Fast Track Diagnostic Tools for Clinical Management of Sepsis: Paradigm Shift from Conventional to Advanced Methods
Abstract
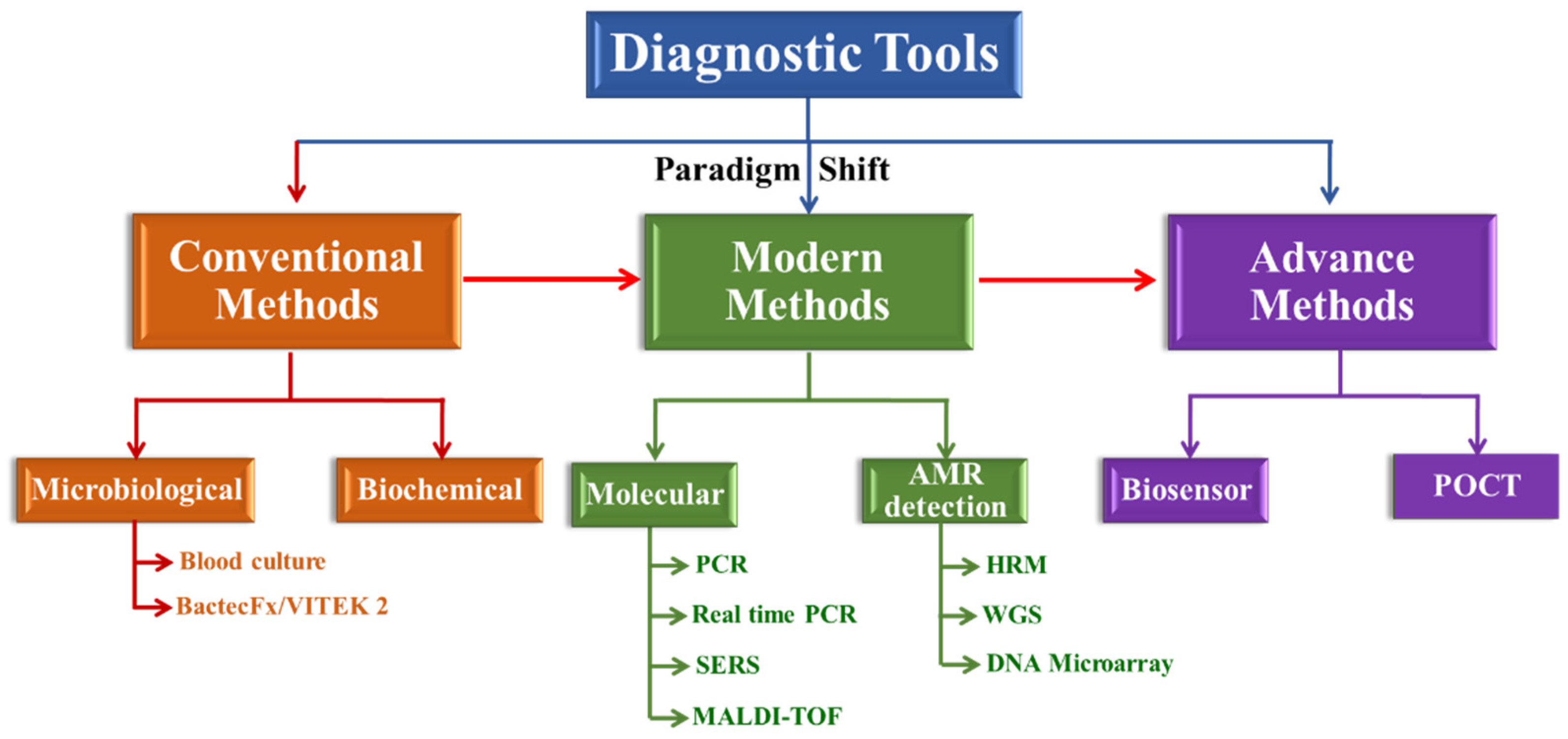
:1. Introduction
2. Conventional Methods for Management of Sepsis
2.1. Microbiological Methods
2.1.1. Identification through Blood Culture/Gram Staining
2.1.2. Identification through Bactec Fx/VITEK 2
2.2. Biochemical Test
3. Modern Methods for Management of Sepsis
3.1. Implementing Molecular Detection for the Identification of Pathogens
3.1.1. PCR
3.1.2. Real-Time PCR
3.1.3. Surface-Enhanced Raman Spectroscopy (SERS)
3.1.4. MALDI-TOF
3.2. Broad-Spectrum Genomic Detection of AMR
3.2.1. High Resolution Melting Analysis Technology
3.2.2. Sequencing
3.2.3. DNA Microarray
4. Advanced Methods for Management of Sepsis
4.1. Biosensors
4.2. Point of Care Test
4.3. CRISPR-Cas9
5. Challenges for a New Improved Method
6. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, S.; Payal, N.; Srivastava, V.K.; Kaushik, S.; Saxena, J.; Jyoti, A. Neutrophil extracellular traps and organ dysfunction in sepsis. Clin. Chim. Acta 2021, 523, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Guirgis, F.; Black, L.P.; DeVos, E.L. Updates and controversies in the early management of sepsis and septic shock. Emerg. Med. Pract. 2018, 20, 1–28. [Google Scholar]
- Kumar, S.; Gupta, E.; Srivastava, V.K.; Kaushik, S.; Saxena, J.; Goyal, L.K.; Mehta, S.; Jyoti, A. Nitrosative stress and cytokines are linked with the severity of sepsis and organ dysfunction. Br. J. Biomed. Sci. 2019, 76, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Gyawali, B.; Ramakrishna, K.; Dhamoon, A.S. Sepsis: The evolution in definition, pathophysiology, and management. SAGE Open Med. 2019, 7, 2050312119835043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudnov, V.A.; Kulabukhov, V.V. Sepsis-3: Updated main definitions, potential problems and next practical steps. Messenger Anesthesiol. Resusc. 2018, 13, 4–11. [Google Scholar] [CrossRef] [Green Version]
- Masoudifar, M.; Gouya, M.M.; Pezeshki, Z.; Eshrati, B.; Afhami, S.; Farzami, M.R.; Seifi, A. Health care-associated infections, including device-associated infections, and antimicrobial resistance in Iran: The national update for 2018. J. Prev. Med. Hyg. 2021, 62, E943. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann-Struzek, C.; Mellhammar, L.; Rose, N.; Cassini, A.; Rudd, K.E.; Schlattmann, P.; Allegranzi, B.; Reinhart, K. Incidence and mortality of hospital-and ICU-treated sepsis: Results from an updated and expanded systematic review and meta-analysis. Crit. Care Med. 2020, 46, 1552–1562. [Google Scholar] [CrossRef]
- Makic, M.B.F.; Bridges, E. CE: Managing sepsis and septic shock: Current guidelines and definitions. Am. J. Nurs. 2018, 118, 34–39. [Google Scholar] [CrossRef]
- Buchman, T.G.; Simpson, S.Q.; Sciarretta, K.L.; Finne, K.P.; Sowers, N.; Collier, M.; Chavan, S.; Oke, I.; Pennini, M.E.; Santhosh, A.; et al. Sepsis among medicare beneficiaries: 1. The burdens of sepsis, 2012–2018. Crit. Care Med. 2020, 48, 276. [Google Scholar] [CrossRef]
- Chávez-Vivas, M.; Cristo-Martínez, D.; Tascón, A.J. Epidemiological characteristics of patients diagnosed with sepsis and septic shock in a hospital in Cali, Colombia. Acta Med. Costarric. 2018, 60, 150–156. [Google Scholar]
- Fleischmann-Struzek, C.; Mikolajetz, A.; Schwarzkopf, D.; Cohen, J.; Hartog, C.S.; Pletz, M.; Gastmeier, P.; Reinhart, K. Challenges in assessing the burden of sepsis and understanding the inequalities of sepsis outcomes between National Health Systems: Secular trends in sepsis and infection incidence and mortality in Germany. Intensive Care Med. 2018, 44, 1826–1835. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Ogura, H.; Kushimoto, S.; Shiraishi, A.; Sugiyama, T.; Deshpande, G.A.; Uchida, M.; Nagata, I.; Saitoh, D.; Fujishima, S.; et al. Variations in infection sites and mortality rates among patients in intensive care units with severe sepsis and septic shock in Japan. J. Intensive Care 2019, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajdács, M. The concept of an ideal antibiotic: Implications for drug design. Molecules 2019, 24, 892. [Google Scholar] [CrossRef] [Green Version]
- van Belkum, A.; Burnham, C.A.D.; Rossen, J.W.; Mallard, F.; Rochas, O.; Dunne, W.M. Innovative and rapid antimicrobial susceptibility testing systems. Nat. Rev. Microbiol. 2020, 18, 299–311. [Google Scholar] [CrossRef]
- Kethireddy, S.; Bilgili, B.; Sees, A.; Kirchner, H.L.; Ofoma, U.R.; Light, R.B.; Mirzanejad, Y.; Maki, D.; Kumar, A.; Layon, A.J.; et al. Culture-negative septic shock compared with culture-positive septic shock: A retrospective cohort study. Crit. Care Med. 2018, 46, 506–512. [Google Scholar] [CrossRef]
- Falcone, M.; Bassetti, M.; Tiseo, G.; Giordano, C.; Nencini, E.; Russo, A.; Graziano, E.; Tagliaferri, E.; Leonildi, A.; Barnini, S.; et al. Time to appropriate antibiotic therapy is a predictor of outcome in patients with bloodstream infection caused by KPC-producing Klebsiella pneumoniae. Crit. Care 2020, 24, 24. [Google Scholar] [CrossRef] [Green Version]
- Nath, P.; Kabir, A.; Khoubafarin Doust, S.; Kreais, Z.J.; Ray, A. Detection of bacterial and viral pathogens using photonic point-of-care devices. Diagnostics 2020, 10, 841. [Google Scholar] [CrossRef]
- Choi, J.A.; Bae, S.M.; Kim, J.W.; Lee, K.J. Development of a Two Triplex Real-Time Polymerase Chain Reaction for Rapid Detection of Six Carbapenemase Genes in Enterobacteriaceae. Osong Public Health Res. Perspect. 2020, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Mejia-Chew, C.; O’Halloran, J.A.; Olsen, M.A.; Stwalley, D.; Kronen, R.; Lin, C.; Salazar, A.S.; Larson, L.; Hsueh, K.; Powderly, W.G.; et al. Effect of infectious disease consultation on mortality and treatment of patients with candida bloodstream infections: A retrospective, cohort study. Lancet Infect. Dis. 2019, 19, 1336–1344. [Google Scholar] [CrossRef]
