Cytomorphometric Analysis of Oral Buccal Mucosa of Dental Colleges’ Students in Sulaimani City
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample of the Study
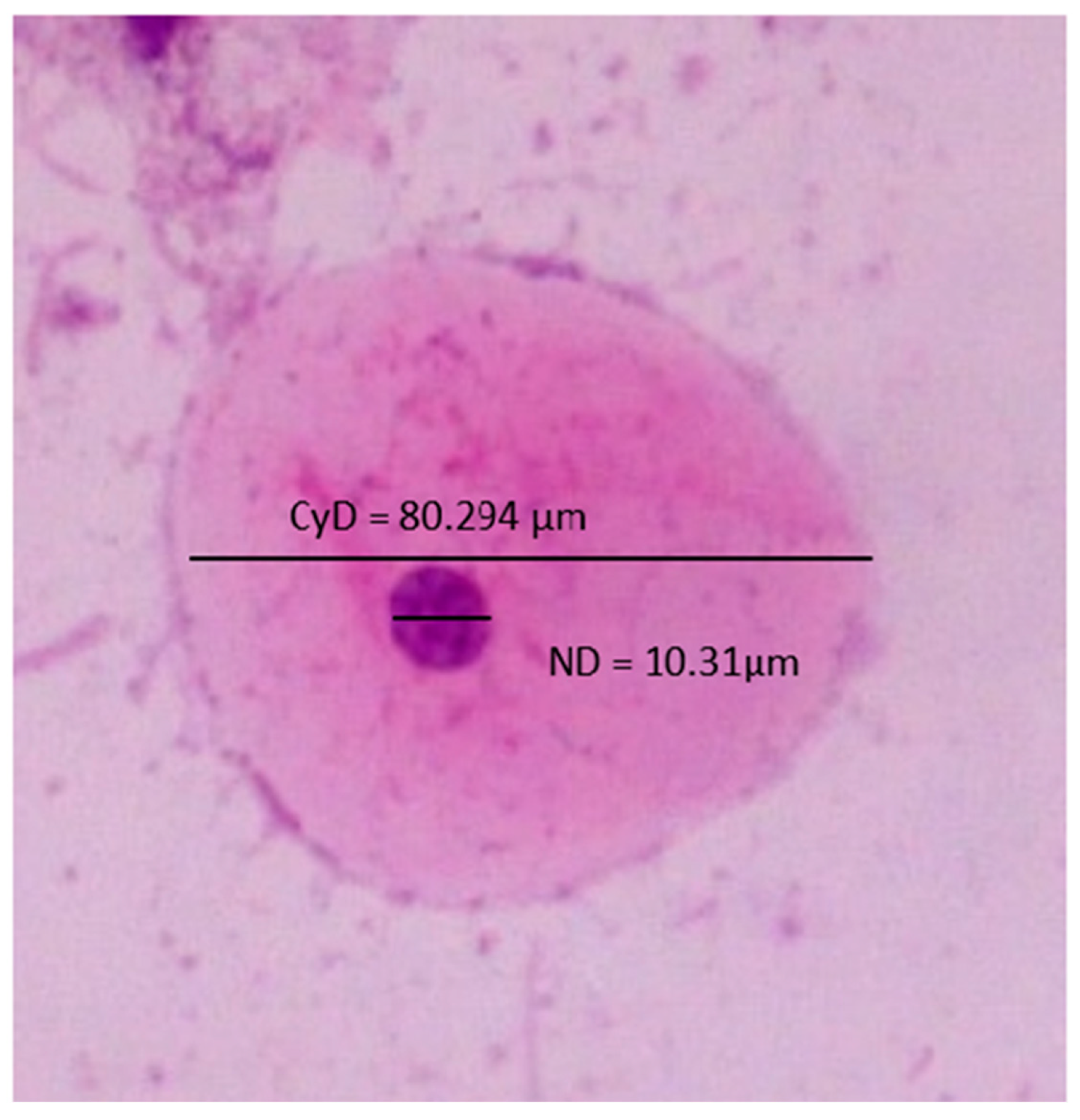
2.2. Quantitative Cytomorphometric Evaluation
2.3. Statistical Analysis
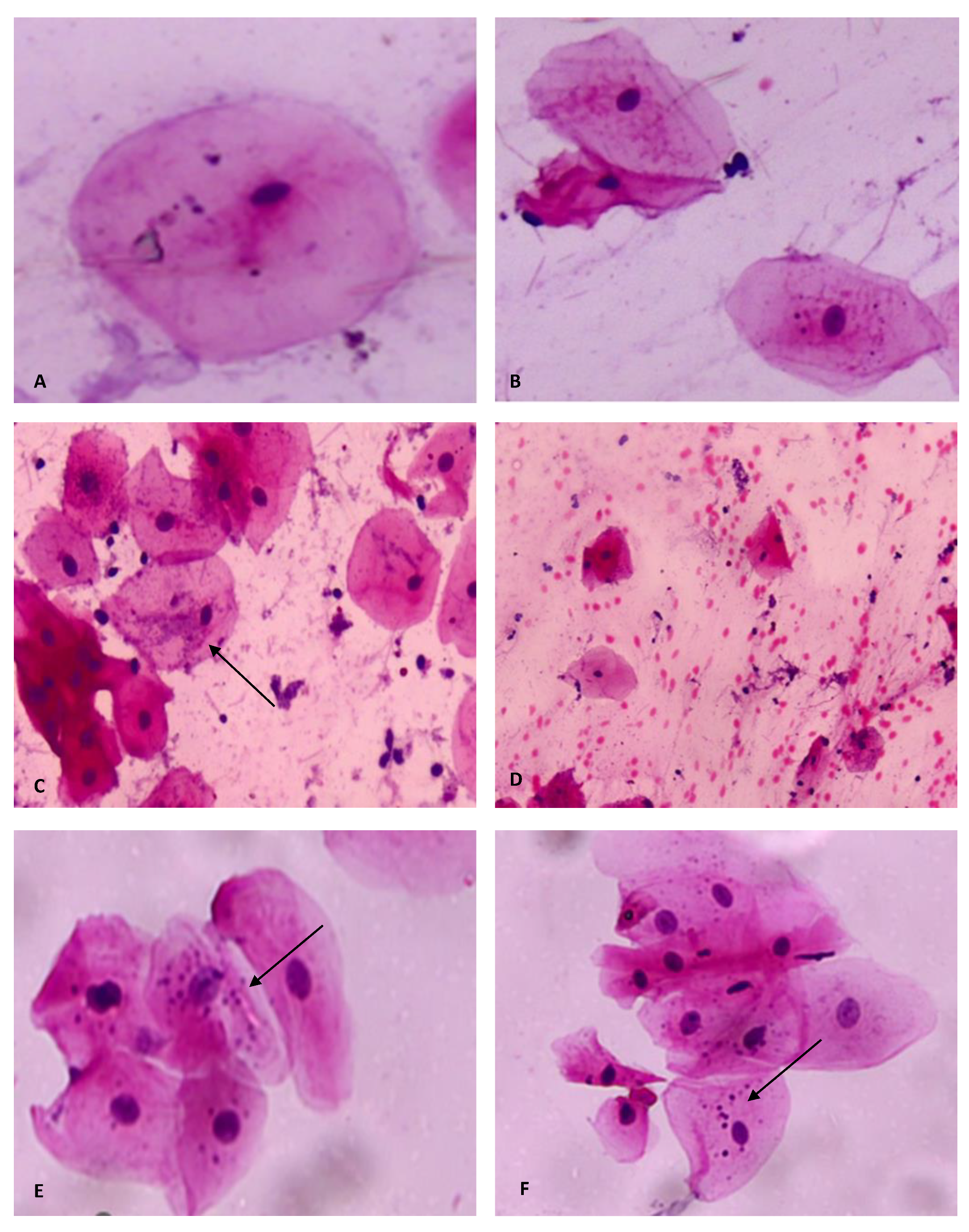
3. Results
4. Discussion
5. Limitations and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Babshet, M.; Nandimath, K.; Pervatikar, S.K.; Naikmasur, V. Efficacy of Oral Brush Cytology in the Evaluation of the Oral Premalignant and Malignant Lesions. J. Cytol. 2011, 28, 165. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, R.; Gupta, A.; Singh, M.; Ibrahim, R. Retraction: Application of Cytology and Molecular Biology in Diagnosing Premalignant or Malignant Oral Lesions. Mol. Cancer 2012, 11, 57. [Google Scholar] [CrossRef] [PubMed]
- Göregen, M.; Murat Akgül, H.; Gündoğdu, C. Th e Cytomorphological Analysis of Buccal Mucosa Cells in Smokers. Turk. J. Med. Sci. 2011, 41, 205–210. [Google Scholar] [CrossRef]
- Nivia, M.; Sunil, S.N.; Rathy, R.; Anilkumar, T.V. Comparative Cytomorphometric Analysis of Oral Mucosal Cells in Normal, Tobacco Users, Oral Leukoplakia and Oral Squamous Cell Carcinoma. J. Cytol. 2015, 32, 253. [Google Scholar] [CrossRef]
- Divani, S.; Exarhou, M.; Theodorou, L.N.; Georgantzis, D.; Skoulakis, H. Advantages and Difficulties of Brush Cytology in the Identification of Early Oral Cancer. Arch. Oncol. 2009, 17, 11–12. [Google Scholar] [CrossRef]
- Da Silva, A.D.; Lima, C.F.; Maraschin, B.J.; Laureano, N.K.; Daroit, N.B.; Brochier, F.; Sant’Ana Filho, M.; Visioli, F.; Rados, P.V. Immunocytochemistry Associated with Oral Exfoliative Cytology: Methodological Analysis. Anal. Quant. Cytol. Histol. 2015, 37, 134–138. [Google Scholar]
- Kazanowska, K.; Hałón, A.; Radwan-Oczko, M. The Role and Application of Exfoliative Cytology in the Diagnosis of Oral Mucosa Pathology—Contemporary Knowledge with Review of the Literature. Adv. Clin. Exp. Med. 2014, 23, 299–305. [Google Scholar] [CrossRef]
