Diagnostic Algorithm for Surgical Management of Limbal Stem Cell Deficiency
Abstract
1. Introduction
2. Materials and Methods
2.1. Medical Records Review
2.2. Ocular Surface Slit-Lamp Biomicroscopy and Staining
2.3. AS-OCT and AS-OCTA
2.4. In Vivo Confocal Microscopy (IVCM)
2.5. Impression Cytology and Immunofluorescent Staining (IC-IF)
2.6. Enzyme-Linked Immunosorbent Assay (ELISA) of the Tear Samples
3. Results
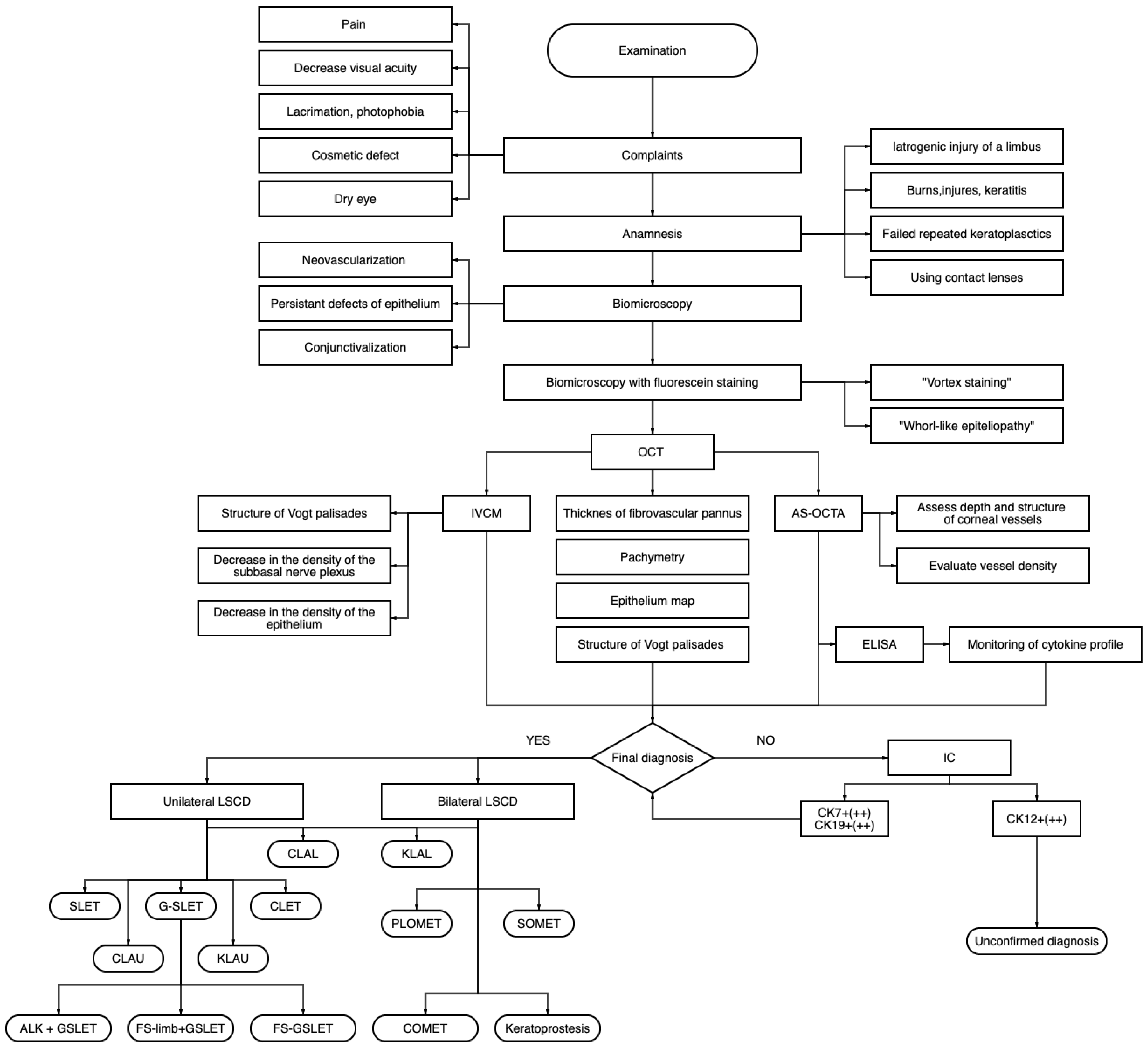
3.1. Comprehensive Diagnostics of Limbal Stem Cell Deficiency
3.1.1. Etiology and Anamnesis
3.1.2. Patient Complaints
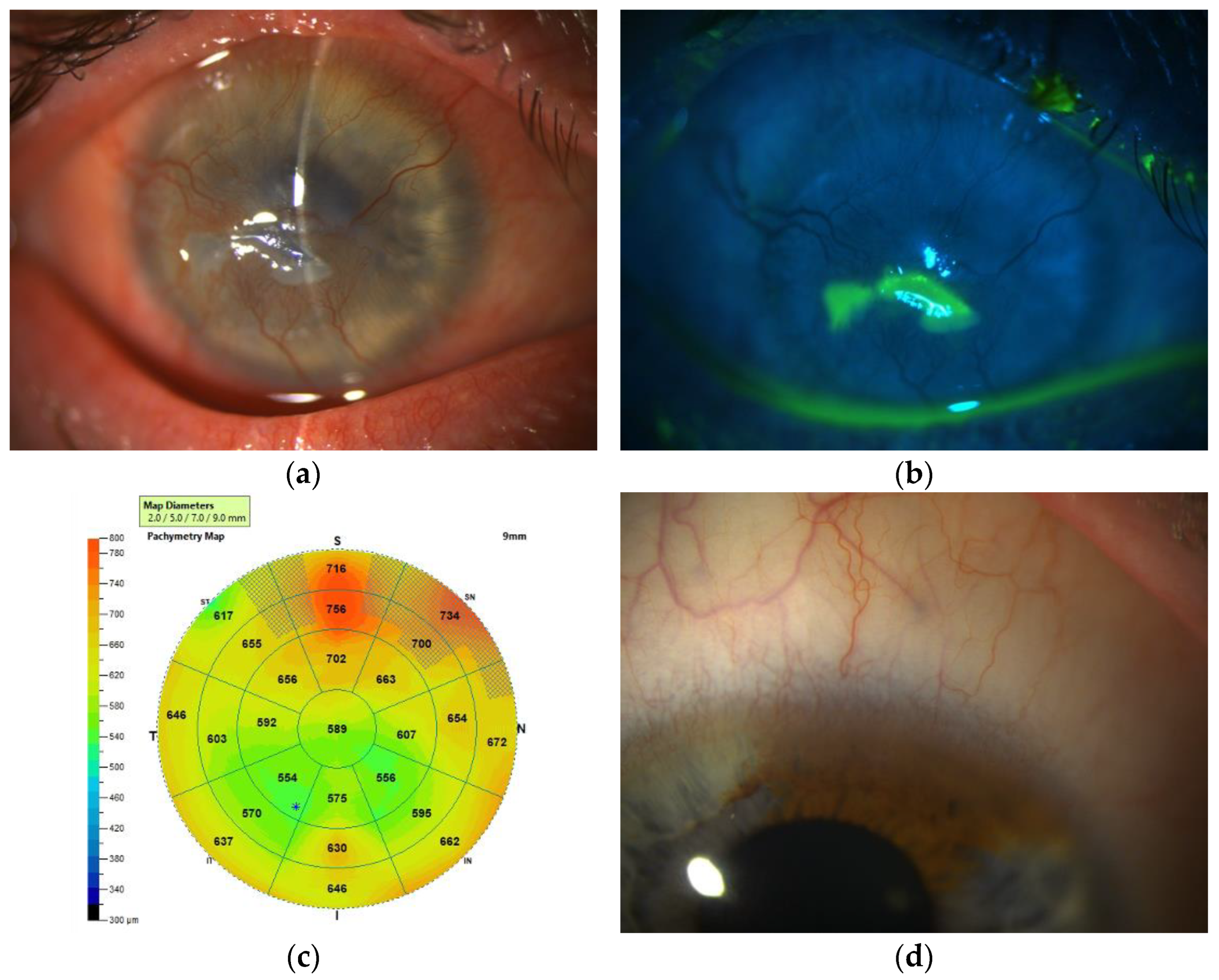
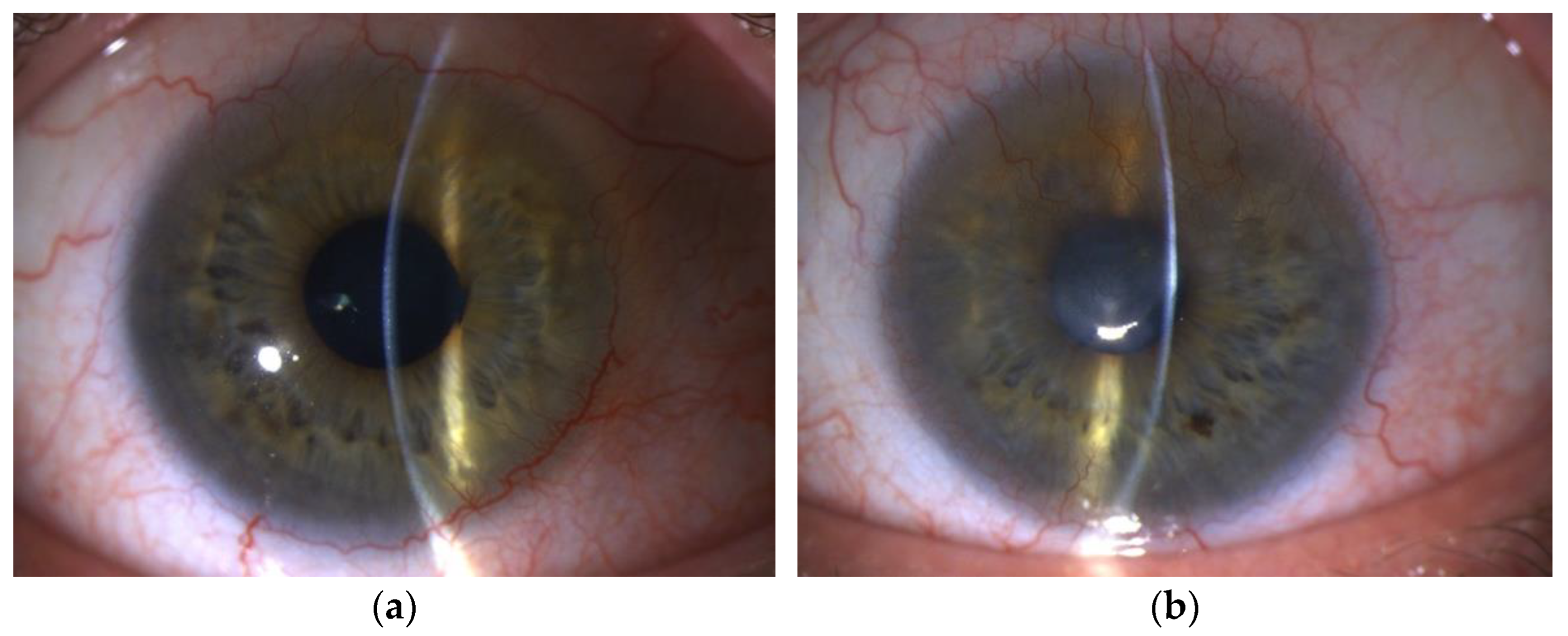
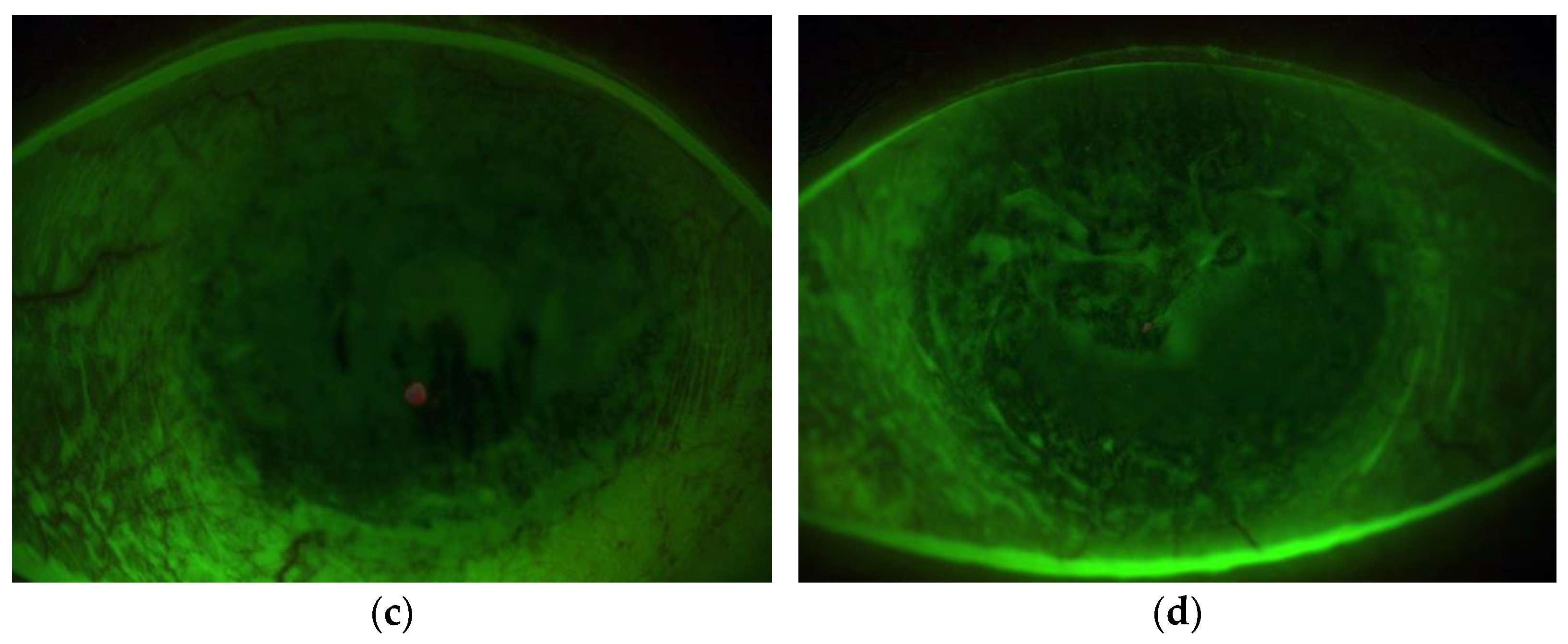
3.1.3. Ocular Surface Biomicroscopy
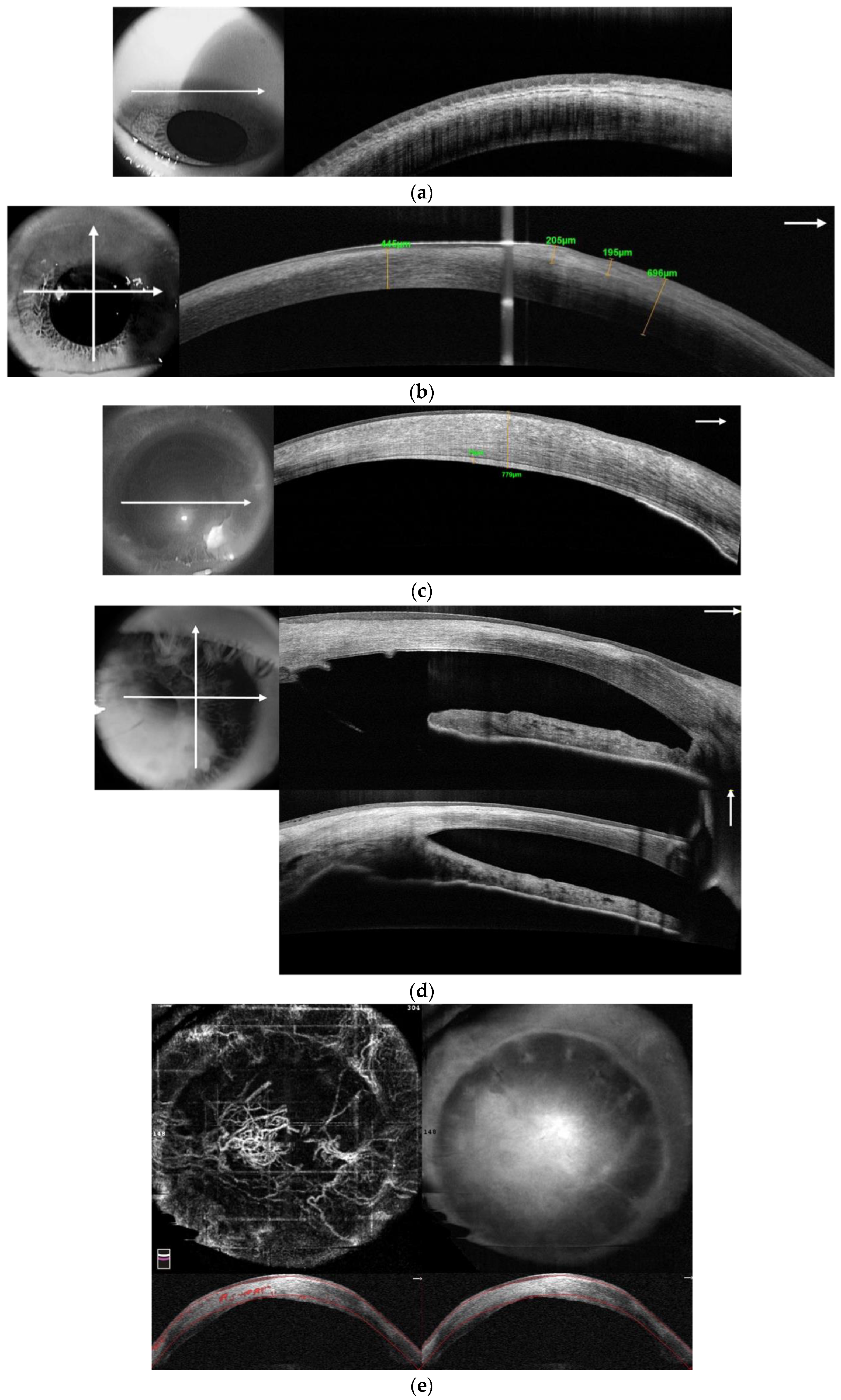
3.1.4. Anterior Segment Optical Coherence Tomography
3.1.5. In Vivo Confocal Microscopy
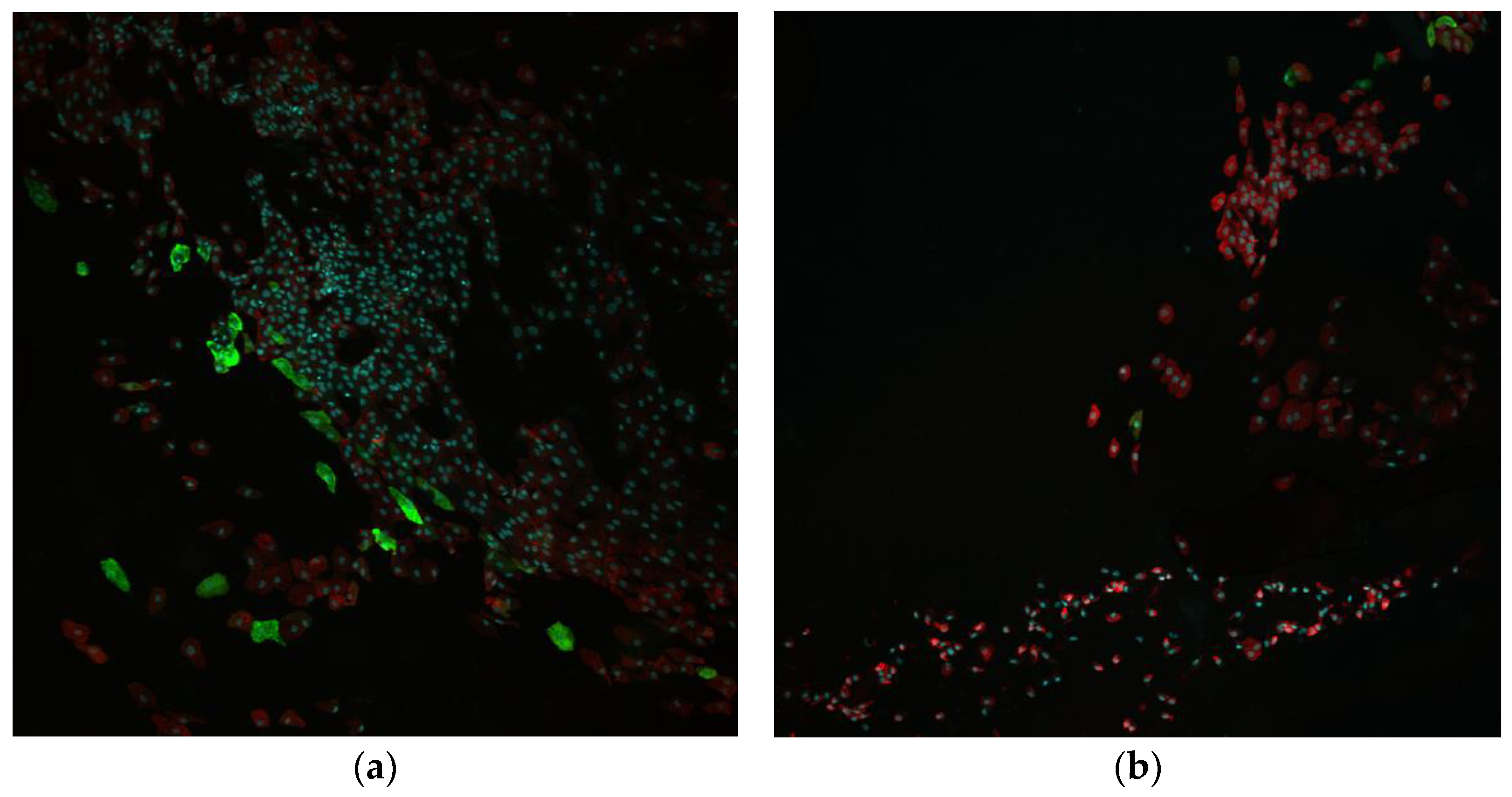
3.1.6. Impression Cytology and Immunofluorescent Staining (IC-IF)
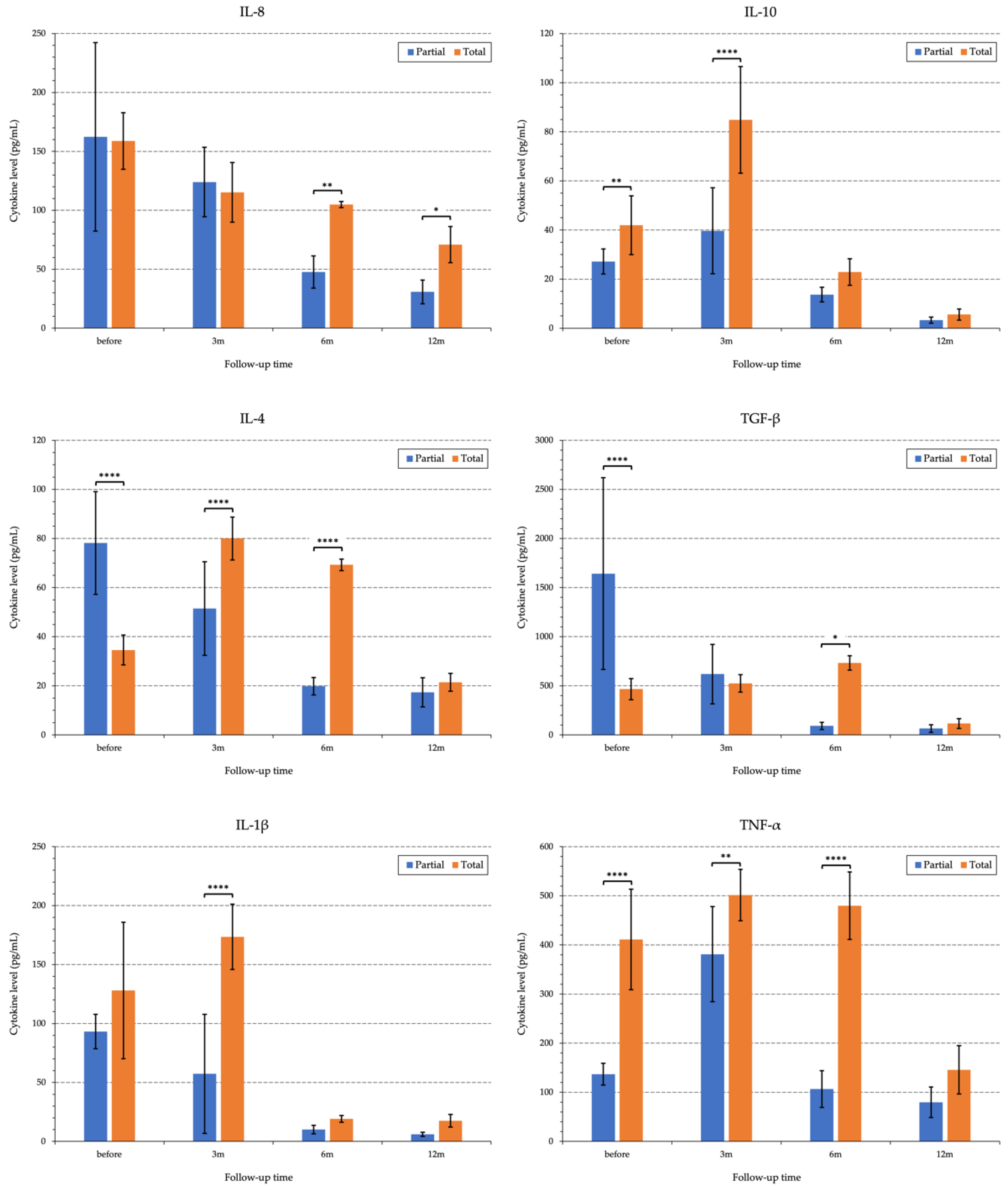
3.1.7. Pro-/and Anti-Inflammatory Cytokines in the Tear Samples
3.2. Surgical Interventions in Patients with Limbal Stem Cell Deficiency
3.2.1. Limbal Stem Cell Transplantation in Patients with Unilateral LSCD
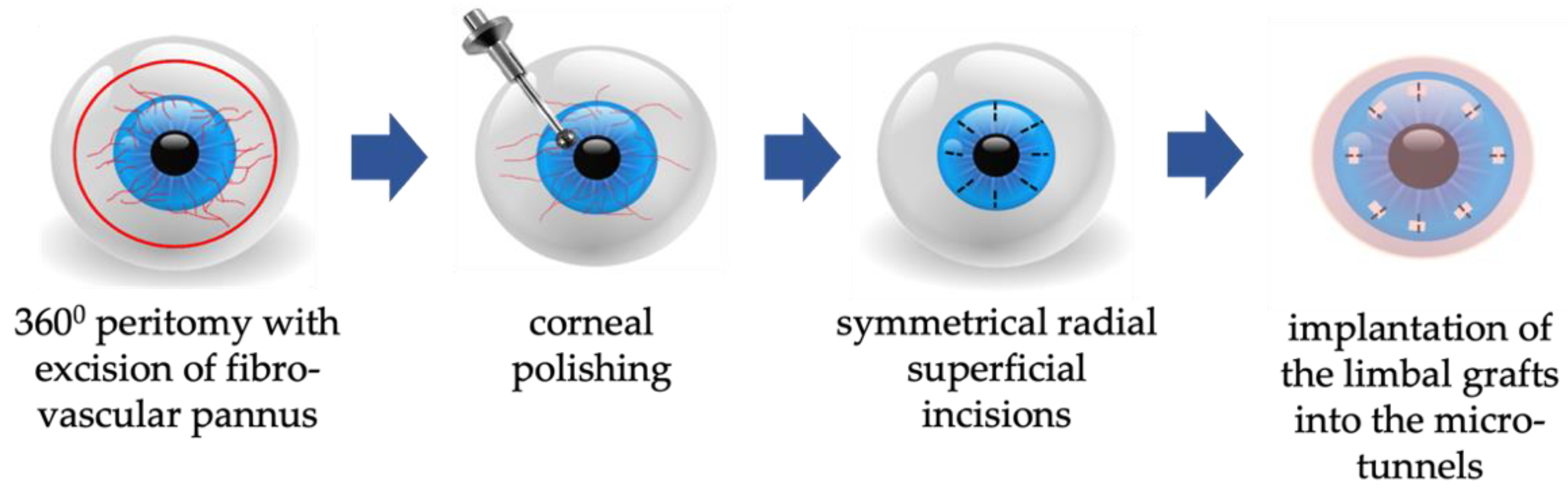
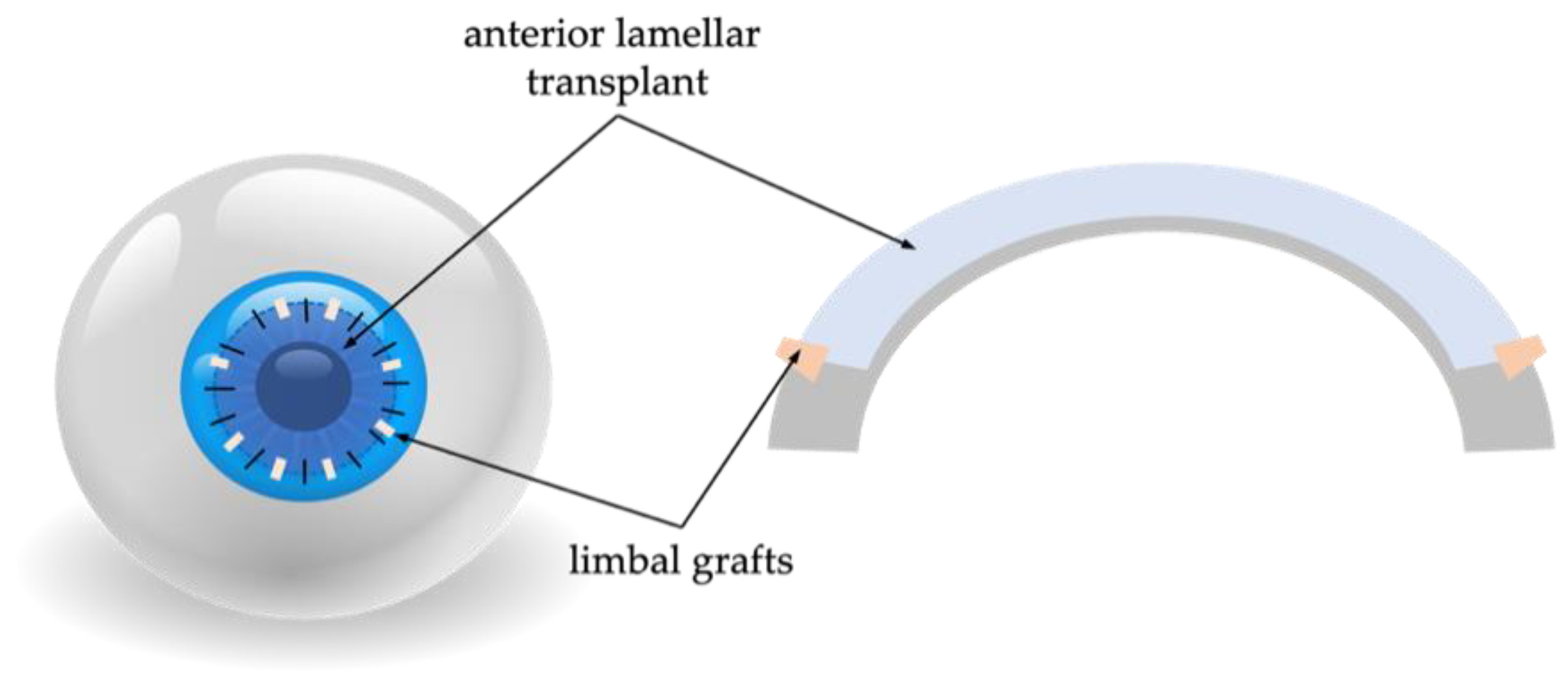
G-SLET in Patients with a Normal Corneal Thickness on the LSCD-Affected Eye
Limbal Stem Cell Transplantation in Patients with Thin Cornea on the LSCD-Affected Eye
3.2.2. Corneal Re-Epithelization in Bilateral LSCD
Allo-Limbal Transplantation
Allogeneic Simple Limbal Epithelial Transplantation
Allogenic Cultivated Limbal Epithelial Stem Cell Transplantation
Cultivated Oral Mucosal Epithelial Transplantation
Transplantation of the Epithelial Cells Generated from Induced Pluripotent Stem Cells
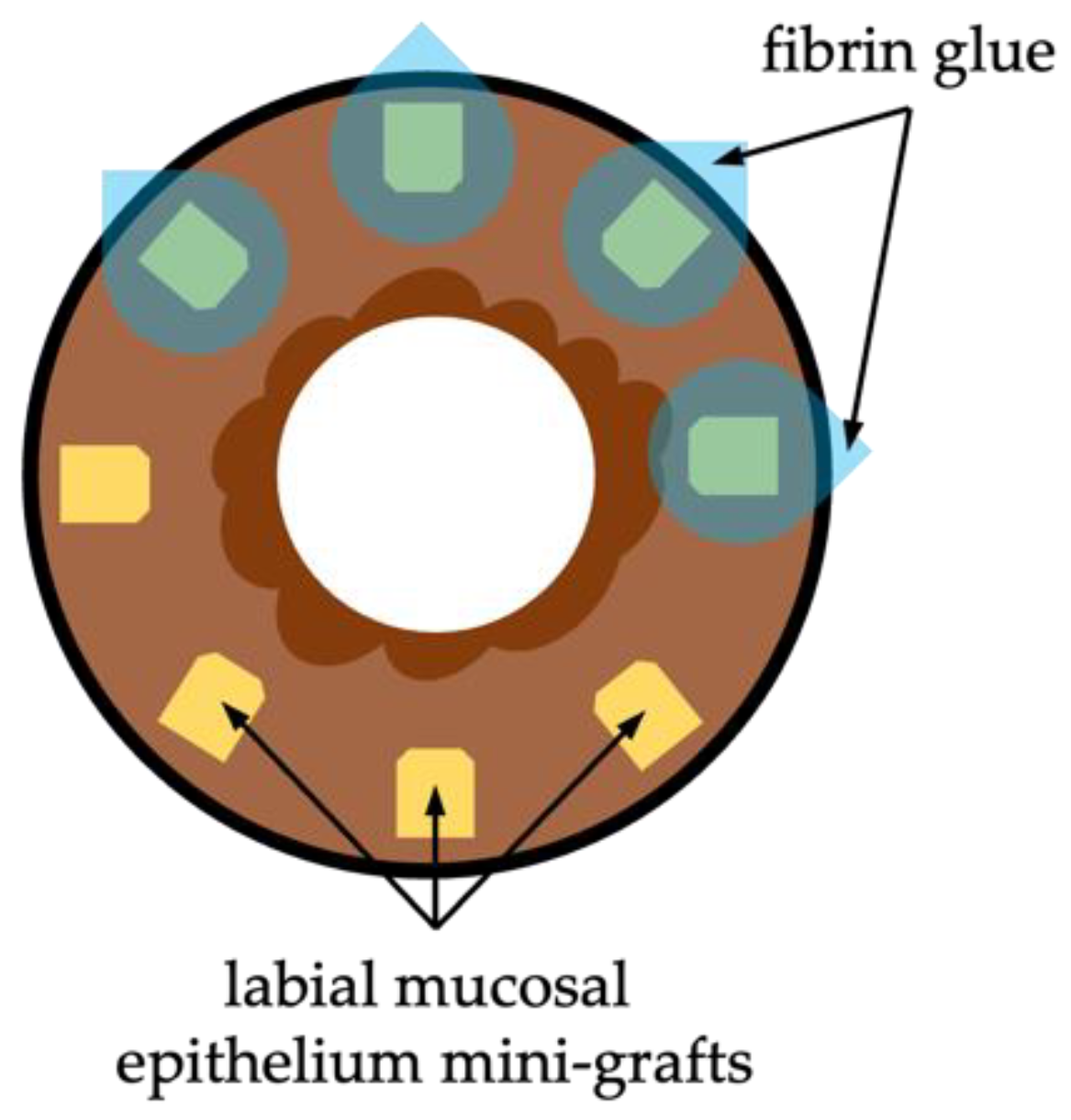
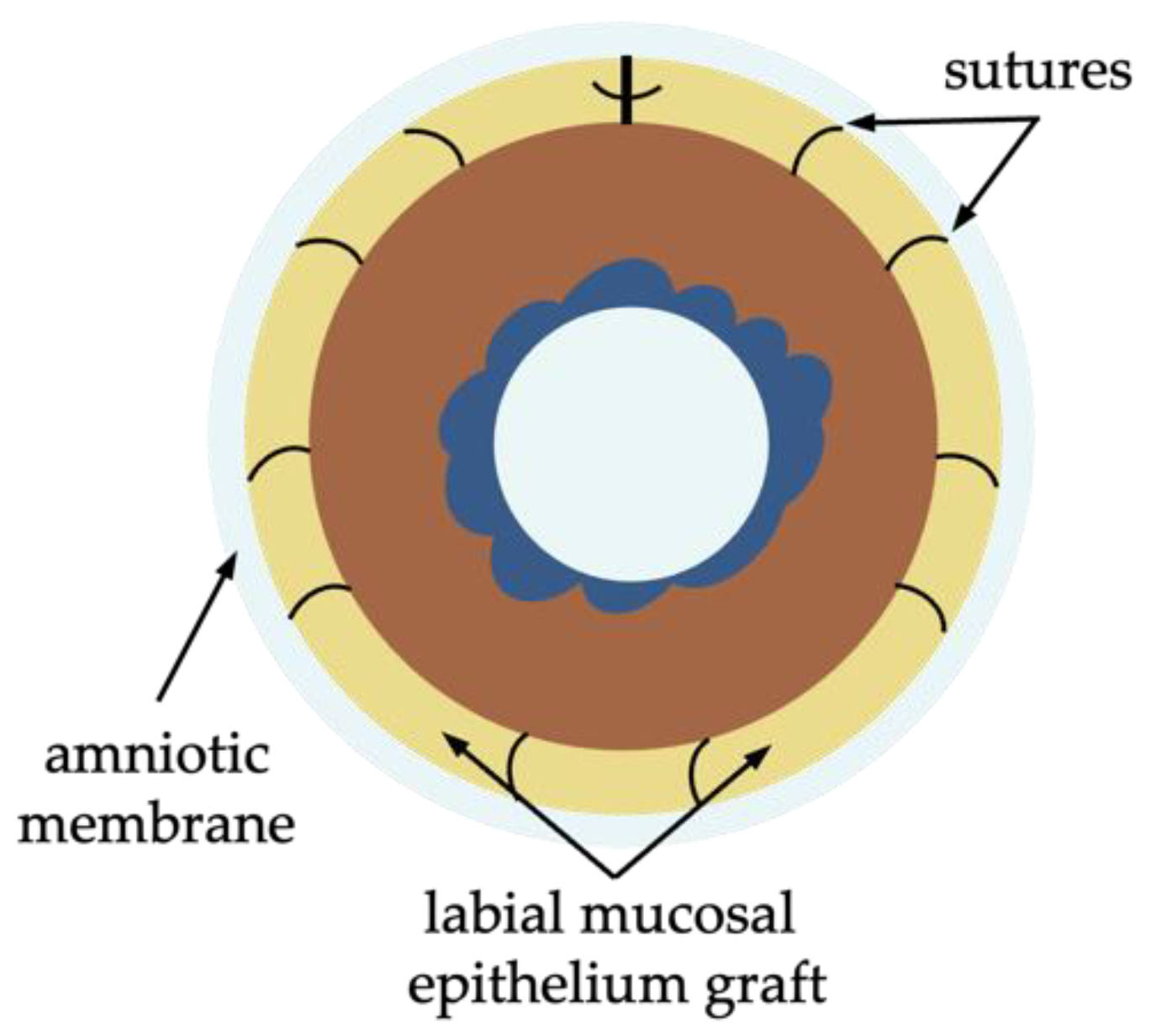
Simple Oral Mucosal Epithelium Transplantation
Paralimbal Oral Mucosal Epithelium Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E. Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Thoft, R.A.; Friend, J. The X, Y, Z hypothesis of corneal epithelial maintenance. Investig. Ophthalmol. Vis Sci. 1983, 24, 1442–1443. [Google Scholar] [PubMed]
- Kohanim, S.; Palioura, S.; Saeed, H.N.; Akpek, E.K.; Amescua, G.; Basu, S.; Blomquist, P.H.; Bouchard, C.S.; Bouchard, J.K.; Gai, K.; et al. Acute and chronic ophthalmic involvement in Stevens-Johnson syndrome/toxic epidermal necrolysis—A comprehensive review and guide to therapy. II. Ophthalmic. Disease Ocul. Surf. 2016, 14, 168–188. [Google Scholar] [CrossRef] [PubMed]