- Edmiston, C.E.; Garcia, R.; Barnden, M.; DeBaun, B.; Johnson, H.B. Rapid diagnostics for bloodstream infections: A primer for infection preventionists. Am. J. Infect. Control 2018, 46, 1060–1068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, H.; Nakao, A.; Sato, K.; Otomo, Y.; Niijima, S.; Shimizu, T. Comparison of time to positivity of pediatric blood cultures obtained within the first year of life and in later years. J. Infect. Chemother. 2020, 26, 813–817. [Google Scholar] [CrossRef] [PubMed]
- Salinas, M.; López-Garrigós, M.; Flores, E.; Leiva-Salinas, C. Current Practice and Regional Variability in Recommendations for Patient Preparation for Laboratory Testing in Primary Care. Lab. Med. 2020, 51, e32–e37. [Google Scholar] [CrossRef] [PubMed]
- Zelellw, D.A.; Dessie, G.; Worku Mengesha, E.; Balew Shiferaw, M.; Mela Merhaba, M.; Emishaw, S. A Systemic Review and Meta-analysis of the Leading Pathogens Causing Neonatal Sepsis in Developing Countries. BioMed Res. Int. 2021, 2021, 6626983. [Google Scholar] [CrossRef] [PubMed]
- Özenci, V.; Strålin, K. Clinical implementation of molecular methods in detection of microorganisms from blood with a special focus on PCR electrospray ionization mass spectrometry. Expert Rev. Mol. Diagn. 2019, 19, 389–395. [Google Scholar] [CrossRef]
- Quirino, A.; Marascio, N.; Peronace, C.; Gallo, L.; Barreca, G.S.; Giancotti, A.; Lamberti, A.G.; Colosimo, M.; Minchella, P.; Trecarichi, E.M.; et al. Direct antimicrobial susceptibility testing (AST) from positive blood cultures using Microscan system for early detection of bacterial resistance phenotypes. Diagn. Microbiol. Infect. Dis. 2021, 101, 115485. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; Yansouni, C.P.; Paquette, K.; Lawandi, A.; Stabler, S.N.; Akhter, M.; Davidson, A.C.; Gavric, M.; Jinah, R.; Saeed, Z.; et al. September. Real-word time-to-positivity of two widely used commercial blood culture systems in patients with severe manifestations of sepsis: An analysis of the FABLED study. Open Forum Infect. Dis. 2020, 7, ofaa371. [Google Scholar] [CrossRef]
- Rule, R.; Paruk, F.; Becker, P.; Neuhoff, M.; Chausse, J.; Said, M. Diagnostic accuracy of the BioFire FilmArray blood culture identification panel when used in critically ill patients with sepsis. J. Microbiol. Methods 2021, 189, 106303. [Google Scholar] [CrossRef]
- Rodrigues, C.; Siciliano, R.F.; Charbel, C.E.; de Carvalho Sarahyba da Silva, L.; Baiardo Redaelli, M.; de Paula Rosa Passetti, A.P.; Franco, M.R.G.; Rossi, F.; Zeigler, R.; De Backer, D.; et al. The effect of a rapid molecular blood test on the use of antibiotics for nosocomial sepsis: A randomized clinical trial. J. Intensive Care 2019, 7, 37. [Google Scholar] [CrossRef]
- Lin, J.F.; Ge, M.C.; Liu, T.P.; Chang, S.C.; Lu, J.J. A simple method for rapid microbial identification from positive monomicrobial blood culture bottles through matrix-assisted laser desorption ionization time-of-flight mass spectrometry. J. Microbiol. Immunol. Infect. 2018, 51, 659–665. [Google Scholar] [CrossRef]
- Wu, Y.; Yao, Y.M.; Ke, H.L.; Ying, L.; Wu, Y.; Zhao, G.J.; Lu, Z.Q. Mdivi-1 protects CD4+ T cells against apoptosis via balancing mitochondrial fusion-fission and preventing the induction of endoplasmic reticulum stress in sepsis. Mediators Inflamm. 2019, 2019, 7329131. [Google Scholar] [CrossRef] [Green Version]
- Ransom, E.M.; Alipour, Z.; Wallace, M.A.; Burnham, C.A.D. Evaluation of optimal blood culture incubation time to maximize clinically relevant results from a contemporary blood culture instrument and media system. J. Clin. Microbiol. 2021, 59, e02459-20. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, A. Influence of Blood Culture Results on Antimicrobial Prescribing in a Private Hospital in North West, South Africa. Ph.D. Thesis, North-West University, Potchefstroom, South Africa, 2020. [Google Scholar]
- Chou, W.K.; Vaikunthan, M.; Schröder, H.V.; Link, A.J.; Kim, H.; Brynildsen, M.P. Synergy screening identifies a compound that selectively enhances the antibacterial activity of nitric oxide. Front. Bioeng. Biotechnol. 2020, 8, 1001. [Google Scholar] [CrossRef] [PubMed]
- Kaczor, A.; Witek, K.; Podlewska, S.; Sinou, V.; Czekajewska, J.; Żesławska, E.; Doroz-Płonka, A.; Lubelska, A.; Latacz, G.; Nitek, W.; et al. Molecular insights into an antibiotic enhancer action of new morpholine-containing 5-arylideneimidazolones in the fight against MDR bacteria. Int. J. Mol. Sci. 2021, 22, 2062. [Google Scholar] [CrossRef]
- Bakhit, M. Antibiotic Resistance: Patient-Clinician Communication and Decision-Making about Antibiotic Use in Primary Care. Ph. D. Thesis, Bond University, Gold Coast, Australia, 2018. [Google Scholar]
- Mehta, Y.; Paul, R.; Rabbani, R.; Acharya, S.P.; Withanaarachchi, U.K. Sepsis Management in Southeast Asia: A Review and Clinical Experience. J. Clin. Med. 2022, 11, 3635. [Google Scholar] [CrossRef]
- Nguyen, M.; Brettin, T.; Long, S.; Musser, J.M.; Olsen, R.J.; Olson, R.; Shukla, M.; Stevens, R.L.; Xia, F.; Yoo, H.; et al. Developing an in silico minimum inhibitory concentration panel test for Klebsiella pneumoniae. Sci. Rep. 2018, 8, 421. [Google Scholar] [CrossRef] [Green Version]
- Kharb, S. Biochemical Tests in Clinical Medicine. In Mind Maps in Clinical Chemistry (Part I); Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; p. 15. [Google Scholar]
- Tavassoly, I.; Goldfarb, J.; Iyengar, R. Systems biology primer: The basic methods and approaches. Essays Biochem. 2018, 62, 487–500. [Google Scholar] [CrossRef]
- Pilecky, M.; Schildberger, A.; Orth-Höller, D.; Weber, V. Pathogen enrichment from human whole blood for the diagnosis of bloodstream infection: Prospects and limitations. Diagn. Microbiol. Infect. Dis. 2019, 94, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.W.; Bi, W. Novel applications of array comparative genomic hybridization in molecular diagnostics. Expert Rev. Mol. Diagn. 2018, 18, 531–542. [Google Scholar] [CrossRef]
