Use of Urine N-Terminal Prohormone of Brain-Natriuretic Peptide (NT-proBNP) as a Non-Invasive Indicator for Renal Function Recovery after Surgical Relief of Hydronephrosis
Abstract
1. Introduction
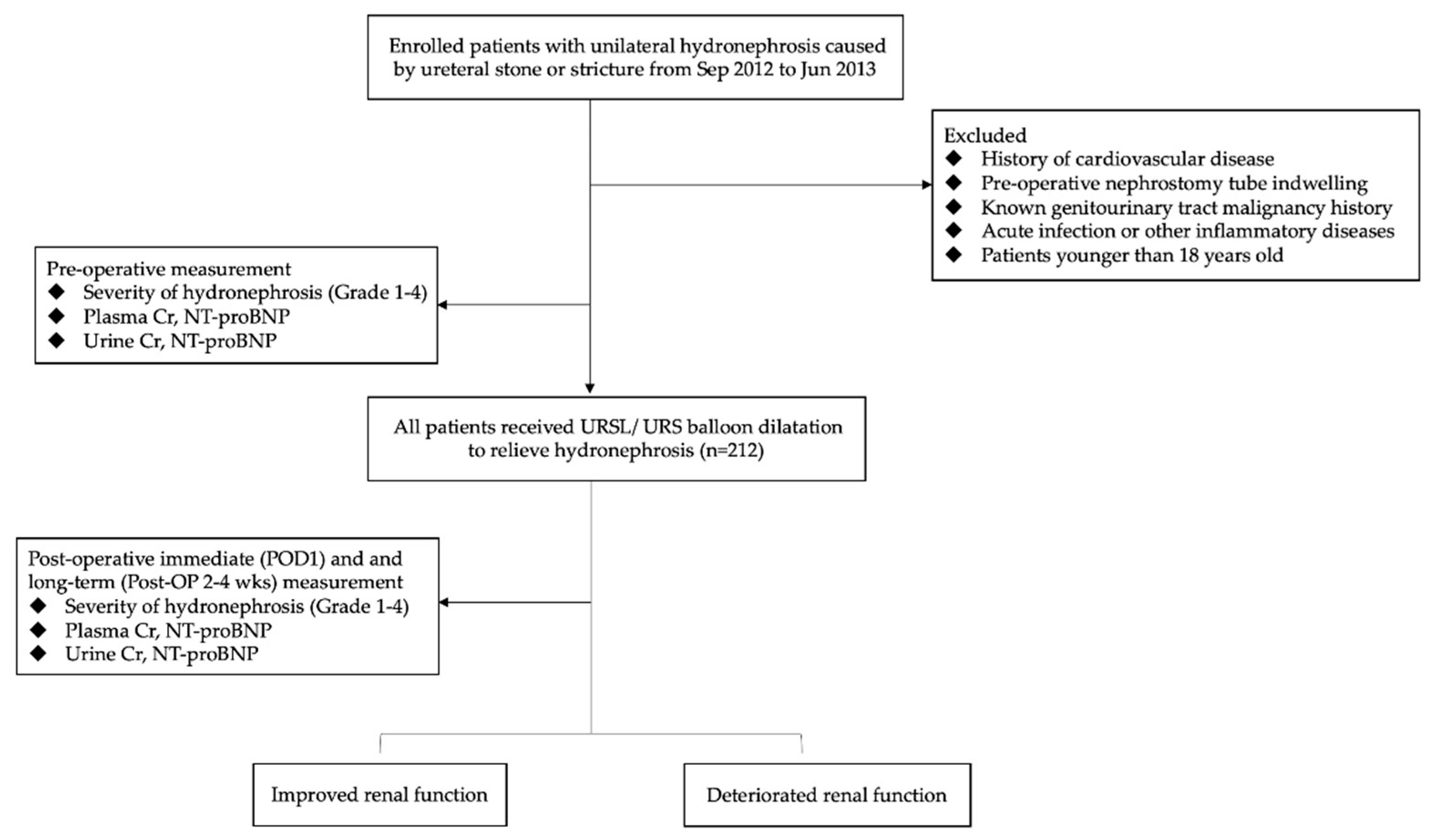
2. Materials and Methods
2.1. Study Participants and Data Collection
2.2. Measurement of Biomedical Variables
2.3. The Procedure of the URS
2.4. Statistical Analysis
3. Results
4. Discussion
4.1. Plasma NT-proBNP, Urine NT-proBNP, and Hydronephrosis
4.2. Cardio-Renal Syndrome and Obstructive Uropathy
4.3. The Recovery of Renal Function after Relieving Hydronephrosis
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patel, K.; Batura, D. An overview of hydronephrosis in adults. Br. J. Hosp. Med. 2020, 81, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rangaswami, J.; Bhalla, V.; Blair, J.E.; Chang, T.I.; Costa, S.; Lentine, K.L.; Lerma, E.V.; Mezue, K.; Molitch, M.; Mullens, W. Cardiorenal syndrome: Classification, pathophysiology, diagnosis, and treatment strategies: A scientific statement from the American Heart Association. Circulation 2019, 139, e840–e878. [Google Scholar] [CrossRef]
- Yeh, H.-M.; Lin, T.-T.; Yeh, C.-F.; Huang, H.-S.; Chang, S.-N.; Lin, J.-W.; Tsai, C.-T.; Lai, L.-P.; Huang, Y.-Y.; Chu, C.-L. Biomarkers and echocardiography for evaluating the improvement of the ventricular diastolic function after surgical relief of hydronephrosis. PLoS ONE 2017, 12, e0188597. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.; Hamm, C. Role of B-type natriuretic peptide (BNP) and NT-proBNP in clinical routine. Heart 2006, 92, 843–849. [Google Scholar] [CrossRef] [PubMed]
- Govoni, A.F. Diagnostic Radiology. In A Textbook of Medical Imaging; Donald, G., Grainger, Allison, D., Adam, A., Adrian, Dixon, D., Livingstone, C., Eds.; Imprint of Harcourt Publishers: London, UK, 2001; Volume 1, p. 973, 1145 illustrations. [Google Scholar]
- Raymond, I.; Groenning, B.A.; Hildebrandt, P.R.; Nilsson, J.C.; Baumann, M.; Trawinski, J.; Pedersen, F. The influence of age, sex and other variables on the plasma level of N-terminal pro brain natriuretic peptide in a large sample of the general population. Heart 2003, 89, 745–751. [Google Scholar] [CrossRef]
- Chow, S.L.; Maisel, A.S.; Anand, I.; Bozkurt, B.; De Boer, R.A.; Felker, G.M.; Fonarow, G.C.; Greenberg, B.; Januzzi, J.L., Jr.; Kiernan, M.S. Role of biomarkers for the prevention, assessment, and management of heart failure: A scientific statement from the American Heart Association. Circulation 2017, 135, e1054–e1091. [Google Scholar] [CrossRef]
