Cell-Free DNA as a New Biomarker of IVF Success, Independent of Any Infertility Factor, Including Endometriosis
Abstract
1. Introduction
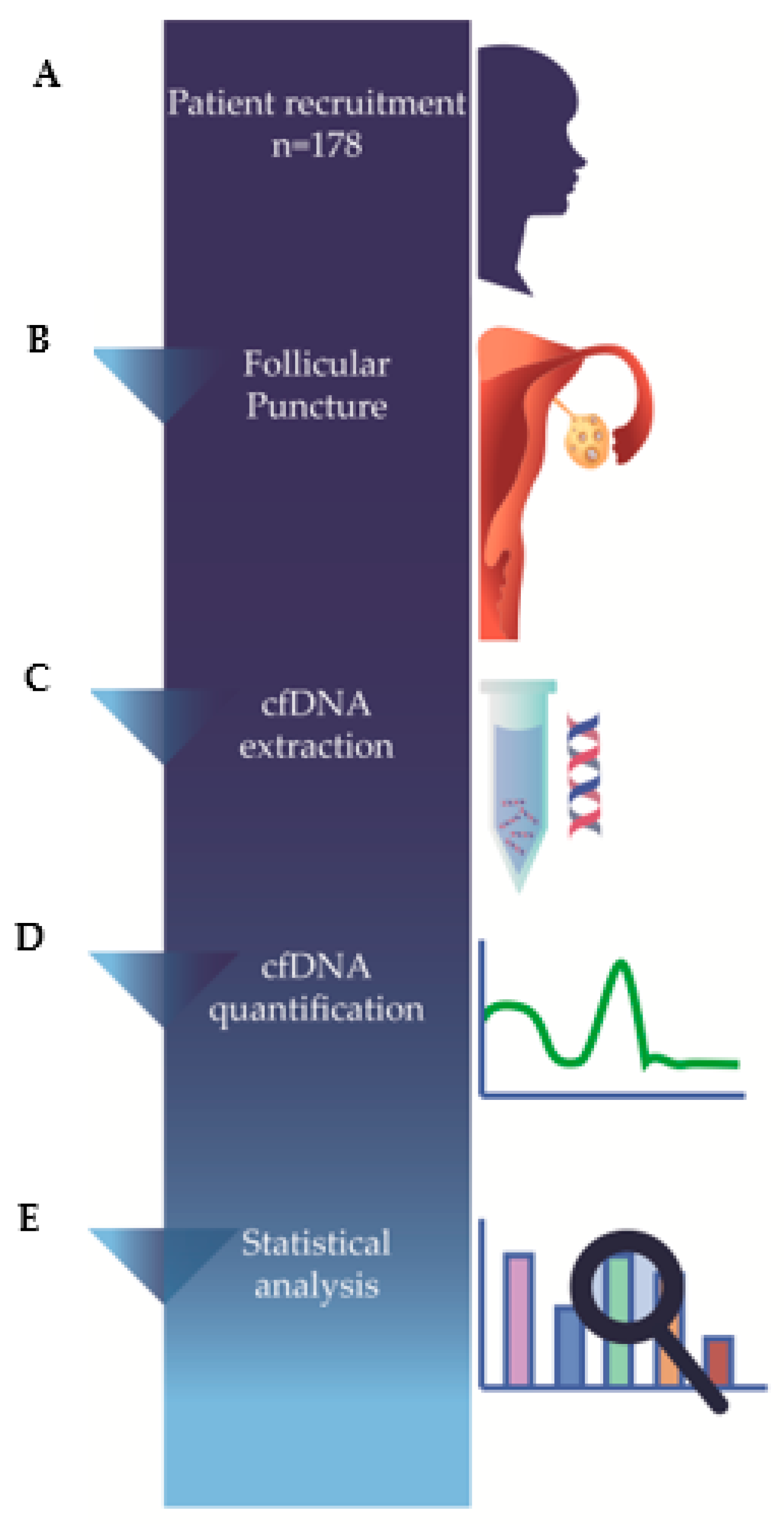
2. Materials and Methods
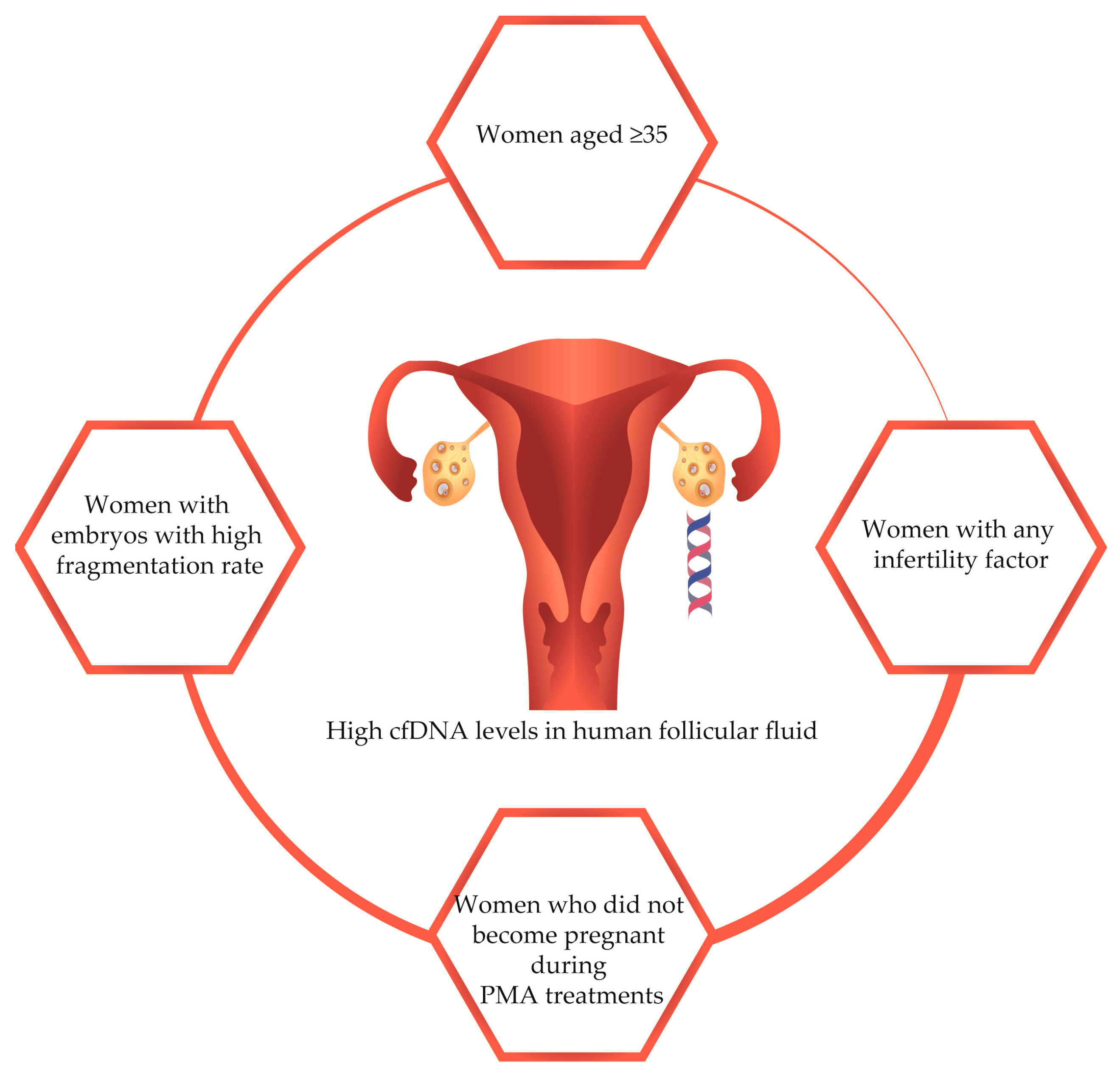
2.1. Study Population
2.2. Ethical Approval
2.3. Follicular Fluid Sample Collection
2.4. Follicular Fluid Preparation
2.5. Cell-Free DNA Extraction
2.6. Cell-Free DNA Quantification
2.7. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zegers-Hochschild, F.; Adamson, G.D.; Dyer, S.; Racowsky, C.; de Mouzon, J.; Sokol, R.; Rienzi, L.; Sunde, A.; Schmidt, L.; Cooke, I.D.; et al. The International Glossary on Infertility and Fertility Care. Hum. Reprod. 2017, 32, 1786–1801. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.K.; Chen, H.H.; Ding, X.P.; Zhang, S.H.; Zhang, J.H. Association of polymorphisms in glutathione S-transferase genes (GSTM1, GSTT1, GSTP1) with idiopathic azoospermia or oligospermia in Sichuan, China. Asian J. Androl. 2015, 17, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Goudakou, M.; Kalogeraki, A.; Matalliotakis, I.; Panagiotidis, Y.; Gullo, G.; Prapas, Y. Cryptic sperm defects may be the cause for total fertilization failure in oocyte donor cycles. Reprod. Biomed. Online 2012, 24, 148–152. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gullo, G.; Cucinella, G.; Perino, A.; Gullo, D.; Segreto, D.; Laganà, A.S.; Buzzaccarini, G.; Donarelli, Z.; Marino, A.; Allegra, A.; et al. The Gender Gap in the Diagnostic-Therapeutic Journey of the Infertile Couple. Int. J. Environ. Res. Public Health. 2021, 18, 6184. [Google Scholar] [CrossRef]
- Balaban, B.; Gardner, D.K. Morphological assessment of blastocyst stage embryos: Types of grading systems and their reported outcomes. In Human Gametes and Preimplantation Embryos: Assessment and Diagnosis; Gardner, D., Sakkas, D., Seli, E., Wells, D., Eds.; Springer Science and Media: New York, NY, USA, 2013; pp. 31–43. [Google Scholar] [CrossRef]
- Rule, K.; Chosed, R.J.; Arthur Chang, T.; David Wininger, J.; Roudebush, W.E. Relationship between blastocoel cell-free DNA and day-5 blastocyst morphology. J. Assist. Reprod. Genet. 2018, 35, 1497–1501. [Google Scholar] [CrossRef]