- Iregbu, K.; Dramowski, A.; Milton, R.; Nsutebu, E.; Howie, S.R.; Chakraborty, M.; Lavoie, P.M.; Costelloe, C.E.; Ghazal, P. Global health systems’ data science approach for precision diagnosis of sepsis in early life. Lancet Infect. Dis. 2021, 22, e143–e152. [Google Scholar] [CrossRef]
- Philips, C.A.; Ahamed, R.; Rajesh, S.; George, T.; Mohanan, M.; Augustine, P. Update on diagnosis and management of sepsis in cirrhosis: Current advances. World J. Hepatol. 2020, 2, 451. [Google Scholar] [CrossRef] [PubMed]
- Sune, D.; Rydberg, H.; Augustinsson, Å.N.; Serrander, L.; Jungeström, M.B. Optimization of 16S rRNA gene analysis for use in the diagnostic clinical microbiology service. J. Microbiol. Methods 2020, 170, 105854. [Google Scholar] [CrossRef] [PubMed]
- Llerena, J.P.; Araujo, P.; Mazzafera, P. Optimization of RT-PCR reactions in studies with genes of lignin biosynthetic route in Saccharum spontaneum. An. Acad. Bras. Cienc. 2018, 90, 509–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreejith, K.R.; Ooi, C.H.; Jin, J.; Dao, D.V.; Nguyen, N.T. Digital polymerase chain reaction technology–recent advances and future perspectives. Lab. Chip. 2018, 18, 3717–3732. [Google Scholar] [CrossRef]
- Manzano, M. Labelled and unlabelled probes for pathogen detection with molecular biology methods and biosensors. Methods Microbiol. 2021, 48, 79–225. [Google Scholar] [CrossRef]
- Ferguson, J.; Duran, J.; Killinen, W.; Wagner, J.; Kulesza, C.; Chatterley, C.; Li, Y. A Field-Deployable and Low-Cost PCR (FLC-PCR) Thermocycler for the Rapid Detection of Environmental E. coli. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 2209–2212. [Google Scholar]
- Dayarathne, M.C.; Mridha, A.U.; Wang, Y. Diagnosis of Fungal Plant Pathogens Using Conventional and Molecular Approaches. In Diagnostics of Plant Diseases; IntechOpen: London, UK, 2020. [Google Scholar]
- Paul, R.; Ostermann, E.; Wei, Q. Advances in point-of-care nucleic acid extraction technologies for rapid diagnosis of human and plant diseases. Biosens. Bioelectron. 2020, 169, 112592. [Google Scholar] [CrossRef]
- Kumar, S.S.; Ghosh, A.R. Assessment of bacterial viability: A comprehensive review on recent advances and challenges. Microbiology 2019, 165, 593–610. [Google Scholar] [CrossRef]
- Jiang, X.W.; Huang, T.S.; Xie, L.; Chen, S.Z.; Wang, S.D.; Huang, Z.W.; Li, X.Y.; Ling, W.P. Development of a diagnostic assay by three-tube multiplex real-time PCR for simultaneous detection of nine microorganisms causing acute respiratory infections. Sci. Rep. 2022, 12, 13306. [Google Scholar] [CrossRef]
- Mota, F.A.; Pereira, S.A.; Araújo, A.R.; Passos, M.L.; Saraiva, M.L.M. Biomarkers in the diagnosis of wounds infection: An analytical perspective. Trends Anal. Chem. 2021, 143, 116405. [Google Scholar] [CrossRef]
- Saha, O.; Islam, M.R.; Rahman, M.S.; Hoque, M.N.; Hossain, M.A.; Sultana, M. Genome-wide diversity and differentiation of two novel multidrug-resistant populations of Pasteurella multocida type B: 2 from fowl cholera. bioRxiv 2020. [Google Scholar] [CrossRef]
- Gunsolus, I.L.; Sweeney, T.E.; Liesenfeld, O.; Ledeboer, N.A. Diagnosing and managing sepsis by probing the host response to infection: Advances, opportunities, and challenges. J. Clin. Microbiol. 2019, 57, e00425-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, D.; Satpathy, G.; Chawla, R.; Venkatesh, P.; Ahmed, N.H.; Panda, S.K. Utility of broad-range 16S rRNA PCR assay versus conventional methods for laboratory diagnosis of bacterial endophthalmitis in a tertiary care hospital. Br. J. Ophthalmol. 2019, 103, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Verbakel, J.Y.; Matheeussen, V.; Loens, K.; Kuijstermans, M.; Goossens, H.; Ieven, M.; Butler, C.C. Performance and ease of use of a molecular point-of-care test for influenza A/B and RSV in patients presenting to primary care. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1453–1460. [Google Scholar] [CrossRef] [PubMed]
- Reta, D.H.; Tessema, T.S.; Ashenef, A.S.; Desta, A.F.; Labisso, W.L.; Gizaw, S.T.; Abay, S.M.; Melka, D.S.; Reta, F.A. Molecular and immunological diagnostic techniques of medical viruses. Int. J. Microbiol. 2020, 2020, 8832728. [Google Scholar] [CrossRef]
- Nik Zuraina, N.M.N.; Mohamad, S.; Hasan, H.; Goni, M.D.; Suraiya, S. Diagnostic performance of an in-house multiplex PCR assay and the retrospective surveillance of bacterial respiratory pathogens at a teaching hospital, Kelantan, Malaysia. Pathog. Glob. Health 2022, 1–13. [Google Scholar] [CrossRef]
- Davidson, K.R.; Ha, D.M.; Schwarz, M.I.; Chan, E.D. Bronchoalveolar lavage as a diagnostic procedure: A review of known cellular and molecular findings in various lung diseases. J. Thorac. Dis. 2020, 12, 4991. [Google Scholar] [CrossRef]
- Cao, X.; Zhao, L.; Zhang, J.; Chen, X.; Shi, L.; Fang, X.; Xie, H.; Chang, Y.; Wang, L. Detection of viable but nonculturable Vibrio parahaemolyticus in shrimp samples using improved real-time PCR and real-time LAMP methods. Food Control 2019, 103, 145–152. [Google Scholar] [CrossRef]
- Yang, W.; Zhang, J.; Ma, R. The prediction of infectious diseases: A bibliometric analysis. Int. J. Environ. Res. Public Health 2020, 7, 6218. [Google Scholar] [CrossRef]
- Pan, Z.; Lu, J.; Wang, N.; He, W.T.; Zhang, L.; Zhao, W.; Su, S. Development of a Taq Man-probe-based multiplex real-time PCR for the simultaneous detection of emerging and reemerging swine coronaviruses. Virulence 2020, 11, 707–718. [Google Scholar] [CrossRef]
- Kadri, K. Polymerase chain reaction (PCR): Principle and applications. In Synthetic Biology-New Interdisciplinary Science; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef] [Green Version]
- Pumford, E.A.; Lu, J.; Spaczai, I.; Prasetyo, M.E.; Zheng, E.M.; Zhang, H.; Kamei, D.T. Developments in integrating nucleic acid isothermal amplification and detection systems for point-of-care diagnostics. Biosens. Bioelectron. 2020, 170, 112674. [Google Scholar] [CrossRef]