- Osorio-Osorno, Y.A.; Arboleda Toro, D.; Arango, J.C.; Parada-Sanchez, M.T. Optimized Liquid-Based Cytology for the Cellular and Molecular Analysis of Oral Keratinocytes: A Promising Diagnostic Tool. Diagn. Cytopathol. 2021, 49, 96–104. [Google Scholar] [CrossRef]
- Palakurthy, P.; Kulkarni, P.G.; Nandan, R.K.; Rao, T.M.; Reddy, D.S.P.; Muddana, K. Cytological Changes in Normal Oral Mucosa of Individuals with Tobacco Habits: A Cytomorphometric Study. J. Contemp. Dent. Pract. 2017, 18, 722–727. [Google Scholar] [CrossRef]
- Sumanthi, J.; Sridhar Reddy, G.; Anuradha, C.H.; Chandhra Sekhar, P.; Krishna Prasad, L.; Ramana Reddy, B.V. A Study on Cytomorphometric Analysis of Exfoliative Buccal Cells in Iron Deficiency Anemic Patients. Contemp. Clin. Dent. 2012, 3, S156. [Google Scholar] [CrossRef]
- Bds, M.T.A.; Garib, B.T. Cytological Features of Oral Cytobrush Smears in Type II Diabetes Mellitus Patients. Tikrit J. Dent. Sci. 2012, 1, 6–12. [Google Scholar]
- Donald, P.M.; George, R.; Sriram, G.; Kavitha, B.; Sivapathasundharam, B. Hormonal Changes in Exfoliated Normal Buccal Mucosal Cells. J. Cytol. 2013, 30, 252. [Google Scholar] [CrossRef]
- Ogden, G.R.; Cowpe, J.G.; Green, M.W. Effect of Radiotherapy on Oral Mucosa Assessed by Quantitative Exfoliative Cytology. J. Clin. Pathol. 1989, 42, 940. [Google Scholar] [CrossRef] [PubMed]
- Srinivasamurthy, B.C.; Balamurugesan, K.; Sathishkumar, N.; Prakash, M.; Bhat, R.V. Cytomorphometric Study of Changes in Buccal Mucosal Cells in Alcoholics. Adv. Biomed. Res. 2020, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Sukegawa, S.; Ono, S.; Nakano, K.; Takabatake, K.; Kawai, H.; Nagatsuka, H.; Furuki, Y. Clinical Study on Primary Screening of Oral Cancer and Precancerous Lesions by Oral Cytology. Diagn. Pathol. 2020, 15, 170. [Google Scholar] [CrossRef] [PubMed]
- Nayar, A.K.; Sundharam, B.S. Cytomorphometric Analysis of Exfoliated Normal Buccal Mucosa Cells. Indian J. Dent. Res. 2003, 14, 87–93. [Google Scholar] [PubMed]