- Georgoudis, P.; Sabatino, F.; Szentmary, N.; Palioura, S.; Fodor, E.; Hamada, S.; Scholl, H.P.N.; Gatzioufas, Z. Ocular mucous membrane pemphigoid: Current state of pathophysiology, diagnostics and treatment. Ophthalmol. Ther. 2019, 8, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Dua, H.S.; Gomes, J.A.; Singh, A. Corneal epithelial wound healing. Br. J. Ophthalmol. 1994, 78, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Elhusseiny, A.M.; Soleimani, M.; Eleiwa, T.K.; ElSheikh, R.H.; Frank, C.R.; Naderan, M.; Yazdanpanah, G.; Rosenblatt, M.I.; Djalilian, A.R. Current and emerging therapies for limbal stem cell deficiency. Stem Cells Transl. Med. 2022, 11, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.X.; Kruse, F.; Gomes, J.A.; Chan, C.C.; Daya, S.; Dana, R.; Figueiredo, F.C.; Figueiredo, S.; Rama, P.; Sangwan, V.; et al. Global consensus on the management of limbal stem cell deficiency. Cornea 2020, 39, 1291–1302. [Google Scholar] [CrossRef] [PubMed]
- Daya, S.M. Conjunctival-limbal autograft. Curr. Opin. Ophthalmol. 2017, 28, 370–376. [Google Scholar] [CrossRef]
- Rama, P.; Matuska, S.; Paganoni, G.; Spinelli, A.; De Luca, M.; Pellegrini, G. Limbal stem-cell therapy and long-term corneal regeneration. N. Engl. J. Med. 2010, 363, 147–155. [Google Scholar] [CrossRef]
- Basu, S.; Sureka, S.P.; Shanbhag, S.S.; Kethiri, A.R.; Singh, V.; Sangwan, V.S. Simple limbal epithelial transplantation: Long-term clinical outcomes in 125 cases of unilateral chronic ocular surface burns. Ophthalmology 2016, 123, 1000–1010. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Nikpoor, N.; Rao Donthineni, P.; Singh, V.; Chodosh, J.; Basu, S. Autologous limbal stem cell transplantation: A systematic review of clinical outcomes with different surgical techniques. Br. J. Ophthalmol. 2020, 104, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Attico, E.; Galaverni, G.; Pellegrini, G. Clinical studies of COMET for total LSCD: A review of the methods and molecular markers for follow-up characterizations. Curr. Ophthalmol. Rep. 2021, 9, 25–37. [Google Scholar] [CrossRef]
- Oie, Y.; Komoto, S.; Kawasaki, R. Systematic review of clinical research on regenerative medicine for the cornea. Jpn. J Ophthalmol. 2021, 65, 169–183. [Google Scholar] [CrossRef]
- Moroz, Z.I.; Vlasova, V.A.; Kovshun, E.V. The history of keratoprosthetics in the S. Fyodorov Eye Microsurgery Federal State Institution. Fyodorov J. Ophthalmic Surg. 2013, 4, 50–55. (In Russian) [Google Scholar]
- Priddy, J.; Bardan, A.S.; Tawfik, H.S.; Liu, C. Systematic review and meta-analysis of the medium- and long-term outcomes of the Boston type 1 keratoprosthesis. Cornea 2019, 38, 1465–1473. [Google Scholar] [CrossRef]