- Miotto, B.A.; Hora, A.S.D.; Taniwaki, S.A.; Brandão, P.E.; Heinemann, M.B.; Hagiwara, M.K. Development and validation of a modified TaqMan based real-time PCR assay targeting the lipl32 gene for detection of pathogenic Leptospira in canine urine samples. Braz. J. Microbiol. 2018, 49, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Barkallah, M.; Elleuch, J.; Smith, K.F.; Chaari, S.; Neila, I.B.; Fendri, I.; Michaud, P.; Abdelkafi, S. Development and application of a real-time PCR assay for the sensitive detection of diarrheic toxin producer Prorocentrum lima. J. Microbiol. Methods. 2020, 178, 106081. [Google Scholar] [CrossRef] [PubMed]
- Marras, S.A.; Tyagi, S.; Antson, D.O.; Kramer, F.R. Color-coded molecular beacons for multiplex PCR screening assays. PLoS ONE 2019, 14, 0213906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inchingolo, R.; Pierandrei, C.; Montemurro, G.; Smargiassi, A.; Lohmeyer, F.M.; Rizzi, A. Antimicrobial resistance in common respiratory pathogens of chronic bronchiectasis patients: A literature review. Antibiotics 2021, 10, 326. [Google Scholar] [CrossRef] [PubMed]
- Candel, F.J.; Sá, M.B.; Belda, S.; Bou, G.; Del Pozo, J.L.; Estrada, O.; Ferrer, R.; del Castillo, J.G.; Julian-Jimenez, A.; Martin-Loeches, I.; et al. Current aspects in sepsis approach. Turning things around. Span. J. Psychol. 2018, 31, 298. [Google Scholar]
- Pashchenko, O.; Shelby, T.; Banerjee, T.; Santra, S. A comparison of optical, electrochemical, magnetic, and colorimetric point-of-care biosensors for infectious disease diagnosis. ACS Infect. Dis. 2018, 4, 1162–1178. [Google Scholar] [CrossRef]
- Han, H.; Sohn, B.; Choi, J.; Jeon, S. Recent advances in magnetic nanoparticle-based microfluidic devices for the pretreatment of pathogenic bacteria. Biomed. Eng. Lett. 2021, 11, 297–307. [Google Scholar] [CrossRef]
- Schmitz, J.E.; Stratton, C.W.; Persing, D.H.; Tang, Y.W. Forty Years of Molecular Diagnostics for Infectious Diseases. J. Clin. Microbiol. 2022, 60, e02446-21. [Google Scholar] [CrossRef]
- Bronder, T.S.; Jessing, M.P.; Poghossian, A.; Keusgen, M.; Schöning, M.J. Detection of PCR-amplified tuberculosis DNA fragments with polyelectrolyte-modified field-effect sensors. Anal. Chem. 2018, 90, 7747–7753. [Google Scholar] [CrossRef]
- Wang, J.; Yang, J.; Gao, S.; Liu, A.; Rashid, M.; Li, Y.; Liu, Z.; Liu, J.; Liu, G.; Luo, J.; et al. Rapid detection and differentiation of Theileria annulata, T. orientalis and T. sinensis using high-resolution melting analysis. Ticks Tick Borne Dis. 2020, 11, 101312. [Google Scholar] [CrossRef]
- Kurbakov, K.A.; Konorov, E.A.; Minaev, M.Y.; Kuznetsova, O.A. Multiplex real-time PCR with HRM for detection of Lactobacillus sakei and Lactobacillus curvatus in Food Samples. Food Technol. Biotechnol. 2019, 57, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pohanka, M. Current trends in the biosensors for biological warfare agents assay. Materials 2019, 12, 2303. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Jiang, L.; Lei, Q.; Yang, J.; Gao, X.; Wang, W.; Zhang, Y.; Kong, T.; Chen, Q.; Li, G. Development and validation of quantitative real-time pcr for the detection of residual CHO host cell DNA and optimization of sample pretreatment method in biopharmaceutical products. Biol. Proced. Online 2019, 21, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Tang, M.; Liu, Y.; Huang, J.; Liu, Z.; Tian, H.; Zheng, Y.; de la Chapelle, M.L.; Zhang, Y.; Fu, W. Surface-enhanced Raman scattering method for the identification of methicillin-resistant Staphylococcus aureus using positively charged silver nanoparticles. Mikrochim. Acta 2019, 186, 1–8. [Google Scholar] [CrossRef]
- Jeong, K.; Stanwix, P.L.; May, E.F.; Aman, Z.M. Surface-Enhanced Raman Scattering Imaging of Cetylpyridinium Chloride Adsorption to a Solid Surface. Anal. Chem. 2022, 94, 14169–14176. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Guo, N.; Zhang, Q.; Wang, T.; Song, P.; Xia, L. An ultrasensitive surface-enhanced Raman scattering sensor for the detection of hydrazine via the Schiff base reaction. J. Hazard. Mater. 2022, 424, 127303. [Google Scholar] [CrossRef]
- Ge, M.; Li, P.; Zhou, G.; Chen, S.; Han, W.; Qin, F.; Nie, Y.; Wang, Y.; Qin, M.; Huang, G.; et al. General surface-enhanced Raman spectroscopy method for actively capturing target molecules in small gaps. J. Am. Chem. Soc. 2021, 143, 7769–7776. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Chen, X.; Zheng, Y.; Song, Y.; Zhang, H.; Zhang, S. Surface-enhanced Raman scattering trace-detection platform based on continuous-rolling-assisted evaporation on superhydrophobic surfaces. ACS Appl. Nano Mater. 2020, 3, 4767–4776. [Google Scholar] [CrossRef]
- Pérez-Jiménez, A.I.; Lyu, D.; Lu, Z.; Liu, G.; Ren, B. Surface-enhanced Raman spectroscopy: Benefits, trade-offs and future developments. Chem. Sci. 2020, 11, 4563–4577. [Google Scholar] [CrossRef] [Green Version]
- Shvalya, V.; Filipič, G.; Zavašnik, J.; Abdulhalim, I.; Cvelbar, U. Surface-enhanced Raman spectroscopy for chemical and biological sensing using nanoplasmonics: The relevance of interparticle spacing and surface morphology. Appl. Phys. Rev. 2020, 7, 031307. [Google Scholar] [CrossRef]
- Zong, C.; Xu, M.; Xu, L.J.; Wei, T.; Ma, X.; Zheng, X.S.; Hu, R.; Ren, B. Surface-enhanced Raman spectroscopy for bioanalysis: Reliability and challenges. Chem. Rev. 2018, 118, 4946–4980. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Long, F.; Chen, W.; Chen, J.; Chu, P.K.; Wang, H. Fundamentals and applications of surface-enhanced Raman spectroscopy–based biosensors. Curr. Opin. Biomed. Eng. 2020, 13, 51–59. [Google Scholar] [CrossRef]
- Sun, J.; Gong, L.; Wang, W.; Gong, Z.; Wang, D.; Fan, M. Surface-enhanced Raman spectroscopy for on-site analysis: A review of recent developments. Luminescence 2020, 35, 808–820. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Hu, Q.; Li, C.; Zhang, F.; Gu, H.; Wang, X.; Li, S.; Xue, L.; Madl, T.; Zhang, Y.; et al. Wide-range, rapid, and specific identification of pathogenic bacteria by Surface-Enhanced Raman Spectroscopy. ACS Sens. 2021, 6, 2911–2919. [Google Scholar] [CrossRef]