- Yanaihara, A.; Mitsukawa, K.; Iwasaki, S.; Otsuki, K.; Kawamura, T.; Okai, T. High concentrations of lactoferrin in the follicular fluid correlate with embryo quality during in vitro fertilization cycles. Fertil. Steril. 2007, 87, 279–282. [Google Scholar] [CrossRef]
- Estes, S.J.; Ye, B.; Qiu, W.; Cramer, D.; Hornstein, M.D.; Missmer, S.A. A proteomic analysis of IVF follicular fluid in women ≤32 years old. Fertil. Steril. 2009, 92, 1569–1578. [Google Scholar] [CrossRef]
- Revelli, A.; Delle Piane, L.; Casano, S.; Molinari, E.; Massobrio, M.; Rinaudo, P. Follicular fluid content and oocyte quality: From single biochemical markers to metabolomics. Reprod Biol. Endocrinol. 2009, 7, 40. [Google Scholar] [CrossRef]
- Baka, S.; Malamitsi-Puchner, A. Novel follicular fluid factors influencing oocyte developmental potential in IVF: A review. Reprod. Biomed. Online. 2006, 12, 500–506. [Google Scholar] [CrossRef]
- Wallace, M.; Cottell, E.; Gibney, M.J.; McAuliffe, F.M.; Wingfield, M.; Brennan, L. An investigation into the relationship between the metabolic profile of follicular fluid, oocyte developmental potential, and implantation outcome. Fertil. Steril. 2012, 97, 1078–1084.e8. [Google Scholar] [CrossRef]
- Angelucci, S.; Ciavardelli, D.; Di Giuseppe, F.; Eleuterio, E.; Sulpizio, M.; Tiboni, G.M.; Giampietro, F.; Palumbo, P.; Di Ilio, C. Proteome analysis of human follicular fluid. Biochim. Et Biophys. Acta (BBA)-Proteins Proteom. 2006, 1764, 1775–1785. [Google Scholar] [CrossRef]
- Stroun, M.; Anker, P.; Lyautey, J.; Lederrey, C.; Maurice, P.A. Isolation and characterization of DNA from the plasma of cancer patients. Eur. J. Cancer Clin. Oncol. 1987, 23, 707–712. [Google Scholar] [CrossRef]
- Volik, S.; Alcaide, M.; Morin, R.D.; Collins, C. Cell-free DNA (cfDNA): Clinical Significance and Utility in Cancer Shaped by Emerging Technologies. Mol. Cancer Res. 2016, 14, 898–908. [Google Scholar] [CrossRef]
- Scalici, E.; Traver, S.; Molinari, N.; Mullet, T.; Monforte, M.; Vintejoux, E.; Hamamah, S. Cell-free DNA in human follicular fluid as a biomarker of embryo quality. Hum Reprod. 2014, 29, 2661–2669. [Google Scholar] [CrossRef]
- Diaz, L.A., Jr.; Bardelli, A. Liquid biopsies: Genotyping circulating tumor DNA. J. Clin. Oncol. 2014, 32, 579–586. [Google Scholar] [CrossRef]
- Schwarzenbach, H.; Hoon, D.S.; Pantel, K. Cell-free nucleic acids as biomarkers in cancer patients. Nat. Rev. Cancer. 2011, 11, 426–437. [Google Scholar] [CrossRef]