- Vazirani, J.; Mariappan, I.; Ramamurthy, S.; Fatima, S.; Basu, S.; Sangwan, V.S. Surgical management of bilateral limbal stem cell deficiency. Ocul. Surf. 2016, 14, 350–364. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Xu, J.; Deng, S.X. The diagnosis of limbal stem cell deficiency. Ocul Surf. 2018, 16, 58–69. [Google Scholar] [CrossRef]
- Singh, R.; Joseph, A.; Umapathy, T.; Tint, N.L.; Dua, H.S. Impression cytology of the ocular surface. Br. J. Ophthalmol. 2005, 89, 1655–1659. [Google Scholar] [CrossRef]
- Poli, M.; Burillon, C.; Auxenfans, C.; Rovere, M.R.; Damour, O. Immunocytochemical diagnosis of limbal stem cell deficiency: Comparative analysis of current corneal and conjunctival biomarkers. Cornea 2015, 34, 817–823. [Google Scholar] [CrossRef]
- Pedrotti, E.; Chierego, C.; Cozzini, T.; Merz, T.; Lagali, N.; De Gregorio, A.; Fasolo, A.; Bonacci, E.; Bonetto, J.; Marchini, J. In Vivo confocal microscopy of the corneal-conjunctival transition in the evaluation of epithelial renewal after SLET. J. Clin. Med. 2020, 9, 3574. [Google Scholar] [CrossRef]
- Pedrotti, E.; Passilongo, M.; Fasolo, A.; Nubile, M.; Parisi, G.; Mastropasqua, R.; Mastropasqua, R.; Ficial, S.; Bertolin, M.; Iorio, E.D.; et al. In Vivo confocal microscopy 1 year after autologous cultured limbal stem cell grafts. Ophthalmology 2015, 122, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.; Samson, C.M.; Deng, S.X. A case of corneal neovascularization misdiagnosed as total limbal stem cell deficiency. Cornea 2018, 37, 1067–1070. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Espana, E.M.; Djalilian, A.R.; Yoo, S.H. En face optical coherence tomography imaging of corneal limbal stem cell niche. In Clinical en Face OCT Atlas; Lumbroso, B., Huang, D., Romano, A., Rispoli, M., Coscas, G., Eds.; Jaypee Brothers Medical Publishers Ltd.: New Delhi, India, 2013; pp. 77–79. [Google Scholar]
- Nubile, M.; Lanzini, M.; Miri, A.; Pocobelli, A.; Calienno, R.; Curcio, C.; Mastropasqua, R.; Dua, H.S.; Mastropasqua, L. In vivo confocal microscopy in diagnosis of limbal stem cell deficiency. Am. J. Ophthalmol. 2013, 155, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Bizheva, K.; Hutchings, N.; Sorbara, L.; Moayed, A.A.; Simpson, T. In vivo volumetric imaging of the human corneo-scleral limbus with spectral domain OCT. Biomed. OptExpress 2011, 2, 1794–1802. [Google Scholar] [CrossRef]
- Haagdorens, M.; Behaegel, J.; Rozema, J.; Van Gerwen, V.; Michiels, S.; Ní Dhubhghaill, S.; Tassignon, M.J.; Zakaria, N. A method for quantifying limbal stem cell niches using OCT imaging. Br. J. Ophthalmol. 2017, 101, 1250–1255. [Google Scholar] [CrossRef]