- Ng Leong, L.; Loke Ian, W.; Davies Joan, E.; Geeranavar, S.; Khunti, K.; Stone Margaret, A.; Chin Derek, T.; Squire Iain, B. Community screening for left ventricular systolic dysfunction using plasma and urinary natriuretic peptides. J. Am. Coll. Cardiol. 2005, 45, 1043–1050. [Google Scholar] [CrossRef]
- NG, L.L.; Geeranavar, S.; Jennings, S.C.; Loke, I.; O’Brien, R.J. Diagnosis of heart failure using urinary natriuretic peptides. Clin. Sci. 2004, 106, 129–133. [Google Scholar] [CrossRef]
- Cortés, R.; Portolés, M.; Salvador, A.; Bertomeu, V.; de Burgos, F.G.; Martínez-Dolz, L.; Lletí, E.R.; Climent, V.; Jordán, A.; Payá, R. Diagnostic and prognostic value of urine NT-proBNP levels in heart failure patients. Eur. J. Heart Fail. 2006, 8, 621–627. [Google Scholar] [CrossRef]
- Corteville, D.C.M.; Bibbins-Domingo, K.; Wu, A.H.B.; Ali, S.; Schiller, N.B.; Whooley, M.A. N-terminal pro-B-type natriuretic peptide as a diagnostic test for ventricular dysfunction in patients with coronary disease: Data from the heart and soul study. Arch. Intern. Med. 2007, 167, 483–489. [Google Scholar] [CrossRef]
- Manzano-Fernández, S.; Januzzi, J.L.; Boronat-García, M.; Pastor, P.; Albaladejo-Otón, M.D.; Garrido, I.P.; Bayes-Genis, A.; Valdés, M.; Pascual-Figal, D.A. Impact of kidney dysfunction on plasma and urinary N-terminal pro-B-type natriuretic peptide in patients with acute heart failure. Congest. Heart Fail. 2010, 16, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Tsutamoto, T.; Sakai, H.; Yamamoto, T.; Nakagawa, Y. Renal Clearance of N-Terminal pro-Brain Natriuretic Peptide Is Markedly Decreased in Chronic Kidney Disease. Circ. Rep. 2019, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Mearns, B.M. NT-proBNP levels: Renal involvement? Nat. Rev. Cardiol. 2009, 6, 613. [Google Scholar] [CrossRef]
- Cortés, R.; Portolés, M.; Roselló-Lletí, E.; Martínez-Dolz, L.; Almenar, L.; Grigorian, L.; Bertomeu, V.; Rivera, M. Impact of glomerular filtration rate on urinary BNP and NT-proBNP levels in heart failure. Peptides 2012, 33, 354–358. [Google Scholar] [CrossRef]
- Linssen, G.C.; Damman, K.; Hillege, H.L.; Navis, G.; van Veldhuisen, D.J.; Voors, A.A. Urinary N-terminal prohormone brain natriuretic peptide excretion in patients with chronic heart failure. Circulation 2009, 120, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Chuasuwan, A.; Kellum, J.A. Cardio-renal syndrome type 3: Epidemiology, pathophysiology, and treatment. Semin. Nephrol. 2012, 32, 31–39. [Google Scholar] [CrossRef]
- Andaloro, V.A., Jr. Mechanism of hypertension produced by ureteral obstruction. Urology 1975, 5, 367–371. [Google Scholar] [CrossRef]