- Queiroz, J.B.; Lima, C.; Burim, R.A.; Brandão, A.A.; Cabral, L.A.; Almeida, J. Exfoliative Cytology of the Oral Mucosa: Comparison of Two Collection Methods. Gen. Dent. 2010, 58, e196–e199. [Google Scholar] [PubMed]
- Khot, K.; Deshmane, S.; Bagri-Manjarekar, K.; Warke, D.; Kotak, K. A Cytomorphometric Analysis of Oral Mucosal Changes in Tobacco Users. J. Nat. Sci. 2015, 6, S22–S24. [Google Scholar] [CrossRef]
- Suresh, T.; Bastian, T.; Ahmed Mujib, B. Cytomorphometric Analysis of Squames Obtained from Normal Mucosa, Leukoplakia and Oral Squamous Cell Carcinoma. J. Oral Maxillofac. Pathol. 2021, 25, 202. [Google Scholar] [CrossRef]
- De Grado, G.F.; Ehlinger, V.; Godeau, E.; Arnaud, C.; Nabet, C.; Benkirane-Jessel, N.; Musset, A.M.; Offner, D. Changes in Tooth Brushing Frequency and Its Associated Factors from 2006 to 2014 among French Adolescents: Results from Three Repeated Cross Sectional HBSC Studies. PLoS ONE 2021, 16, e0249129. [Google Scholar] [CrossRef]
- Sakalauskiene, Ž.; Vehkalahti, M.M.; Murtomaa, H.; Mačiulskiene, V. Factors Related to Gender Differences in Toothbrushin g Among Lithuanian Middle-Aged University Employees. Medicina 2011, 47, 25. [Google Scholar] [CrossRef]
- Azodo, C.C.; Unamatokpa, B. Gender Difference in Oral Health Perception and Practices among Medical House Officers. Russ. Open Med. J. 2012, 1, 0208. [Google Scholar] [CrossRef]
- Mamai-Homata, E.; Koletsi-Kounari, H.; Margaritis, V. Gender Differences in Oral Health Status and Behavior of Greek Dental Students: A Meta-Analysis of 1981, 2000, and 2010 Data. J. Int. Soc. Prev. Community Dent. 2016, 6, 60. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, T.V.; Kawecki, M.M.; Cunningham, C.; Bovaird, I.; Morgan, R.; Rhodes, K.; Watkins, R. Mouthwash Use in General Population: Results from Adult Dental Health Survey in Grampian, Scotland. J. Oral Maxillofac. Res. 2010, 1, 2. [Google Scholar] [CrossRef]
- Lachenmeier, D.W. Mouthwash Controversies. Br. Dent. J. 2010, 208, 95–96. [Google Scholar] [CrossRef] [PubMed]