- Pisetsky, D.S.; Fairhurst, A.M. The origin of extracellular DNA during the clearance of dead and dying cells. Autoimmunity. 2007, 40, 281–284. [Google Scholar] [CrossRef]
- Gahan, P.B. Biology of circulating nucleic acids and possible roles in diagnosis and treatment in diabetes and cancer. Infect. Disord. Drug Targets. 2012, 12, 360–370. [Google Scholar] [CrossRef]
- Marzese, D.M.; Hirose, H.; Hoon, D.S. Diagnostic and prognostic value of circulating tumor-related DNA in cancer patients. Expert Rev. Mol. Diagn. 2013, 13, 827–844. [Google Scholar] [CrossRef]
- Lo, Y.M.; Corbetta, N.; Chamberlain, P.F.; Rai, V.; Sargent, I.L.; Redman, C.W.; Wainscoat, J.S. Presence of fetal DNA in maternal plasma and serum. Lancet. 1997, 350, 485–487. [Google Scholar] [CrossRef]
- Traver, S.; Assou, S.; Scalici, E.; Haouzi, D.; Al-Edani, T.; Belloc, S.; Hamamah, S. Cell-free nucleic acids as non-invasive biomarkers of gynecological cancers, ovarian, endometrial and obstetric disorders and fetal aneuploidy. Hum. Reprod. Updat. 2014, 20, 905–923. [Google Scholar] [CrossRef] [PubMed]
- Scalici, E.; Mullet, T.; Hoa, A.F.; Gala, A.; Loup, V.; Anahory, T.; Belloc, S.; Hamamah, S. Les acides nucléiques circulants et infertilité [Circulating nucleic acids and infertility]. Gynécologie Obs. Fertil. 2015, 43, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Zachariah, R.; Schmid, S.; Radpour, R.; Buerki, N.; Fan, A.X.; Hahn, S.; Holzgreve, W.; Zhong, X.Y. Circulating cell-free DNA as a potential biomarker for minimal and mild endometriosis. Reprod. Biomed. Online. 2009, 18, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Czamanski-Cohen, J.; Sarid, O.; Cwikel, J.; Levitas, E.; Lunenfeld, E.; Douvdevani, A.; Har-Vardi, I. Decrease in cell free DNA levels following participation in stress reduction techniques among women undergoing infertility treatment. Arch. Women's Ment. Health. 2014, 17, 251–253. [Google Scholar] [CrossRef] [PubMed]
- Traver, S.; Scalici, E.; Mullet, T.; Molinari, N.; Vincens, C.; Anahory, T.; Hamamah, S. Cell-free DNA in Human Follicular Microenvironment: New Prognostic Biomarker to Predict in vitro Fertilization Outcomes. PLoS ONE. 2015, 10, e0136172. [Google Scholar] [CrossRef][Green Version]