- Wise, H.M., Jr. Hypertension Resulting From Hydronephrosis. JAMA 1975, 231, 491–492. [Google Scholar] [CrossRef]
- Oparil, S. Hypertension in a 74-year-old man with hydronephrosis and coronary disease. Hypertension 1985, 7, 824–833. [Google Scholar] [CrossRef]
- Abramson, M.; Jackson, B. Hypertension and unilateral hydronephrosis. J. Urol. 1984, 132, 746–748. [Google Scholar] [CrossRef]
- Selmi, V.; Sarı, S.; Caniklioğlu, M.; Öztekin, Ü.; Taspinar, M.S.; Işıkay, L. Effect of Endoscopic Ureteral Stone Treatment on Kidney Function. Cureus 2021, 13. [Google Scholar] [CrossRef]
- Shokeir, A.A.; Shoma, A.M.; Abubieh, E.A.; Nasser, M.A.; Eassa, W.; El-Asmy, A. Recoverability of renal function after relief of acute complete ureteral obstruction: Clinical prospective study of the role of renal resistive index. Urology 2002, 59, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Khalaf, I.M.; Shokeir, A.A.; El-Gyoushi, F.I.; Amr, H.S.; Amin, M.M. Recoverability of renal function after treatment of adult patients with unilateral obstructive uropathy and normal contralateral kidney: A prospective study. Urology 2004, 64, 664–668. [Google Scholar] [CrossRef] [PubMed]
- Klahr, S.; Harris, K.; Purkerson, M.L. Effects of obstruction on renal functions. Pediatric Nephrol. 1988, 2, 34–42. [Google Scholar] [CrossRef] [PubMed]



| Grade of Hydronephrosis | |||
|---|---|---|---|
| Low Grade | High Grade | p-Value | |
| No. pts | 108 | 103 | |
| Age (yrs) | 52.8 (12.8) | 56.2 (13.2) | 0.06 |
| Male sex (%) | 60.2% | 67.0% | |
| BMI (kg/m2) | 25.9 (4.2) | 28.2 (28.7) | 0.41 |
| Overweight (%) | 56.5% | 56.3% | |
| MAP (mmHg) | 103.1 (14.1) | 98.8 (14.5) | 0.03 |
| Hypertension (%) | 60.2% | 44.7% | |
| eGFR (mL/min/1.73 m2) | 90.7 (23.3) | 74.1 (28.7) | <0.001 |
| CKD (%) | 11.1% | 33.0% | |
| Urine output (L/day) | 1.2 (0.6) | 1.3 (0.6) | 0.46 |
| Urine creatinine (mg/mL) | 33.2 (31.9) | 45.5 (26.8) | 0.09 |
| Stone size (cm) | 1.1 (1.8) | 1.0 (1.2) | 0.46 |
| Plasma NT-proBNP (ng/mL) | 2.9 (1.8) | 2.8 (2.8) | 0.65 |
| Urine NT-proBNP (pg/mg Cr) | 11.1 (7.1) | 6.7 (5.7) | 0.006 |
| Variables | Baseline | Immediate | p-Value | Long-Term | p-Value |
|---|---|---|---|---|---|
| eGFR(mL/min/1.73 m2) | 82.3 (27.1) | 82.2 (26.7) | 0.89 | 84.87 (27.79) | 0.004 |
| P NT-proBNP (ng/mL) | 2.9 (2.3) | 3.0 (2.3) | 0.16 | 2.2 (3.1) | 0.93 |
| U NT-proBNP (pg/mL Cr) | 9.4 (6.9) | 15.0 (13.1) | <0.001 | N/A | |
| Urine output (mL/day) | 1244.8 (592.2) | 1694.6 (771.7) | <0.001 | 1398.2 (338.5) | 0.003 |
| Immediate | Long-Term | ||||||
|---|---|---|---|---|---|---|---|