- Hussain, H.Y.; Abdul Satar, B.A. Prevalence and Determinants of Tobacco Use among Iraqi Adolescents: Iraq GYTS 2012. Tob. Induc. Dis. 2013, 11, 14. [Google Scholar] [CrossRef]
- Al Ansari, M.; Dawson, A.; Conigrave, K. Alcohol among Young People in Iraq: A Systematic Scoping Review. Discov. Psychol. 2022, 2, 16. [Google Scholar] [CrossRef]
- Halimi1, L.; Haghdoost2, A.A.; Alizadeh3, S.M. Prevalence of Cigarette Smoking among Iranian Women: A Systematic Review and Meta-Analysis. Med. J. Islam. Repub. Iran 2013, 27, 132. [Google Scholar]
- Sweis, N.J. Smoking Behavior among Jordanians: Physical, Psychological, Social, and Economic Reasons. Value Heal. Reg. Issues 2018, 16, 5–8. [Google Scholar] [CrossRef]
- Seifi, S.; Feizi, F.; Mehdizadeh, M.; Khafri, S.; Ahmadi, B. Evaluation of Cytological Alterations of Oral Mucosa in Smokers and Waterpipe Users. Cell J. 2014, 15, 302. [Google Scholar]
- Gadelkarim Ahmed, H.; Ali Alqufeye, M.; Ali Alsulaiman, G.; Lafy Alenezi, L.; Khaled Almuslumani, R.; Mohammed Alhussain, G.; Khaled Alenezi, A.; Fahad Algharbi, A.; Eid Alanazi, A.; Khleef Alshammari, N.; et al. Patterns of Oral Inflammatory Cells Infiltrate Associated with Cigarette Smoking Medical Science. Med. Sci. 2020, 24, 2817–2825. [Google Scholar]
- Proia, N.K.; Paszkiewicz, G.M.; Nasca, M.A.S.; Franke, G.E.; Pauly, J.L. Smoking and Smokeless Tobacco-Associated Human Buccal Cell Mutations and Their Association with Oral Cancer—A Review. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1061–1077. [Google Scholar] [CrossRef] [PubMed]
- Abdelaziz, M.S.; Osman, T.E. Detection of Cytomorphological Changes in Oral Mucosa among Alcoholics and Cigarette Smokers. Oman Med. J. 2011, 26, 349. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.G.E.; Omer, A.S.A.; Abd Algaffar, S.A. Cytological Study of Exfoliative Buccal Mucosal Cells of Qat Chewers in Yemen. Diagn. Cytopathol. 2011, 39, 796–800. [Google Scholar] [CrossRef]
- Ojeda, J.E.Q.; Aguilar-Medina, M.; Olimón-Andalón, V.; Jau, R.A.G.; Ham, A.A.; Quintana, J.G.R.; De Lourdes Silva-Benítez, E.; Sanchez-Schmitz, G.; Ramos-Payán, R. Increased Micronuclei Frequency in Oral and Lingual Epithelium of Treated Diabetes Mellitus Patients. Biomed Res. Int. 2018, 2018, 4898153. [Google Scholar] [CrossRef]