- Pyrak, E.; Krajczewski, J.; Kowalik, A.; Kudelski, A.; Jaworska, A. Surface enhanced Raman spectroscopy for DNA biosensors—How far are we? Molecules 2019, 24, 4423. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Jang, Y.; Kim, N.J.; Kim, H.; Yi, G.C.; Shin, Y.; Kim, M.H.; Yoon, S. Study of chemical enhancement mechanism in non-plasmonic surface enhanced Raman spectroscopy (SERS). Front. Chem. 2019, 7, 582. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.Y.; Lin, Y.C.; Cheng, W.C.; Lin, Y.T.; Teng, L.J.; Wang, J.K.; Wang, Y.L. Rapid antibiotic susceptibility testing of bacteria from patients’ blood via assaying bacterial metabolic response with surface-enhanced Raman spectroscopy. Sci. Rep. 2020, 10, 12538. [Google Scholar] [CrossRef]
- Wang, K.; Li, S.; Petersen, M.; Wang, S.; Lu, X. Detection and characterization of antibiotic-resistant bacteria using surface-enhanced Raman spectroscopy. Nanomaterials 2018, 8, 762. [Google Scholar] [CrossRef] [Green Version]
- Tahir, M.A.; Dina, N.E.; Cheng, H.; Valev, V.K.; Zhang, L. Surface-enhanced Raman spectroscopy for bioanalysis and diagnosis. Nanoscale 2021, 13, 11593–11634. [Google Scholar] [CrossRef]
- Dizaji, A.N.; Ozek, N.S.; Aysin, F.; Calis, A.; Yilmaz, A.; Yilmaz, M. Combining vancomycin-modified gold nanorod arrays and colloidal nanoparticles as a sandwich model for the discrimination of Gram-positive bacteria and their detection via surface-enhanced Raman spectroscopy (SERS). Analyst 2021, 146, 3642–3653. [Google Scholar] [CrossRef]
- Ahmad, W.; Wang, J.; Li, H.; Jiao, T.; Chen, Q. Trends in the bacterial recognition patterns used in surface enhanced Raman spectroscopy. Trends Anal. Chem. 2021, 142, 116310. [Google Scholar] [CrossRef]
- Kumar, M.; Shergill, S.P.S.; Tandel, K.; Sahai, K.; Gupta, R.M. Direct antimicrobial susceptibility testing from positive blood culture bottles in laboratories lacking automated antimicrobial susceptibility testing systems. Med. J. Armed Forces India 2019, 75, 450–457. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, Y.; Bai, Y.; Song, Z.; Chu, W.; Zhao, M.; Hao, Y.; Lu, Z. Rapid method for direct identification of positive blood cultures by MALDI-TOF MS. Exp. Ther. Med. 2020, 20, 235. [Google Scholar] [CrossRef]
- Dai, Y.; Xu, X.; Yan, X.; Li, D.; Cao, W.; Tang, L.; Hu, M.; Jiang, C. Evaluation of a rapid and simplified protocol for direct identification of microorganisms from positive blood cultures by using Matrix Assisted Laser Desorption Ionization Time-Of-Flight Mass Spectrometry (MALDI-TOF MS). Front. Cell. Infect. Microbiol. 2021, 11, 632679. [Google Scholar] [CrossRef]
- Kayin, M.; Mert, B.; Aydemir, S.; Özenci, V. Comparison of rapid BACpro® II, Sepsityper® kit and in-house preparation methods for direct identification of bacteria from blood cultures by MALDI-TOF MS with and without Sepsityper® module analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2133–2143. [Google Scholar] [CrossRef] [Green Version]
- Homolová, R.; Bogdanová, K.; Bardoň, J.; Kolář, M. Direct identification of bacteria in blood cultures by MALDI-TOF MS. Clin. Microbiol. Infect. 2020, 26, 45–50. [Google Scholar]
- Tsuchida, S.; Nakayama, T. MALDI-Based Mass Spectrometry in Clinical Testing: Focus on Bacterial Identification. Appl. Sci. 2022, 12, 2814. [Google Scholar] [CrossRef]
- Perini, M.; Batisti Biffignandi, G.; Di Carlo, D.; Pasala, A.R.; Piazza, A.; Panelli, S.; Zuccotti, G.V.; Comandatore, F. MeltingPlot, a user-friendly online tool for epidemiological investigation using High Resolution Melting data. BMC Bioinform. 2021, 22, 76. [Google Scholar] [CrossRef]
- Ahmed, M.O.; Baptiste, K.E. Vancomycin-resistant enterococci: A review of antimicrobial resistance mechanisms and perspectives of human and animal health. Microb. Drug Resist. 2018, 24, 590–606. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Xiu, L.; Wang, L.; Zhang, L.; Wang, F.; Peng, J. Rapid Detection of Antimicrobial Resistance in Mycoplasma genitalium by High-Resolution Melting Analysis with Unlabeled Probes. Microbiol. Spectr. 2022, 10, e01014-22. [Google Scholar] [CrossRef]
- Dehshiri, M.; Khoramrooz, S.S.; Zoladl, M.; Khosravani, S.A.; Parhizgari, N.; Motazedian, M.H.; Jahedi, S.; Sharifi, A. The frequency of Klebsiella pneumonia encoding genes for CTX-M, TEM-1 and SHV-1 extended-spectrum beta lactamases enzymes isolated from urinary tract infection. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 4. [Google Scholar] [CrossRef] [Green Version]
- Shalmashi, H.; Farajnia, S.; Sadeghi, M.; Tanoumand, A.; Veissi, K.; Hamishekar, H.; Gotaslou, R. Detection of ESBLs types blaCTX-M, blaSHV and blaTEM resistance genes among clinical isolates of Pseudomonas aeruginosa. Gene Rep. 2022, 28, 101637. [Google Scholar] [CrossRef]
- Zarabadi-Pour, M.; Peymani, A.; Habibollah-Pourzereshki, N.; Sarookhani, M.R.; Karami, A.A.; Javadi, A. Detection of Extended-Spectrum ß-Lactamases among Acinetobacter Baumannii Isolated from Hospitals of Qazvin, Iran. Ethiop. J. Health Sci. 2021, 31, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Abdar, M.H.; Taheri-Kalani, M.; Taheri, K.; Emadi, B.; Hasanzadeh, A.; Sedighi, A.; Pirouzi, S.; Sedighi, M. Prevalence of extended-spectrum beta-lactamase genes in Acinetobacter baumannii strains isolated from nosocomial infections in Tehran, Iran. GMS Hyg. Infect. Control 2019, 14, 318. [Google Scholar] [CrossRef]
- Jordt, H.; Stalder, T.; Kosterlitz, O.; Ponciano, J.M.; Top, E.M.; Kerr, B. Coevolution of host–plasmid pairs facilitates the emergence of novel multidrug resistance. Nat. Ecol. Evol. 2020, 4, 863–869. [Google Scholar] [CrossRef]