| eGFR ↓/ = (n = 136) | eGFR ↑ (n = 74) | p-Value | eGFR ↓/ = (n = 120) | eGFR ↑ (n = 74) | p-Value | ||
| Baseline eGFR | 90.1 (24.9) | 69.0 (26.4) | <0.001 * | 89.4 (24.4) | 72.4 (26.6) | <0.001 * | |
| P NT-proBNP | Baseline | 2.7 (1.8) | 3.2 (3.2) | 0.35 | 2.7 (1.7) | 3.0 (3.2) | 0.54 |
| △ | 0.2 (0.9) | −0.1 (1.0) | 0.11 | 0.3 (0.9) | −0.03 (1.0) | 0.11 | |
| U NT-proBNP | Baseline | 10.3 (7.1) | 7.2 (6.0) | 0.08 | 11.1 (7.1) | 6.7 (5.7) | 0.006 * |
| △ | 4.7 (14.5) | 7.6 (15.5) | 0.44 | 5.2 (16.8) | 6.1 (11.0) | 0.82 | |
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p-Value | OR | 95% CI | p-Value | |
| Sex | ||||||
| Female Male | 1.0 (ref.) | |||||
| 1.4 | 0.65–2.13 | 0.30 | ||||
| BMI | ||||||
| Overweight Normal | 1.0 (ref.) | |||||
| 0.87 | 0.48–1.57 | 0.65 | ||||
| Baseline CKD | ||||||
| Yes No | 1.0 (ref.) | 1.0 (ref.) | ||||
| 0.22 | 0.11–0.4 | 0.001 | 0.63 | 0.21–1.92 | 0.417 | |
| Hydronephrosis | ||||||
| High Low | 1.0 (ref.) | |||||
| 0.71 | 0.40–1.26 | 0.242 | ||||
| UTI | ||||||
| Yes | 1.0 (ref.) | |||||
| No | 1.44 | 0.81–2.58 | 0.215 | |||
| Hypertension | ||||||
| Yes | 1.0 (ref.) | |||||
| No | 1.54 | 0.87–2.71 | 0.114 | |||
| U NT-proBNP | ||||||
| High | 1.0 (ref.) | |||||
| Low | 3.34 | 1.19–9.38 | 0.022 | 3.24 | 1.09–9.70 | 0.035 |
| P NT-proBNP | ||||||
| High | 1.0 (ref.) | |||||
| Low | 0.92 | 0.73–1.14 | 0.43 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.M.; Liu, C.J.; Lu, Z.H.; Huang, H.S. Use of Urine N-Terminal Prohormone of Brain-Natriuretic Peptide (NT-proBNP) as a Non-Invasive Indicator for Renal Function Recovery after Surgical Relief of Hydronephrosis. Diagnostics 2023, 13, 247. https://doi.org/10.3390/diagnostics13020247
Liu CM, Liu CJ, Lu ZH, Huang HS. Use of Urine N-Terminal Prohormone of Brain-Natriuretic Peptide (NT-proBNP) as a Non-Invasive Indicator for Renal Function Recovery after Surgical Relief of Hydronephrosis. Diagnostics. 2023; 13(2):247. https://doi.org/10.3390/diagnostics13020247
Chicago/Turabian StyleLiu, Chia Min, Chan Jung Liu, Ze Hong Lu, and Ho Shiang Huang. 2023. "Use of Urine N-Terminal Prohormone of Brain-Natriuretic Peptide (NT-proBNP) as a Non-Invasive Indicator for Renal Function Recovery after Surgical Relief of Hydronephrosis" Diagnostics 13, no. 2: 247. https://doi.org/10.3390/diagnostics13020247
APA StyleLiu, C. M., Liu, C. J., Lu, Z. H., & Huang, H. S. (2023). Use of Urine N-Terminal Prohormone of Brain-Natriuretic Peptide (NT-proBNP) as a Non-Invasive Indicator for Renal Function Recovery after Surgical Relief of Hydronephrosis. Diagnostics, 13(2), 247. https://doi.org/10.3390/diagnostics13020247