- Jadhav, K.; Gupta, N.; Ahmed Mujib, B.R. Micronuclei: An Essential Biomarker in Oral Exfoliated Cells for Grading of Oral Squamous Cell Carcinoma. J. Cytol. 2011, 28, 7. [Google Scholar] [CrossRef]
- Casartelli, G.; Bonatti, S.; De Ferrari, M.; Scala, M.; Mereu, P.; Margarino, G.; Abbondandolo, A. Micronucleus Frequencies in Exfoliated Buccal Cells in Normal Mucosa, Precancerous Lesions and Squamous Cell Carcinoma. Anal. Quant. Cytol. Histol. 2000, 22, 486–492. [Google Scholar]
- Kharisma, Y.; Damayanti, M.M.; Yulianto, F.A.; Rahimah, S.B.; Maharani, W.; Rachmawati, M.; Sastramihardja, H.S.; Abdul’ Aziiz, M.A.; Halim, M.I. Folic Acid Usual Doses Decrease the Buccal Micronucleus Frequency on Smokers. Glob. Med. Health Commun. 2019, 7, 131–135. [Google Scholar] [CrossRef]
- Dehghannezhad, M.; Naderi, N.J.; Semyari, H. Micronucleus Assay of Buccal Mucosa Cells in Waterpipe (Hookah) Smokers: A Cytologic Study. Iran. J. Pathol. 2020, 15, 75. [Google Scholar] [CrossRef]
- Shashikala, R.; Indira, A.P.; Manjunath, G.; Rao, K.; Akshatha, B. Role of Micronucleus in Oral Exfoliative Cytology. J. Pharm. Bioallied Sci. 2015, 7, S409. [Google Scholar] [CrossRef]
- Al-Kasser, M.K. Air Pollution in Iraq Sources and Effects. In Proceedings of the IOP Conference Series: Earth and Environmental Science, First International Virtual Conference on Environment & Natural Resources, Al Diwaniyah, Iraq, 24–25 March 2021; Volume 790. [Google Scholar] [CrossRef]
- Srilatha, T.; Manthapuri, S.; Shylaja, S.; Ramanand, O.V.; Reddy, E.S.; Vamshi, V.R. Cytomorphometric Analysis of Exfoliated Buccal Mucosal Cells in Smokers and Patients with Hypertension: A Quantitative Analysis. J. Int. Oral Health 2021, 13, 53. [Google Scholar] [CrossRef]