- Bizheva, K.; Tan, B.; MacLellan, B.; Hosseinaee, Z.; Mason, E.; Hileeto, D.; Sorbara, L. In-vivo imaging of the palisades of Vogt and the limbal crypts with sub-micrometer axial resolution optical coherence tomography. Biomed. Opt. Express. 2017, 8, 4141–4151. [Google Scholar] [CrossRef]
- Le, Q.; Yang, Y.; Deng, S.X.; Xu, J. Correlation between the existence of the palisades of Vogt and limbal epithelial thickness in limbal stem cell deficiency. Clin. Exp. Ophthalmol. 2017, 45, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Sim, D.A.; Keane, P.A.; Sng, C.C.; Egan, C.A.; Tufail, A.; Wilkins, M.R. Optical coherence tomography angiography for anterior segment vasculature imaging. Ophthalmology 2015, 122, 1740–1747. [Google Scholar] [CrossRef]
- Oie, Y.; Nishida, K. Evaluation of Corneal Neovascularization Using Optical Coherence Tomography Angiography in Patients With Limbal Stem Cell Deficiency. Cornea 2017, 36 (Suppl. S1), S72–S75. [Google Scholar] [CrossRef]
- Binotti, W.W.; Nosé, R.M.; Koseoglu, N.D.; Dieckmann, G.M.; Kenyon, K.; Hamrah, P. The utility of anterior segment optical coherence tomography angiography for the assessment of limbal stem cell deficiency. Ocul. Surf. 2021, 19, 94–103. [Google Scholar] [CrossRef]
- Nanji, A.; Redd, T.; Chamberlain, W.; Schallhorn, J.M.; Chen, S.; Ploner, S.; Ploner, S.; Maier, A.; Fujimoto, J.G.; Jia, Y.; et al. Application of Corneal Optical Coherence Tomography Angiography for Assessment of Vessel Depth in Corneal Neovascularization. Cornea 2020, 39, 598–604. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.; Smallwood, R.; Brooks, A.M.; Chan, E.; Fagan, X. Anterior segment optical coherence tomography angiography. J. Vis. Commun. Med. 2019, 42, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Le, Q.; Zhao, F.; Hong, J.; Xu, J.; Zheng, T.; Sun, X. Application of in vivo laser scanning confocal microscopy for evaluation of ocular surface diseases: Lessons learned from pterygium, meibomian gland disease, and chemical burns. Cornea 2011, 30 (Suppl. S1), S25–S28. [Google Scholar] [CrossRef] [PubMed]
- Cruzat, A.; Pavan-Langston, D.; Hamrah, P. In vivo confocal microscopy of corneal nerves: Analysis and clinical correlation. Semin. Ophthalmol. 2010, 25, 171–177. [Google Scholar] [CrossRef]
- Lum, E.; Golebiowski, B.; Swarbrick, H.A. Mapping the corneal sub-basal nerve plexus in orthokeratology lens wear using in vivo laser scanning confocal microscopy. Invest. Ophthalmol. Vis. Sci. 2012, 53, 1803–1809. [Google Scholar] [CrossRef]
- Vera, L.S.; Gueudry, J.; Delcampe, A.; Roujeau, J.C.; Brasseur, G.; Muraine, M. In vivo confocal microscopic evaluation of corneal changes in chronic Stevens-Johnson syndrome and toxic epidermal necrolysis. Cornea 2009, 28, 401–407. [Google Scholar] [CrossRef]