- Prapas, Y.; Petousis, S.; Panagiotidis, Y.; Gullo, G.; Kasapi, L.; Papadeothodorou, A.; Prapas, N. Injection of embryo culture supernatant to the endometrial cavity does not affect outcomes in IVF/ICSI or oocyte donation cycles: A randomized clinical trial. Eur. J. Obstet. Gynecol. Reprod. Biol. 2012, 162, 169–173. [Google Scholar] [CrossRef]
- Umetani, N.; Kim, J.; Hiramatsu, S.; Reber, H.A.; Hines, O.J.; Bilchik, A.J.; Hoon, D.S. Increased integrity of free circulating DNA in sera of patients with colorectal or periampullary cancer: Direct quantitative PCR for ALU repeats. Clin. Chem. 2006, 52, 1062–1069. [Google Scholar] [CrossRef]
- Mandel, P.; Metais, P. Les acides nucléiques du plasma sanguin chez l’homme [Nuclear Acids in Human Blood Plasma]. CR Seances Soc. Biol. Fil. 1948, 142, 241–243. [Google Scholar]
- Ralla, B.; Stephan, C.; Meller, S.; Dietrich, D.; Kristiansen, G.; Jung, K. Nucleic acid-based biomarkers in body fluids of patients with urologic malignancies. Crit. Rev. Clin. Lab. Sci. 2014, 51, 200–231. [Google Scholar] [CrossRef]
- Swarup, V.; Rajeswari, M.R. Circulating (cell-free) nucleic acids--a promising, non-invasive tool for early detection of several human diseases. FEBS Lett. 2007, 581, 795–799. [Google Scholar] [CrossRef]
- Jahr, S.; Hentze, H.; Englisch, S.; Hardt, D.; Fackelmayer, F.O.; Hesch, R.D.; Knippers, R. DNA fragments in the blood plasma of cancer patients: Quantitations and evidence for their origin from apoptotic and necrotic cells. Cancer Res. 2001, 61, 1659–1665. [Google Scholar]
- Burgio, S.; Polizzi, C.; Buzzaccarini, G.; Laganà, A.S.; Gullo, G.; Perricone, G.; Perino, A.; Cucinella, G.; Alesi, M. Psychological variables in medically assisted reproduction: A systematic review. Menopausal Rev. 2022, 21, 47–63. [Google Scholar] [CrossRef]
- Habib, N.; Buzzaccarini, G.; Centini, G.; Moawad, G.N.; Ceccaldi, P.F.; Gitas, G.; Alkatout, I.; Gullo, G.; Terzic, S.; Sleiman, Z. Impact of lifestyle and diet on endometriosis: A fresh look to a busy corner. Menopausal Rev. 2022, 21, 124–132. [Google Scholar] [CrossRef]
- Scutiero, G.; Iannone, P.; Bernardi, G.; Bonaccorsi, G.; Spadaro, S.; Volta, C.A.; Greco, P.; Nappi, L. Oxidative Stress and Endometriosis: A Systematic Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 7265238. [Google Scholar] [CrossRef]