- Balan, U.; Gonsalves, N.; Jose, M.; Girish, K.L. Symptomatic Changes of Oral Mucosa during Normal Hormonal Turnover in Healthy Young Menstruating Women. J. Contemp. Dent. Pract. 2012, 13, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Balan, U.; Luqman, M.; Soliman, A.M.M.; Almubarak, H. Cytomorphometric Changes of Oral Mucosa during Normal Hormonal Turnovers in Healthy Young Menstruating Women. King Khalid Univ. J. Health Sci. 2018, 3, 8. [Google Scholar] [CrossRef]
- Meenakshi, S.; Jagannathan, N.; Swarnameenakshi, S. Cytomorphometric Analysis of Oral Epithelial Cells in Menstrual Cycle. Int. J. Pharma. Res. Health Sci. 2017, 5, 1695–1697. [Google Scholar] [CrossRef]
- Gowhar, O. Comparison of Exfoliative Cytology of Tongue and Buccal Mucosa among Smokers and Non-Smokers Using PAP Stain and AgNOR Counts. Int. J. Contemp. Med. Res. 2015, 4, 2393–2915. [Google Scholar]
- Deswal, R.; Narwal, V.; Dang, A.; Pundir, C.S. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261. [Google Scholar] [CrossRef]
- Wendland, N.; Opydo-Szymaczek, J.; Mizgier, M.; Jarząbek-Bielecka, G. Subgingival Microflora in Adolescent Females with Polycystic Ovary Syndrome and Its Association with Oral Hygiene, Gingivitis, and Selected Metabolic and Hormonal Parameters. Clin. Oral Investig. 2021, 25, 1485–1496. [Google Scholar] [CrossRef]
- Tanguturi, S.C.; Nagarakanti, S. Polycystic Ovary Syndrome and Periodontal Disease: Underlying Links—A Review. Indian J. Endocrinol. Metab. 2018, 22, 267. [Google Scholar] [CrossRef]
- Farook, F.F.; Ng, K.T.; MNM, N.; Koh, W.J.; Teoh, W.Y. Association of Periodontal Disease and Polycystic Ovarian Syndrome: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. Open Dent. J. 2020, 13, 478–487. [Google Scholar] [CrossRef]
| Variables | Male 39 | Female 54 | Total % 93 | Pearson Chi-Square Test | |
|---|---|---|---|---|---|
| Tooth brushing | Once | 18 (19.4%) | 8 (8.6%) | 26 (28%) | p = 0.001 |
| Twice | 21 (22.6%) | 46 (49.4%) | 67 (72%) | ||
| Mouthwash | Yes | 4 (4.3%) | 8 (8.6%) | 12 (12.9%) | p = 0.518 |
| No | 35 (37.6%) | 46 (49.5%) | 81 (87.1%) | ||
| Smoking | Yes | 12 (12.9%) | 0 (0%) | 12 (12.9%) | p = 0.000 |
| No | 27 (29%) | 54 (58%) | 81 (87%) | ||