- Zhivov, A.; Winter, K.; Hovakimyan, M.; Peschel, S.; Harder, V.; Schober, H.C.; Kundt, G.; Baltrusch, S.; Guthoff, R.F.; Stachs, O. Imaging and quantification of subbasal nerve plexus in healthy volunteers and diabetic patients with or without retinopathy. PLoS ONE 2013, 8, e52157. [Google Scholar] [CrossRef]
- Lagali, N. Laser-Scanning in vivo Confocal Microscopy of the Cornea: Imaging and Analysis Methods for Preclinical and Clinical Applications. In Confocal Laser Microscopy-Principles and Applications in Medicine, Biology, and the Food Sciences; Lagali, N., Ed.; IntechOpen Limited: London, UK, 2013; pp. 51–80. [Google Scholar]
- Jirsova, K.; Dudakova, L.; Kalasova, S.; Vesela, V.; Merjava, S. The OV-TL 12/30 clone of anti-cytokeratin 7 antibody as a new marker of corneal conjunctivalization in patients with limbal stem cell deficiency. Invest. Ophthalmol. Vis. Sci. 2011, 52, 5892–5898. [Google Scholar] [CrossRef]
- Moll, R.; Divo, M.; Langbein, L. The human keratins: Biology and pathology. Histochem. Cell. Biol. 2008, 129, 705–733. [Google Scholar] [CrossRef]
- Ramirez-Miranda, A.; Nakatsu, M.N.; Zarei-Ghanavati, S.; Nguyen, C.V.; Deng, S.X. Keratin 13 is a more specific marker of conjunctival epithelium than keratin 19. Mol. Vis. 2011, 17, 1652–1661. [Google Scholar]
- Roy, R.; Boisjoly, H.M.; Wagner, E.; Langlois, A.; Bernard, P.M.; Bazin, R.; Laughrea, P.A.; Dubé, I. Pretransplant and posttransplant antibodies in human corneal transplantation. Transplantation 1992, 54, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J. Management of limbal stem cell deficiency: A historical perspective, past, present, and future. Cornea 2015, 34 (Suppl. S10), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Basu, S.; MacNeil, S.; Balasubramanian, D. Simple limbal epithelial transplantation (SLET): A novel surgical technique for the treatment of unilateral limbal stem cell deficiency. Br. J. Ophthalmol. 2012, 96, 931–934. [Google Scholar] [CrossRef] [PubMed]
- Malyugin, B.E.; Gerasimov, M.Y.; Borzenok, S.A. Glueless simple limbal epithelial transplantation: The report of the first 2 cases. Cornea 2020, 39, 1588–1591. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S.; Basu, S.; Vemuganti, G.K.; Sejpal, K.; Subramaniam, S.V.; Bandyopadhyay, S.; Krishnaiah, S.; Gaddipati, S.; Tiwari, S.; Balasubramanian, D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: A 10-year study. Br. J. Ophthalmol. 2011, 95, 1525–1529. [Google Scholar] [CrossRef]
- Daya, S.M.; Chan, C.C.; Holland, E.J. Cornea Society nomenclature for ocular surface rehabilitative procedures. Cornea 2011, 30, 1115–1119. [Google Scholar] [CrossRef]