- Agarwal, A.; Aponte-Mellado, A.; Premkumar, B.J.; Shaman, A.; Gupta, S. The effects of oxidative stress on female reproduction: A review. Reprod. Biol. Endocrinol. 2012, 10, 49. [Google Scholar] [CrossRef]
- Sedensky, M.M.; Morgan, P.G. Mitochondrial respiration and reactive oxygen species in mitochondrial aging mutants. Exp. Gerontol. 2006, 41, 237–245. [Google Scholar] [CrossRef]
- Pisoschi, A.M.; Pop, A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015, 97, 55–74. [Google Scholar] [CrossRef]
- Franks, S.; Stark, J.; Hardy, K. Follicle dynamics and anovulation in polycystic ovary syndrome. Hum. Reprod. Updat. 2008, 14, 367–378. [Google Scholar] [CrossRef]
- Dewailly, D.; Andersen, C.Y.; Balen, A.; Broekmans, F.; Dilaver, N.; Fanchin, R.; Griesinger, G.; Kelsey, T.W.; La Marca, A.; Lambalk, C.; et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum. Reprod. Updat. 2014, 20, 370–385. [Google Scholar] [CrossRef]
- Stigliani, S.; Anserini, P.; Venturini, P.L.; Scaruffi, P. Mitochondrial DNA content in embryo culture medium is significantly associated with human embryo fragmentation. Hum. Reprod. 2013, 28, 2652–2660. [Google Scholar] [CrossRef]
| Variable | Mean | n | Min-Max | SD | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q115) | p-Value (q115) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247) | p-Value (q247) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247/q115) | p-Value (q247/q115) |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 34 | - | 19–39 | 3.69 | - | - | - | - | - | - |
| <35 years | - | 92 | - | - | 1.3 ± 1.7 [1.0–1.7] | 0.259 | 1.7 ± 2.9 [1.1–2.3] | 0.670 | 9.7 ± 1.1 [9.5–9.9] | 0.017 |
| ≥35 years | - | 86 | - | - | 1.5 ± 1.8 [1.1–1.9] | 1.3 ± 1.8 [0.9–1.7] | 10.1 ± 1.2 [9.9–10.4] | |||
| BMI (kg/m2) | 24 | - | 17–33 | 3.70 | - | - | - | - | - | - |
| ≤25 kg/m2 | - | 123 | - | - | 1.5 ± 1.7 [1.2–1.8] | 0.612 | 1.6 ± 2.7 [1.1–2.1] | 0.427 | 9.9 ± 1.2 [9.7–10.2] | 0.582 |
| >25 kg/m2 | - | 55 | - | - | 1.4 ± 1.6 [0.9–1.8] | 1.3 ± 1.7 [0.8–1.7] | 9.8 ± 1.2 [9.5–10.2] | |||
| Ethnicity | - | - | - | - | - | - | - | - | - | - |
| Caucasian | - | 174 | - | - | 1.4 ± 1.7 [1.1–1.8] | 0.922 | 1.5 ± 2.4 [1.1–1.8] | 0.743 | 9.9 ± 1.1 [9.7–10.1] | 0.065 |
| Gypsy | - | 2 | - | - | 0.9 ± 1.2 [1.0–1.2] | 2.2 ± 2.5 [2.0–2.5] | 9.1 ± 0.7 [2.7–15.6] | |||