| Drinking alcohol | Yes | 5 (5.4%) | 0 (0%) | 5 (5.4%) | p = 0.007 |
| No | 34 (36.6%) | 54 (58%) | 88 (94.6%) | ||
| Physical hormonal changes | Yes | 0 (0%) | 8 (14.8%) | 8 (14.8%) | p = 0.000 |
| No | 0 (0%) | 46 (85.2%) | 46 (85.2%) | ||
| Polycystic ovary syndrome | Yes | 0 (0%) | 4 (7.4%) | 4 (7.4%) | p = 0.000 |
| No | 0 (0%) | 50 (92.6%) | 50 (92.6%) |
| Variables | Male | Female | Total % | Pearson Chi-Square Test | |
|---|---|---|---|---|---|
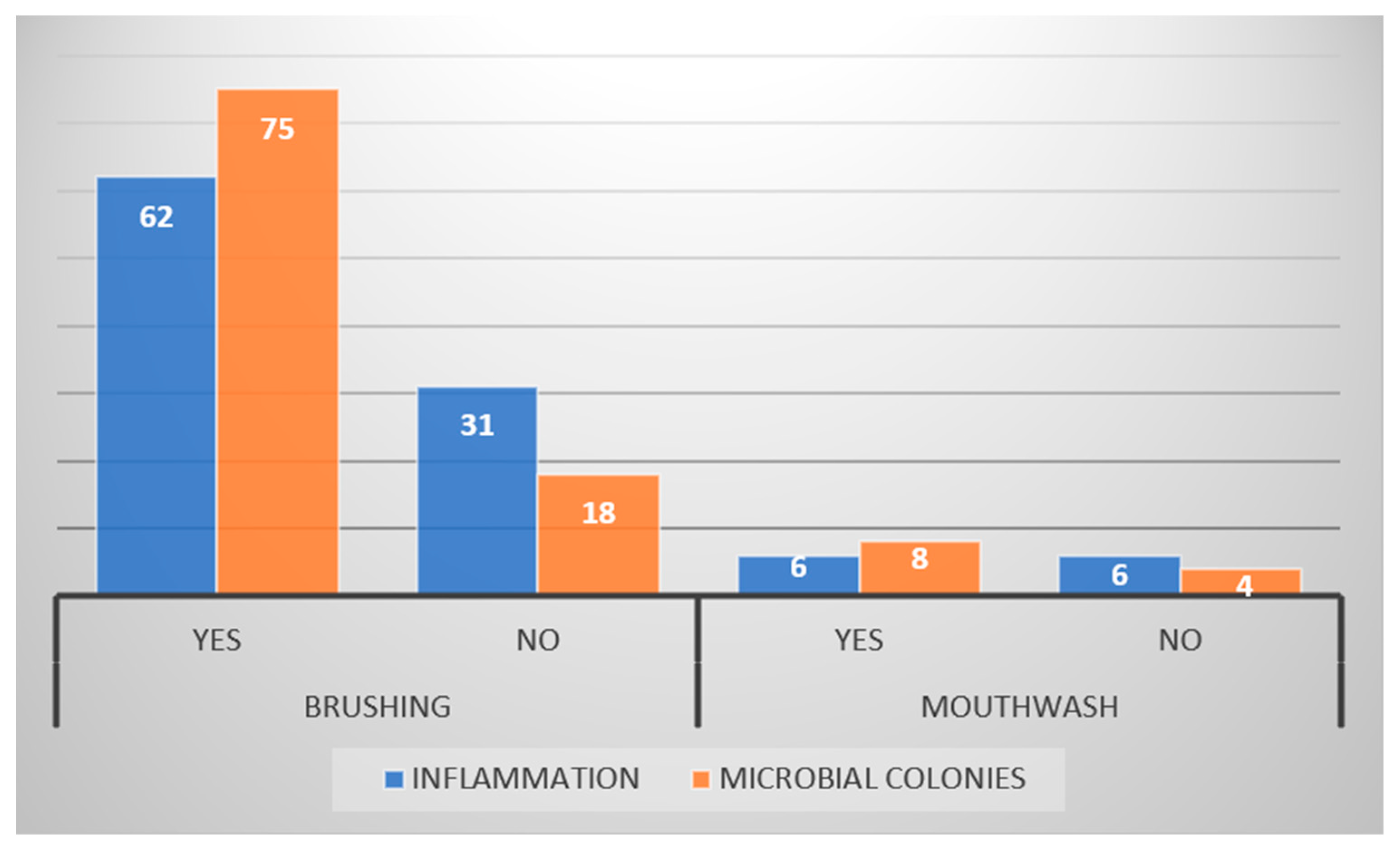
| Inflammation | Yes | 25 (26.9%) | 37 (39.8%) | 62 (66.7%) | p = 0.500 |
| No | 14 (15%) | 17 (18.3%) | 31 (33.3%) | ||
| Microbial colonies | Yes | 34 (36.6%) | 41 (44%) | 75 (80.6%) | p = 0.111 |
| No | 5 (5.4%) | 13 (14%) | 18 (19.4%) | ||
| Keratinization | Yes | 27 (29%) | 31 (33.4%) | 58 (62.4%) | p = 0.245 |
| No | 12 (12.9%) | 23 (24.7%) | 35 (37.6%) | ||
| Micronuclei | Yes | 37 (39.8%) | 47 (50.5%) | 84 (90.3%) | p = 0.207 |
| No | 2 (2.2%) | 7 (7.5%) | 9 (9.7%) | ||
| Overlapping | Yes | 39 (41.9%) | 54 (58.1%) | 93 (100%) | |
| Hemorrhage | Yes | 4 (4.3%) | 6 (6.4%) | 10 (10.7%) | p = 0.896 |
| No | 35 (37.6%) | 48 (51.7%) | 83 (89.3%) |
| Variables | Inflammation | Microbial Colonies | Micronuclei | Keratinization | Pearson Chi-Square Test | |
|---|---|---|---|---|---|---|
| Smoking (12, 12.9%) | Yes | 6 (50%) | 10 (83.3%) | 11 (91.7%) | 9 (75%) | p = 0.260 p = 0.951 p = 0.866 p = 0.333 |
| No | 6 (50%) | 2 (16.7%) | 1 (8.3%) | 3 (25%) | ||
| Alcohol drinking (5, 5.4%) | Yes | 2 (40%) | 5 (100%) | 5 (100%) | 4 (80%) | p = 0.430 p = 0.258 p = 0.452 p = 0.403 |
| No | 3 (60%) | 0 | 0 | 1 (20%) | ||
| Physical hormonal changes (8, 14.8%) | Yes | 4 (50%) | 6 (75%) | 7 (87.5%) | 5 (62.5%) | p = 0.644 p = 0.304 p = 0.451 p = 0.484 |
| No | 4 (50%) | 2 (25%) | 1 (12.5%) | 3 (37.5%) | ||
| Polycystic ovary syndrome (4, 7.4%) | Yes | 2 (50%) | 3 (75%) | 4 (100%) | 2 (50%) | p = 0.452 p = 0.240 p = 0.298 p = 0.484 |
| No | 2 (50%) | 1 (25%) | 0 | 2 (50%) |