- Chan, C.C.; Biber, J.M.; Holland, E.J. The modified Cincinnati procedure: Combined conjunctival limbal autografts and keratolimbal allografts for severe unilateral ocular surface failure. Cornea 2012, 31, 1264–1272. [Google Scholar] [CrossRef]
- Cheung, A.Y.; Eslani, M.; Kurji, K.H.; Wright, E.; Sarnicola, E.; Govil, A.; Holland, E.J. Long-term outcomes of living-related conjunctival limbal allograft compared with keratolimbal allograft in patients with limbal stem cell deficiency. Cornea 2020, 39, 980–985. [Google Scholar] [CrossRef]
- Titiyal, J.S.; Sharma, N.; Agarwal, A.K.; Prakash, G.; Tandon, R.; Vajpayee, R. Live related versus cadaveric limbal allograft in limbal stem cell deficiency. Ocul. Immunol. Inflamm. 2015, 23, 232–239. [Google Scholar] [CrossRef]
- Le, Q.; Chauhan, T.; Yung, M.; Tseng, C.H.; Deng, S.X. Outcomes of limbal stem cell transplant: A meta-analysis. JAMA Ophthalmol. 2020, 138, 660–670. [Google Scholar] [CrossRef]
- Miller, A.K.; Young, J.W.; Wilson, D.J.; Dunlap, J.; Chamberlain, W. Transmission of donor-derived breast carcinoma as a recurrent mass in a keratolimbal allograft. Cornea 2017, 36, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Sepsakos, L.; Cheung, A.Y.; Nerad, J.A.; Mogilishetty, G.; Holland, E.J. Donor-derived conjunctival-limbal melanoma after a keratolimbal allograft. Cornea 2017, 36, 1415–1418. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Saeed, H.N.; Paschalis, E.I.; Chodosh, J. Keratolimbal allograft for limbal stem cell deficiency after severe corneal chemical injury: A systematic review. Br. J. Ophthalmol. 2018, 102, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.J.; Mogilishetty, G.; Skeens, H.M.; Hair, D.B.; Neff, K.D.; Biber, J.M.; Chan, C.C. Systemic immunosuppression in ocular surface stem cell transplantation: Results of a 10-year experience. Cornea 2012, 31, 655–661. [Google Scholar] [CrossRef]
- Schwab, I.R.; Reyes, M.; Isseroff, R.R. Successful transplantation of bioengineered tissue replacements in patients with ocular surface disease. Cornea 2000, 19, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Shortt, A.J.; Bunce, C.; Levis, H.J.; Blows, P.; Doré, C.J.; Vernon, A.; Secker, G.A.; Tuft, S.J.; Daniels, J.T. Three-year outcomes of cultured limbal epithelial allografts in aniridia and Stevens-Johnson syndrome evaluated using the Clinical Outcome Assessment in Surgical Trials assessment tool. Stem Cells Transl. Med. 2014, 3, 265–275. [Google Scholar] [CrossRef]
- Behaegel, J.; Tassignon, M.J.; Lagali, N.; Consejo, A.; Koppen, C.; Ní Dhubhghaill, S. Outcomes of human leukocyte antigen-matched allogeneic cultivated limbal epithelial transplantation in aniridia-associated keratopathy-A single-center retrospective analysis. Cornea 2022, 41, 69–77. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, N.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef]