| Afro-Europeans | - | 2 | - | - | 1.3 ± 0.9 [0.7–9.9] | 0.4 ± 0.4 [0.3–4.3] | 11.7 ± 2.4 [10.2–33.8] | |||
| Age at menarche (years) | 12 | - | 8–18 | 1.82 | - | - | - | - | - | - |
| ≤12 years | - | 102 | - | - | 1.4 ± 1.6 [1.0–1.7] | 0.675 | 1.7 ± 2.8 [1.1–2.2] | 0.403 | 9.8 ± 1.2 [9.6–10.1] | 0.348 |
| 13–14 years | - | 52 | - | - | 1.4 ± 1.7 [0.9–1.9] | 1.1 ± 1.3 [0.7–1.5] | 10.0 ± 1.2 [9.7–10.4] | |||
| ≥15 years | - | 21 | - | - | 1.7 ± 2.2 [0.7–2.7] | 1.4 ± 2.3 [0.4–2.5] | 10.2 ± 1.1 [9.7–10.7] | |||
| Smoking Habits | - | - | - | - | - | - | - | - | - | - |
| Never | - | 130 | - | - | 1.4 ± 1.7 [1.1–1.7] | 0.998 | 1.5 ± 2.5 [1.0–1.9] | 0.741 | 10.0 ± 1.2 [9.7–10.2] | 0.572 |
| Previous | - | 21 | - | - | 1.4 ± 1.5 [0.7–2.1] | 1.7 ± 2.6 [0.5–2.9] | 9.9 ± 1.2 [9.4–10.4] | |||
| Present | - | 27 | - | - | 1.4 ± 1.7 [1.1–1.7] | 1.2 ± 1.5 [0.6–1.8] | 9.7 ± 1.7 [9.2–10.2] | |||
| Infertility length (months) | 53 | - | 14–192 | 32.8 | - | - | - | - | - | - |
| <24 months | - | 9 | - | - | 0.6 ± 0.8 [0.3–1.2] | 0.277 | 0.5 ± 0.3 [0.3–0.8] | 0.476 | 9.7 ± 1.02 [8.9–10.5] | 0.435 |
| 24–48 months | - | 91 | - | - | 1.5 ± 1.8 [1.1–1.9] | 1.6 ± 2.3 [1.1–2.1] | 9.8 ± 1.2 [9.6–10.1] | |||
| >48 months | - | 78 | - | - | 1.3 ± 1.6 [1.0–1.7] | 1.5 ± 2.4 [0.9–2.1] | 10.0 ± 1.1 [9.8–10.3] |
| Variable | n | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q115) | p-Value (q115) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247) | p-Value (q247) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247/q115) | p-Value (q247/q115) |
|---|---|---|---|---|---|---|---|
| Endometriosis | 45 | 1.2 ± 1.4 [0.8–1.6] | 0.528 | 1.3 ± 1.5 [0.8–1.7] | 0.376 | 9.9 ± 1.3 [9.5–10.3] | 0.822 |
| PCOS | 60 | 1.2 ± 1.5 [0.8–1.6] | 0.359 | 1.6 ± 3.5 [0.7–2.5] | 0.566 | 10.0 ± 1.2 [9.6–10.3] | 0.599 |
| POF | 40 | 1.6 ± 1.9 [1.0–2.2] | 0.494 | 1.5 ± 2.1 [0.8–2.2] | 0.999 | 10.0 ± 1.01 [9.7–10.4] | 0.326 |
| Idiopathic | 44 | 1.8 ± 2.1 [1.1–2.4] | 0.187 | 1.6 ± 1.5 [1.1–2.0] | 0.033 | 9.8 ± 1.1 [9.4–10.1] | 0.375 |
| Variable | Mean | n | Min-Max | SD | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q115) | p-Value (q115) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247) | p-Value (q247) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247/q115) | p-Value (q247/q115) |