- Maharjan, M.; Sah, A.K.; Pyakurel, S.; Thapa, S.; Maharjan, S.; Adhikari, N.; Rijal, K.R.; Ghimire, P.; Thapa Shrestha, U. Molecular Confirmation of Vancomycin-Resistant Staphylococcus aureus with vanA Gene from a Hospital in Kathmandu. Int. J. Microbiol. 2021, 2021, 3847347. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.F.; Mobashery, S. β-Lactams against the Fortress of the Gram-Positive Staphylococcus aureus Bacterium. Chem. Rev. 2020, 121, 3412–3463. [Google Scholar] [CrossRef] [PubMed]
- Leonard, H.; Colodner, R.; Halachmi, S.; Segal, E. Recent advances in the race to design a rapid diagnostic test for antimicrobial resistance. ACS Sens. 2018, 3, 2202–2217. [Google Scholar] [CrossRef]
- Schürch, A.C.; Arredondo-Alonso, S.; Willems, R.J.L.; Goering, R.V. Whole genome sequencing options for bacterial strain typing and epidemiologic analysis based on single nucleotide polymorphism versus gene-by-gene–based approaches. Clin. Microbiol. Infect. 2018, 24, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Fujii, H.; Kakiuchi, S.; Tsuji, M.; Nishimura, H.; Yoshikawa, T.; Yamada, S.; Omura, N.; Inagaki, T.; Shibamura, M.; Harada, S.; et al. Application of next-generation sequencing to detect acyclovir-resistant herpes simplex virus type 1 variants at low frequency in thymidine kinase gene of the isolates recovered from patients with hematopoietic stem cell transplantation. J. Virol. Methods 2018, 251, 123–128. [Google Scholar] [CrossRef]
- Yan, Q.; Wi, Y.M.; Thoendel, M.J.; Raval, Y.S.; Greenwood-Quaintance, K.E.; Abdel, M.P.; Jeraldo, P.R.; Chia, N.; Patel, R. Evaluation of the CosmosID bioinformatics platform for prosthetic joint-associated sonicate fluid shotgun metagenomic data analysis. J. Clin. Microbiol. 2019, 57, e01182-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friães, A.; Mamede, R.; Ferreira, M.; Melo-Cristino, J.; Ramirez, M. Annotated Whole-Genome Multilocus Sequence Typing Schema for Scalable High-Resolution Typing of Streptococcus pyogenes. J. Clin. Microbiol. 2022, 60, e00315-22. [Google Scholar] [CrossRef] [PubMed]
- Palleja, A.; Mikkelsen, K.H.; Forslund, S.K.; Kashani, A.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Liang, S.; Feng, Q.; Zhang, C.; et al. Recovery of gut microbiota of healthy adults following antibiotic exposure. Nat. Microbiol. 2018, 3, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.F.; Olm, M.R.; Morowitz, M.J.; Banfield, J.F. Machine learning leveraging genomes from metagenomes identifies influential antibiotic resistance genes in the infant gut microbiome. MSystems 2018, 3, e00123-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sturaro, L.L.; Gonoi, T.; Busso-Lopes, A.F.; Tararam, C.A.; Levy, C.E.; Lyra, L.; Trabasso, P.; Schreiber, A.Z.; Kamei, K.; Moretti, M.L. Visible DNA microarray system as an adjunctive molecular test in identification of pathogenic fungi directly from a blood culture bottle. J. Clin. Microbiol. 2018, 56, e01908-17. [Google Scholar] [CrossRef] [Green Version]
- Dhanjal, D.S.; Chopra, C.; Chopra, R.S. Metagenomic DNA sequencing: Technological advances and applications. In Metagenomics: Techniques, Applications, Challenges and Opportunities; Springer: Berlin/Heidelberg, Germany, 2020; pp. 37–53. [Google Scholar] [CrossRef]
- Schaack, D.; Siegler, B.H.; Tamulyte, S.; Weigand, M.A.; Uhle, F. The immunosuppressive face of sepsis early on intensive care unit—A large-scale microarray meta-analysis. PloS ONE 2018, 13, 0198555. [Google Scholar] [CrossRef] [PubMed]
- Carroll, K.C.; Reid, J.L.; Thornberg, A.; Whitfield, N.N.; Trainor, D.; Lewis, S.; Wakefield, T.; Davis, T.E.; Church, K.G.; Samuel, L.; et al. Clinical performance of the novel GenMark Dx ePlex blood culture ID Gram-positive panel. J. Clin. Microbiol. 2020, 58, e01730-19. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhang, F.; Cong, Y.; Zhao, Y. Identification of potential genes and miRNAs associated with sepsis based on microarray analysis. Mol. Med. Rep. 2018, 17, 6227–6234. [Google Scholar] [CrossRef]
- Kuchibiro, T.; Hirano, A.; Ogasawara, S.; Nakamura, T. The microcolony detection method (MCD), a simple and rapid screening test for antimicrobial resistance bacteria on positive blood cultures. Heliyon 2020, 6, 05494. [Google Scholar] [CrossRef]
- She, R.C.; Bender, J.M. Advances in rapid molecular blood culture diagnostics: Healthcare impact, laboratory implications, and multiplex technologies. J. Appl. Lab. Med. 2019, 3, 617–630. [Google Scholar] [CrossRef] [Green Version]
- Huang, T.D.; Melnik, E.; Bogaerts, P.; Evrard, S.; Glupczynski, Y. Evaluation of the ePlex blood culture identification panels for detection of pathogens in bloodstream infections. J. Clin. Microbiol. 2019, 57, e01597-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żukowska, M.E. Advanced methods of bacteriological identification in a clinical microbiology laboratory. J. Pre Clin. Clin. Res. 2021, 15, 68–72. [Google Scholar] [CrossRef]
- Fournier, C.; Aires-de-Sousa, M.; Nordmann, P.; Poirel, L. Occurrence of CTX-M-15-and MCR-1-producing Enterobacterales in pigs in Portugal: Evidence of direct links with antibiotic selective pressure. Int. J. Antimicrob. Agents 2020, 55, 105802. [Google Scholar] [CrossRef]
- Kumar, S.; Tripathy, S.; Jyoti, A.; Singh, S.G. Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review. Biosens. Bioelectron. 2019, 124, 205–215. [Google Scholar] [CrossRef]
- Kundu, S.; Tabassum, S.; Kumar, R. A perspective on sepsis pathogenesis, biomarkers and diagnosis: A concise survey. Med. Devices Sens. 2020, 3, 10089. [Google Scholar] [CrossRef]
- Min, J.; Nothing, M.; Coble, B.; Zheng, H.; Park, J.; Im, H.; Weber, G.F.; Castro, C.M.; Swirski, F.K.; Weissleder, R.; et al. Integrated biosensor for rapid and point-of-care sepsis diagnosis. ACS Nano 2018, 12, 3378–3384. [Google Scholar] [CrossRef]
- Levy, M.M.; Gesten, F.C.; Phillips, G.S.; Terry, K.M.; Seymour, C.W.; Prescott, H.C.; Friedrich, M.; Iwashyna, T.J.; Osborn, T.; Lemeshow, S. Mortality changes associated with mandated public reporting for sepsis. The results of the New York state initiative. Am. J. Respir. Crit. Care Med. 2018, 198, 1406–1412. [Google Scholar] [CrossRef]