- Utheim, T.P. Concise review: Transplantation of cultured oral mucosal epithelial cells for treating limbal stem cell deficiency-current status and future perspectives. Stem Cells 2015, 33, 1685–1695. [Google Scholar] [CrossRef]
- Malyugin, B.; Borzenok, S.; Gerasimov, M. Clinical outcomes of autologous cultured oral mucosal epithelium transplantation for treatment of limbal stem cell deficiency. Fyodorov J. Ophthalmic Surg. 2020, 4, 77–85. [Google Scholar] [CrossRef]
- Cabral, J.V.; Jackson, C.J.; Utheim, T.P.; Jirsova, K. Ex vivo cultivated oral mucosal epithelial cell transplantation for limbal stem cell deficiency: A review. Stem Cell Res. Ther. 2020, 11, 301. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, F.C.; Glanville, J.M.; Arber, M.; Carr, E.; Rydevik, G.; Hogg, J.; Okonkwo, A.; Figueiredo, G.; Lako, M.; Whiter, F.; et al. A systematic review of cellular therapies for the treatment of limbal stem cell deficiency affecting one or both eyes. Ocul. Surf. 2021, 20, 48–61. [Google Scholar] [CrossRef]
- Baradaran-Rafii, A.; Delfazayebaher, S.; Aghdami, N.; Taghiabadi, E.; Bamdad, S.; Roshandel, D. Midterm outcomes of penetrating keratoplasty after cultivated oral mucosal epithelial transplantation in chemical burn. Ocul. Surf. 2017, 15, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, R.; Ishikawa, Y.; Sasamoto, Y.; Katori, R.; Nomura, N.; Ichikawa, T.; Ichikawa, T.; Araki, S.; Takeshi Soma, T.; Kawasaki, S.; et al. Coordinated ocular development from human iPS cells and recovery of corneal function. Nature 2016, 531, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Mikhailova, A.; Ilmarinen, T.; Ratnayake, A.; Petrovski, G.; Uusitalo, H.; Skottman, H.; Rafat, M. Human pluripotent stem cell-derived limbal epithelial stem cells on bioengineered matrices for corneal reconstruction. Exp. Eye Res. 2016, 146, 26–34. [Google Scholar] [CrossRef]
- Kobold, S.; Guhr, A.; Mah, N.; Bultjer, N.; Seltmann, S.; Wulczyn, A.E.S.; Stacey, G.; Jie, H.; Liu, W.; Löser, P.; et al. Manually curated database of clinical studies involving cell products derived from human pluripotent stem cells. Stem Cell Rep. 2020, 15, 546–555. [Google Scholar] [CrossRef]
- Inamochi, A.; Tomioka, A.; Kitamoto, K.; Miyai, T.; Usui, T.; Aihara, M.; Yamagami, S. Simple oral mucosal epithelial transplantation in a rabbit model. Sci. Rep. 2019, 9, 18088. [Google Scholar] [CrossRef]
- Umfress, A.C.; Mawn, L.A.; Joos, K.M.; Donahue, S.P.; Schmitt, A.D.; Shieh, C. Surgical management of large bilateral epibulbar dermoids with autologous oral mucous membrane transplantation. Am. J. Ophthalmol. Case Rep. 2020, 20, 100982. [Google Scholar] [CrossRef]
