.; Ivan, D.; A Reed, J.; López-Terrada, D.; Lazar, A.J.F.; Prieto, V.G. COL1A1, PDGFB chimeric transcripts are not present in indeterminate fibrohistiocytic lesions of the skin. Am. J. Dermatopathol. 2010, 32, 149–153. [Google Scholar] [CrossRef]



| Studies | Dermatofibromas with Aberrant CD34 Protein Expression/Total Cases | Contributions of Each Study | References |
|---|---|---|---|
| 1 | 7/30 (23%) | A broad panel of immunostains is needed in DFs with CD34 positivity | [21] |
| 2 | 4/30 (13%) | A combination of CD34, FXIIIa, and Stromelysin-3 immunostains can separate DFs from DFSPs | [22] |
| 3 | 4/19 (21%) | The expressions of extracellular matrix proteins play a role in the development of DFs | [23] |
| 4 | 1/26 (3%) | A combination of CD34 and FXIIIa immune markers can separate DFs from DFSPs | [24] |
| 5 | 4/23 (17%) | A combination of CD34 and Stromelysin-3 immunostains can help to separate DFs from DFSPs | [25] |
| 6 | 1/19(5%) | The inclusion of the CD163 marker (hemoglobin scavenger receptor) can help to separate DFs from DFSPs | [26] |
| 7 | 8/22 (36%) | The use of HMGA1 and HMGA2 (members of the high mobility group protein family genes) immune markers can help to separate DFs from DFSPs | [27] |
| 8 | 5/20 (25%) | The overexpression of tenascin at the dermal-epidermal junction overlying the lesion in DFs but not in DFSPs helps separate these tumors. | [28] |
| 9 | 12/30 (40%) | A combination of CD34 and FXIIIa can separate DFs from DFSP | [29] |
| 10 | 1/13 (7%) | There is no convincing evidence indicating the derivation of DFs from cells with the vascular or hematopoietic origin | [30] |
| 11 | 2/26 (7%) | Atrophic variants of DFSP and DFs represent distinct entities that can be separated by the use of immunostains such as CD34, Factor XIIIa, and metallothionein | [31] |
| 12 | 2/20 (10%) | The deep penetrating DFs and DFSP represent distinct entities | [32] |
| Studies | Ultrastructural Findings | Number of Cases of Dermatofibromas | References |
|---|---|---|---|
| 1 | Spindle cells and dense collagen with the mesh-like appearance | 2 cases | [4] |
| 2 | Multiple capillary vessels having prominent endothelium and a perivascular ovoid or spindled cells showing intracytoplasmic lipid material and subplasmalemmal densities but lacking cell processes | 10 cases | [33] |
| 3 | Cells with histiocytic and fibroblastic features | 11 cases | [3] |
| 4 | Cells with phagocytized elastic fibers | Atrophic variant (a single case) | [15] |
| 5 | Fibrocytes amid fibrillary collagen and pools of mucin | Myxoid variant (7 cases) | [12] |
| 6 | Cells with abundant endoplasmic reticulum and Golgi complex, several intermediate filaments | Myofibroblastic variant (36 cases) | [13] |
| 7 | Cells with pools of phagolysosomes and glycogen granules | Granular variant (5 cases) | [14] |
| 8 | Most of the cells are Fibroblast-like and histiocyte-like, showing numerous rough endoplasmic reticulum, free ribosomes, bundles of filaments, macropinocytosis vesicles, and a basement membrane-like material on the outer cell surface. Some cells resembling smooth muscle | 9 cases | [2] |
| No of Case | Age | Sex | Localization | Recurrence or Distant Metastasis | IHC | ||||
|---|---|---|---|---|---|---|---|---|---|
| CD34 % of positive cells | Factor XIIIa | D2-40 | S100 | Ki67 | |||||
| 1 | 57 | Male | Upper arm | None | 35 | + | + | - | 0.0% |
| 2 | 48 | Male | Upper back | None | 35 | + | + | - | 1% |
| 3 | 57 | Female | Shoulder | None | 60 | + | + | - | 1% |
| 4 | 41 | Female | Left leg | None | 60 | + | + | - | 1% |
| 5 | 41 | Male | Left forearm | None | 60 | + | + | - | 0.0% |
| 6 | 30 | Male | Left hip | None | 75 | + | + | - | 1% |
| 7 | 45 | Male | Left scapula | None | 60 | + | + | - | 1% |
| 8 | 53 | Female | Foot right | None | 35 | + | + | - | 1% |
| 9 | 34 | Male | Shoulder | None | 60 | + | + | - | 0.0% |
| 10 | 35 | Male | Left thigh | None | 60 | + | + | - | 1% |
| 11 | 49 | Male | Left thigh | None | 60 | + | + | - | 0.0% |
| Cases | Age | Sex | Site | Recurrence or Distant Metastasis | IHC | ||||
|---|---|---|---|---|---|---|---|---|---|
| CD34 % of positive cells | Factor XIIIa | D2-40 | S100 | Ki 67 | |||||
| 1 | 52 | Female | Right deltoid | None | 0 | + | + | - | 0.0% |
| 2 | 41 | Female | Left leg | None | 0 | + | + | - | 0.0% |
| 3 | 42 | Female | Chest wall | None | 0 | + | + | - | 0.0% |
| 4 | 45 | Male | Upper back | None | 5 | + | + | - | 1% |
| 5 | 81 | Female | Upper arm | None | 0 | + | + | - | 0.0% |
| 6 | 38 | Female | Left thigh | None | 5 | + | + | - | 1% |
| 7 | 63 | Female | Right leg | None | 5 | + | + | - | 0.0% |
| 8 | 52 | Male | Left forearm | None | 0 | + | + | - | 1% |
| 9 | 58 | Female | Leg | None | 5 | + | + | - | 0.0% |
| 10 | 75 | Male | Left lower leg | None | 0 | + | + | - | 1% |
| 11 | 72 | Female | Upper back | None | 0 | + | + | - | 0.0% |
| 12 | 21 | Female | Left leg | None | 5 | + | + | - | 1% |
| 13 | 39 | Female | Left leg | None | 0 | + | + | - | 1% |
| 14 | 10 | Female | Right cheek | None | 0 | + | + | - | 0.0% |
| 15 | 24 | Female | Left-arm | None | 0 | + | + | - | 0.0% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussein, M.R.A.; Abdelwahed Hussein, T.M.R. Dermatofibromas with Aberrant Expression of CD34 Protein: A Systematic Review and a Reappraisal of Clinicopathological Features and Histogenesis. Diagnostics 2023, 13, 185. https://doi.org/10.3390/diagnostics13020185
Hussein MRA, Abdelwahed Hussein TMR. Dermatofibromas with Aberrant Expression of CD34 Protein: A Systematic Review and a Reappraisal of Clinicopathological Features and Histogenesis. Diagnostics. 2023; 13(2):185. https://doi.org/10.3390/diagnostics13020185
Chicago/Turabian StyleHussein, Mahmoud Rezk Abdelwahed, and Toka Mahmoud Rezk Abdelwahed Hussein. 2023. "Dermatofibromas with Aberrant Expression of CD34 Protein: A Systematic Review and a Reappraisal of Clinicopathological Features and Histogenesis" Diagnostics 13, no. 2: 185. https://doi.org/10.3390/diagnostics13020185
APA StyleHussein, M. R. A., & Abdelwahed Hussein, T. M. R. (2023). Dermatofibromas with Aberrant Expression of CD34 Protein: A Systematic Review and a Reappraisal of Clinicopathological Features and Histogenesis. Diagnostics, 13(2), 185. https://doi.org/10.3390/diagnostics13020185