|---|---|---|---|---|---|---|---|---|---|---|
| AMH (ng/mL) | 3.40 | - | 0.02–27 | 3.64 | - | - | - | - | - | - |
| ≤1 ng/mL | - | 35 | - | - | 1.6 ± 1.9 [0.9–2.3] | 0.479 | 1.7 ± 2.2 [0.9–2.5] | 0.534 | 9.9 ± 1.2 [9.5–10.3] | 0.940 |
| >1 ng/mL | - | 139 | - | - | 1.4 ± 1.7 [1.1–1.7] | 1.4 ± 2.5 [1.0–1.9] | 9.9 ± 1.2 [9.7–10.1] | |||
| AFC | 9.98 | - | 3–27 | 4.51 | - | - | - | - | - | - |
| ≤10 | - | 110 | - | - | 1.4 ± 1.7 [1.0–1.7] | 0.533 | 1.6 ± 2.3 [1.2–2.1] | 0.466 | 9.8 ± 1.1 [9.6–10.1] | 0.300 |
| >10 | - | 62 | - | - | 1.5 ± 1.8 [1.1–2.0] | 1.3 ± 2.7 [0.6–2.0] | 10.0 ± 1.2 [9.7–10.4] | |||
| Days of stimulation | 10.7 | - | 8–14 | 1.18 | - | - | - | - | - | - |
| ≤10 | - | 99 | - | - | 1.3 ± 1.8 [0.9–1.6] | 0.251 | 1.2 ± 2.0 [0.8–1.6] | 0.070 | 9.9 ± 1.1 [9.7–10.2] | 0.483 |
| >10 | - | 78 | - | - | 1.6 ± 1.7 [1.2–2.0] | 1.9 ± 2.8 [1.2–2.5] | 9.9 ± 1.3 [9.6–10.2] | |||
| Total dose of gonadotropins (IU/I) | 2383 | - | 150–3900 | 783.7 | - | - | - | - | - | - |
| <3000 IU/I | - | 113 | - | - | 1.5 ± 1.8 [1.2–1.9] | 0.317 | 1.5 ± 1.8 [1.2–1.9] | 0.625 | 9.9 ± 1.2 [9.7–10.1] | 0.838 |
| ≥3000 IU/I | - | 64 | - | - | 1.4 ± 1.8 [0.9–1.8] | 1.2 ± 1.6 [0.8–1.6] | 9.9 ± 1.1 [9.7–10.2] |
| Variable | Mean | n | Min-Max | SD | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q115) | p-Value (q115) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247) | p-Value (q247) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247/q115) | p-Value (q247/q115) |
|---|---|---|---|---|---|---|---|---|---|---|
| Retrieved oocytes | 9 | - | 0–32 | 5.85 | - | - | - | - | - | - |
| ≤6 | - | 37 | - | - | 1.7 ± 2.0 [1.0–2.4] | 0.268 | 1.7 ± 2.3 [0.9–2.5] | 0.614 | 10.0 ± 1.3 [9.5–10.4] | 0.661 |
| >6 | - | 140 | - | - | 1.3 ± 1.6 [1.1–1.7] | 1.4 ± 2.4 [1.0–1.8] | 9.9 ± 1.2 [9.7–10.1] | |||
| Mature oocytes | 8 | - | 0–27 | 5.41 | - | - | - | - | - | - |
| <8 | - | 101 | - | - | 1.5 ± 1.7 [1.1–1.8] | 0.315 | 1.4 ± 1.8 [1.1–1.8] | 0.848 | 9.9 ± 1.1 [9.7–10.1] | 0.378 |
| 8–12 | - | 35 | - | - | 1.7 ± 2.1 [1.0–2.5] | 1.1 ± 0.9 [0.7–1.4] | 10.1 ± 1.1 [9.7–10.5] | |||
| ≥13 | - | 40 | - | - | 0.9 ± 1.1 [0.6–1.3] | 2.0 ± 4.1 [0.7–3.3] | 9.7 ± 1.3 [9.3–10.2] | |||
| Total embryo number | 5 | - | 0–20 | 4.03 | - | - | - | - | - | - |