- Kara, N.; Dogan, L. Simple oral mucosal epithelial transplantation in a patient with bilateral limbal stem cell deficiency. Eye Contact Lens. 2021, 47, 65–67. [Google Scholar] [CrossRef]
- Ngowyutagon, P.; Prabhasawat, P.; Chirapapaisan, C.; Jaru-Ampornpan, P.; Pornpanich, K.; Ekpo, P.; Sukon, N.; Matamnan, S. Successful ocular surface reconstruction in complete ankyloblepharon using a simple oral mucosal epithelial transplantation technique: A case report. Cornea 2021, 40, 1482–1486. [Google Scholar] [CrossRef]
- Gong, D.; Yan, C.; Yu, F.; Yan, D.; Wu, N.; Chen, L.; Zhang, S.; Zhang, Y. Direct oral mucosal epithelial transplantation supplies stem cells and promotes corneal wound healing for the treatment of refractory persistent corneal epithelial defects. Exp. Eye Res. 2022, 215, 108934. [Google Scholar] [CrossRef] [PubMed]
- Choe, H.R.; Yoon, C.H.; Kim, M.K. Ocular surface reconstruction using circumferentially treated autologous oral mucosal graft transplantation for limbal stem cell deficiency. Korean J. Ophthalmol. 2019, 33, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sheha, H.; Fu, Y.; Giegengack, M.; Tseng, S.C. Oral mucosal grafting with amniotic membrane transplantation for total limbal stem cell deficiency. Am. J. Ophthalmol. 2011, 152, 739–747.e1. [Google Scholar] [CrossRef] [PubMed]
- Gerasimov, M.Y.; Ostrovskiy, D.S.; Shatskikh AVBorzenok, S.A.; Malyugin, B.E. Labial mucosal epithelium grafting in an ex vivo humana donor corneal model. Exp. Eye Res. 2022, 216, 108931. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malyugin, B.; Kalinnikova, S.; Isabekov, R.; Ostrovskiy, D.; Knyazer, B.; Gerasimov, M. Diagnostic Algorithm for Surgical Management of Limbal Stem Cell Deficiency. Diagnostics 2023, 13, 199. https://doi.org/10.3390/diagnostics13020199
Malyugin B, Kalinnikova S, Isabekov R, Ostrovskiy D, Knyazer B, Gerasimov M. Diagnostic Algorithm for Surgical Management of Limbal Stem Cell Deficiency. Diagnostics. 2023; 13(2):199. https://doi.org/10.3390/diagnostics13020199
Chicago/Turabian StyleMalyugin, Boris, Svetlana Kalinnikova, Ruslan Isabekov, Dmitriy Ostrovskiy, Boris Knyazer, and Maxim Gerasimov. 2023. "Diagnostic Algorithm for Surgical Management of Limbal Stem Cell Deficiency" Diagnostics 13, no. 2: 199. https://doi.org/10.3390/diagnostics13020199
APA StyleMalyugin, B., Kalinnikova, S., Isabekov, R., Ostrovskiy, D., Knyazer, B., & Gerasimov, M. (2023). Diagnostic Algorithm for Surgical Management of Limbal Stem Cell Deficiency. Diagnostics, 13(2), 199. https://doi.org/10.3390/diagnostics13020199