- Alam, N.; Oskam, E.; Stassen, P.M.; van Exter, P.; van de Ven, P.M.; Haak, H.R.; Holleman, F.; van Zanten, A.; van Leeuwen-Nguyen, H.; Bon, V.; et al. Prehospital antibiotics in the ambulance for sepsis: A multicentre, open label, randomised trial. Lancet Respir. Med. 2018, 6, 40–50. [Google Scholar] [CrossRef]
- Cheng, M.P.; Stenstrom, R.; Paquette, K.; Stabler, S.N.; Akhter, M.; Davidson, A.C.; Gavric, M.; Lawandi, A.; Jinah, R.; Saeed, Z.; et al. Blood culture results before and after antimicrobial administration in patients with severe manifestations of sepsis: A diagnostic study. Ann. Intern. Med. 2019, 171, 547–554. [Google Scholar] [CrossRef]
- Peltan, I.D.; Mitchell, K.H.; Rudd, K.E.; Mann, B.A.; Carlbom, D.J.; Rea, T.D.; Butler, A.M.; Hough, C.L.; Brown, S.M. Prehospital care and emergency department door-to-antibiotic time in sepsis. Ann. Am. Thorac. Soc. 2018, 15, 1443–1450. [Google Scholar] [CrossRef]
- Rello, J.; Van Engelen, T.S.R.; Alp, E.; Calandra, T.; Cattoir, V.; Kern, W.V.; Netea, M.G.; Nseir, S.; Opal, S.M.; van de Veerdonk, F.L.; et al. Towards precision medicine in sepsis: A position paper from the European Society of Clinical Microbiology and Infectious Diseases. Clin. Microbiol. Infect. 2018, 24, 1264–1272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V. Targeting macrophage immunometabolism: Dawn in the darkness of sepsis. Int. Immunopharmacol. 2018, 58, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Antonioli, L.; Blandizzi, C.; Fornai, M.; Pacher, P.; Lee, H.T.; Haskó, G. P2X4 receptors, immunity, and sepsis. Curr. Opin. Pharmacol. 2019, 47, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Mirasoli, M.; Bonvicini, F.; Lovecchio, N.; Petrucci, G.; Zangheri, M.; Calabria, D.; Costantini, F.; Roda, A.; Gallinella, G.; Caputo, D.; et al. On-chip LAMP-BART reaction for viral DNA real-time bioluminescence detection. Sens. Actuators B Chem. 2018, 262, 1024–1033. [Google Scholar] [CrossRef]
- Nasseri, B.; Soleimani, N.; Rabiee, N.; Kalbasi, A.; Karimi, M.; Hamblin, M.R. Point-of-care microfluidic devices for pathogen detection. Biosens. Bioelectron. 2018, 117, 112–128. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.; Hassan, U.; Seymour, C.; Angus, D.C.; Isbell, T.S.; White, K.; Weir, W.; Yeh, L.; Vincent, A.; Bashir, R. Point-of-care sensors for the management of sepsis. Nat. Biomed. Eng. 2018, 2, 640–648. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, A.; Andini, N.; Yang, S. A culture’shift: Application of molecular techniques for diagnosing polymicrobial infections. Biotechnol. Adv. 2019, 37, 476–490. [Google Scholar] [CrossRef]
- Thurtle-Schmidt, D.M.; Lo, T.W. Molecular biology at the cutting edge: A review on CRISPR/CAS9 gene editing for undergraduates. Biochem. Mol. Biol. Educ. 2018, 46, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Butiuc-Keul, A.; Farkas, A.; Carpa, R.; Iordache, D. CRISPR-Cas system: The powerful modulator of accessory genomes in prokaryotes. Microb. Physiol. 2022, 32, 2–17. [Google Scholar] [CrossRef]
- Mohamadi, S.; Bostanabad, S.Z.; Mirnejad, R. CRISPR arrays: A review on its mechanism. J. Appl. Biotechnol. Rep. 2020, 7, 81–86. [Google Scholar] [CrossRef]
- Majumdar, S.; Terns, M.P. CRISPR RNA-guided DNA cleavage by reconstituted Type IA immune effector complexes. Extremophiles 2020, 23, 19–33. [Google Scholar] [CrossRef] [PubMed]
- Gouw, A.M. Challenging the therapy/enhancement distinction in CRISPR gene Editing. In The Palgrave Handbook of Philosophy and Public Policy; Palgrave Macmillan: Cham, Switzerland, 2018; pp. 493–508. [Google Scholar] [CrossRef]
- Koonin, E.V. CRISPR: A new principle of genome engineering linked to conceptual shifts in evolutionary biology. Biol. Philos. 2018, 34, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewart, D.T.; Peterson, E.J.; Steer, C.J. Gene editing for inflammatory disorders. Ann. Rheum. Dis. 2019, 78, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knott, G.J.; Doudna, J.A. CRISPR-Cas guides the future of genetic engineering. Science 2018, 361, 866–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Graham, S.; Gao, L.; Tam, J.; Levesque, M.C. Editing the immune system in vivo in mice using CRISPR/Cas9 ribonucleoprotein (RNP)-mediated gene editing of transplanted hematopoietic stem cells. Methods 2021, 194, 30–36. [Google Scholar] [CrossRef]
- Reyes, M.; Filbin, M.R.; Bhattacharyya, R.P.; Billman, K.; Eisenhaure, T.; Hung, D.T.; Levy, B.D.; Baron, R.M.; Blainey, P.C.; Goldberg, M.B.; et al. An immune-cell signature of bacterial sepsis. Nat. Med. 2020, 26, 333–340. [Google Scholar] [CrossRef]
- Ma, L.; Li, Q.; Cai, S.; Peng, H.; Huyan, T.; Yang, H. The role of NK cells in fighting the virus infection and sepsis. Int. J. Med. Sci. 2021, 18, 3236. [Google Scholar] [CrossRef]
- Wu, M.; Hu, N.; Du, X.; Wei, J. Application of CRISPR/Cas9 technology in sepsis research. Brief Funct. Genomics 2020, 19, 229–234. [Google Scholar] [CrossRef]
- Cai, M.; Li, S.; Shuai, Y.; Li, J.; Tan, J.; Zeng, Q. Genome-wide CRISPR-Cas9 viability screen reveals genes involved in TNF-α-induced apoptosis of human umbilical vein endothelial cells. J. Cell. Physiol. 2019, 234, 9184–9193. [Google Scholar] [CrossRef]
- Grigoriev, E.V.; Salakhov, R.R.; Golubenko, M.V.; Ponasenko, A.V.; Shukevich, D.L.; Matveeva, V.G.; Radivilko, A.S.; Tsepokina, A.V.; Velikanova, E.A.; Kornelyuk, R.S.; et al. Mitochondrial DNA as DAMP in critical conditions. Bull. Sib. Med. 2019, 18, 134–143. [Google Scholar] [CrossRef]
- Panicker, S.; Balijepalli, S.; Zhang, B.; Swamy, S.; Sherman, M.A.; Raghavendran, K.; Suresh, M.V. Role of Toll-like Receptor-9 in Lung Injury. J. Nat. Sci. 2019, 5, 551. [Google Scholar]
- Pustylnikov, S.; Costabile, F.; Beghi, S.; Facciabene, A. Targeting mitochondria in cancer: Current concepts and immunotherapy approaches. Transl. Res. 2018, 202, 35–51. [Google Scholar] [CrossRef] [PubMed]