| ≤2 | - | 47 | - | - | 1.7 ± 1.8 [1.1–1.9] | 0.257 | 1.5 ± 2.1 [0.9–2.2] | 0.832 | 10.1 ± 1.2 [9.7–10.5] | 0.210 |
| >2 | - | 131 | - | - | 1.3 ± 1.7 [1.0–1.6] | 1.5 ± 2.5 [1.0–1.9] | 9.8 ± 1.1 [9.6–10.1] |
| Variable | n | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q115) | p-Value (q115) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247) | p-Value (q247) | FF cfDNA (ng/uL) Mean ± SD [95%CI] (q247/q115) | p-Value (q247/q115) |
|---|---|---|---|---|---|---|---|
| % embryo fragmentation | - | - | - | - | - | - | - |
| % fragmentation < 20 | 37 | 1.1 ± 1.3 [0.6–1.5] | 0.107 | 1.4 ± 1.3 [0.9–1.9] | 0.937 | 9.5 ± 1.0 [9.2–9.9] | 0.007 |
| % fragmentation ≥ 20 | 81 | 1.6 ± 1.8 [1.2–2.0] | 1.5 ± 2.6 [0.9–2.0] | 10.2 ± 1.2 [9.9–10.4] | |||
| βhCG analysis result | - | - | - | - | - | - | - |
| Positive | 44 | 1.6 ± 1.7 [1.1–2.1] | 0.146 | 1.3 ± 1.4 [0.9–1.8] | 0.043 | 9.9 ± 1.0 [9.5–10.2] | 0.377 |
| Negative | 84 | 1.3 ± 1.7 [1.0–1.7] | 1.4 ± 2.6 [0.8–2.0] | 10.1 ± 1.2 [9.8–10.4] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casteleiro Alves, M.M.; Oliani, L.; Almeida, M.; Cardoso, H.J.; Oliani, A.H.; Breitenfeld, L.; Ramalhinho, A.C. Cell-Free DNA as a New Biomarker of IVF Success, Independent of Any Infertility Factor, Including Endometriosis. Diagnostics 2023, 13, 208. https://doi.org/10.3390/diagnostics13020208
Casteleiro Alves MM, Oliani L, Almeida M, Cardoso HJ, Oliani AH, Breitenfeld L, Ramalhinho AC. Cell-Free DNA as a New Biomarker of IVF Success, Independent of Any Infertility Factor, Including Endometriosis. Diagnostics. 2023; 13(2):208. https://doi.org/10.3390/diagnostics13020208
Chicago/Turabian StyleCasteleiro Alves, Maria Manuel, Luísa Oliani, Micaela Almeida, Henrique José Cardoso, António Hélio Oliani, Luiza Breitenfeld, and Ana Cristina Ramalhinho. 2023. "Cell-Free DNA as a New Biomarker of IVF Success, Independent of Any Infertility Factor, Including Endometriosis" Diagnostics 13, no. 2: 208. https://doi.org/10.3390/diagnostics13020208
APA StyleCasteleiro Alves, M. M., Oliani, L., Almeida, M., Cardoso, H. J., Oliani, A. H., Breitenfeld, L., & Ramalhinho, A. C. (2023). Cell-Free DNA as a New Biomarker of IVF Success, Independent of Any Infertility Factor, Including Endometriosis. Diagnostics, 13(2), 208. https://doi.org/10.3390/diagnostics13020208