| Variables | Gender | Smoking | Drinking Alcohol | Physical Hormonal Changes | Polycystic Ovary Syndrome | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Yes | No | Yes | No | Yes | No | Yes | No | |
| CYD | 66.341 | 65.971 | 66.399 | 66.086 | 64.223 | 66.234 | 65.441 | 66.064 | 66.326 | 65.943 |
| MIN/MAX | 56.20/77.75 | 48.20/81.26 | 58.02/76.29 | 48.20/81.26 | 58.02/76.23 | 48.20/81.26 | 53.27/78.56 | 48.20/81.26 | 56.65/78.01 | 48.20/81.26 |
| ND | 8.149 | 8.201 | 8.013 | 8.203 | 8.178 | 8.179 | 8.366 | 8.172 | 9.461 | 8.099 |
| MIN/MAX | 6.80/9.89 | 6.22/10.24 | 7.53/8.87 | 6.22/10.24 | 7.53/8.87 | 6.22/10.24 | 7.16/10.24 | 6.22/10.04 | 8.70/10.24 | 6.22/9.93 |
| NCR | 12.351 | 12.543 | 12.179 | 12.506 | 12.828 | 12.443 | 12.875 | 12.485 | 14.468 | 12.389 |
| ANOVA | ND p = 0.755 CyD p = 0.806 N: C p = 0.549 | ND p = 0.435 CyD p = 0.887 N: C p = 0.486 | ND p = 0.998 CyD p = 0.540 N: C p = 0.580 | ND p = 0.775 CyD p = 0.946 N: C p = 0.668 | ND p = 0.003 CyD p = 0.965 N: C p = 0.023 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, D.K.; Ibraheem, B.F.; Mohammad, D.N.; Garib, B.T.; Hamied, M.A.-S. Cytomorphometric Analysis of Oral Buccal Mucosa of Dental Colleges’ Students in Sulaimani City. Diagnostics 2023, 13, 234. https://doi.org/10.3390/diagnostics13020234
Mahmood DK, Ibraheem BF, Mohammad DN, Garib BT, Hamied MA-S. Cytomorphometric Analysis of Oral Buccal Mucosa of Dental Colleges’ Students in Sulaimani City. Diagnostics. 2023; 13(2):234. https://doi.org/10.3390/diagnostics13020234
Chicago/Turabian StyleMahmood, Darya Khalid, Ban Falih Ibraheem, Dena Nadhim Mohammad, Balkees Taha Garib, and Marwa Abdul-Salam Hamied. 2023. "Cytomorphometric Analysis of Oral Buccal Mucosa of Dental Colleges’ Students in Sulaimani City" Diagnostics 13, no. 2: 234. https://doi.org/10.3390/diagnostics13020234
APA StyleMahmood, D. K., Ibraheem, B. F., Mohammad, D. N., Garib, B. T., & Hamied, M. A.-S. (2023). Cytomorphometric Analysis of Oral Buccal Mucosa of Dental Colleges’ Students in Sulaimani City. Diagnostics, 13(2), 234. https://doi.org/10.3390/diagnostics13020234