- Zhong, R.; Miao, L.; Zhang, H.; Tan, L.; Zhao, Y.; Tu, Y.; Prieto, M.A.; Simal-Gandara, J.; Chen, L.; He, C.; et al. Anti-inflammatory activity of flavonols via inhibiting MAPK and NF-κB signaling pathways in RAW264. 7 macrophages. Curr. Res. Food Sci. 2022, 5, 1176–1184. [Google Scholar] [CrossRef] [PubMed]
- Baglaenko, Y.; Macfarlane, D.; Marson, A.; Nigrovic, P.A.; Raychaudhuri, S. Genome editing to define the function of risk loci and variants in rheumatic disease. Nat. Rev. Rheumatol. 2021, 17, 462–474. [Google Scholar] [CrossRef]
- Khan, S.H. Genome-editing technologies: Concept, pros, and cons of various genome-editing techniques and bioethical concerns for clinical application. Mol. Ther. Nucleic. Acids. 2019, 16, 326–334. [Google Scholar] [CrossRef] [Green Version]
- Chew, W.L. Immunity to CRISPR Cas9 and Cas12a therapeutics. Wiley Interdiscip. Rev. Syst. Biol. Med. 2018, 10, 1408. [Google Scholar] [CrossRef]
- Yan, W.X.; Hunnewell, P.; Alfonse, L.E.; Carte, J.M.; Keston-Smith, E.; Sothiselvam, S.; Garrity, A.J.; Chong, S.; Makarova, K.S.; Koonin, E.V.; et al. Functionally diverse type V CRISPR-Cas systems. Science 2019, 363, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Gupta, Y.; Ghrera, A.S. Recent advances in gold nanoparticle-based lateral flow immunoassay for the detection of bacterial infection. Arch. Microbiol. 2021, 203, 3767–3784. [Google Scholar] [CrossRef]
- Costa, S.P.; Carvalho, C.M. Burden of bacterial bloodstream infections and recent advances for diagnosis. Pathog. Dis. 2022, 80, ftac027. [Google Scholar] [CrossRef]
- Di Gaudio, F.; Indelicato, S.; Indelicato, S.; Tricoli, M.R.; Stampone, G.; Bongiorno, D. Improvement of a rapid direct blood culture microbial identification protocol using MALDI-TOF MS and performance comparison with SepsiTyper kit. J. Microbiol. Methods. 2018, 155, 1–7. [Google Scholar] [CrossRef]
- Mirza, F.H.; Baig, F.A.; Syed, S.; Kumar, A.; Shahid, M.A. Role of Presepsin and Comparison with Conventional Markers for Early Diagnosis and Differentiation of Sepsis. J. Hunan Univ. Nat. Sci. 2021, 48, 72–77. [Google Scholar]
- Scerbo, M.H.; Kaplan, H.B.; Dua, A.; Litwin, D.B.; Ambrose, C.G.; Moore, L.J.; Murray, C.C.K.; Wade, C.E.; Holcomb, J.B. Beyond blood culture and Gram stain analysis: A review of molecular techniques for the early detection of bacteremia in surgical patients. Surg. Infect. 2016, 17, 294–302. [Google Scholar] [CrossRef] [PubMed]
- Schenz, J.; Weigand, M.A.; Uhle, F. Molecular and biomarker-based diagnostics in early sepsis: Current challenges and future perspectives. Expert Rev. Mol. Diagn. 2019, 19, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- Behera, B.; Vishnu, G.A.; Chatterjee, S.; Sreekumar, N.; Nagabhushan, A.; Rajendran, N.; Prathik, B.H.; Pandya, H.J. Emerging technologies for antibiotic susceptibility testing. Biosens. Bioelectron. 2019, 142, 111552. [Google Scholar] [CrossRef] [PubMed]
- Peker, N.; Couto, N.; Sinha, B.; Rossen, J.W. Diagnosis of bloodstream infections from positive blood cultures and directly from blood samples: Recent developments in molecular approaches. Clin. Microbiol. Infect. 2018, 24, 944–955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabak, Y.P.; Vankeepuram, L.; Ye, G.; Jeffers, K.; Gupta, V.; Murray, P.R. Blood culture turnaround time in US acute care hospitals and implications for laboratory process optimization. J. Clin. Microbiol. 2018, 56, e00500–e00518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.R.; Sambrook, J. Analysis and normalization of real-time polymerase chain reaction (PCR) experimental data. Cold Spring Harb. Protoc. 2018, 2018, 095000. [Google Scholar] [CrossRef]
- Dailey, P.J.; Elbeik, T.; Holodniy, M. Companion and complementary diagnostics for infectious diseases. Expert Rev. Mol. Diagn. 2020, 20, 619–636. [Google Scholar] [CrossRef]
- Briggs, N.; Campbell, S.; Gupta, S. Advances in rapid diagnostics for bloodstream infections. Diagn. Microbiol. Infect. Dis. 2021, 99, 115219. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, D.; Varghese, D.; Stephens, J.; Ansari, W.; Martin, S.; Charbonneau, C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 35. [Google Scholar] [CrossRef]
- Ulrich, P.S.; Bastian, I.N.; Chen, D.J. Clinical Significance of BD Bactec FX Blood Culture Incubation Beyond 96 Hours (4 Days). J. Clin. Microbiol. 2022, 60, e00549-22. [Google Scholar] [CrossRef] [PubMed]
- Maugeri, G.; Lychko, I.; Sobral, R.; Roque, A.C. Identification and antibiotic-susceptibility profiling of infectious bacterial agents: A review of current and future trends. Biotechnol. J. 2019, 14, 1700750. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.Y.; Lee, C.H.; Hsieh, C.C.; Hong, M.Y.; Chen, M.J.; Lee, C.C. Differential effects of inappropriate empirical antibiotic therapy in adults with community-onset gram-positive and gram-negative aerobe bacteremia. J. Infect. Chemother. 2020, 26, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kang, S.; Vikesland, P.J. Surface-enhanced Raman spectroscopy of bacterial metabolites for bacterial growth monitoring and diagnosis of viral infection. Environ. Sci. Technol. 2021, 55, 9119–9128. [Google Scholar] [CrossRef]
- Tsuchida, S.; Umemura, H.; Nakayama, T. Current status of matrix-assisted laser desorption/ionization–time-of-flight mass spectrometry (MALDI-TOF MS) in clinical diagnostic microbiology. Molecules 2020, 25, 4775. [Google Scholar] [CrossRef]
- Tjandra, K.C.; Ram-Mohan, N.; Abe, R.; Hashemi, M.M.; Lee, J.H.; Chin, S.M.; Yang, S. Diagnosis of Bloodstream Infections: An Evolution of Technologies towards Accurate and Rapid Identification and Antibiotic Susceptibility Testing. Antibiotics 2022, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Brenner, T.; Decker, S.O.; Grumaz, S.; Stevens, P.; Bruckner, T.; Schmoch, T.; Sohn, K. Next-generation sequencing diagnostics of bacteremia in sepsis (Next GeneSiS-Trial): Study protocol of a prospective, observational, noninterventional, multicenter, clinical trial. Medicine 2018, 97, 9868. [Google Scholar] [CrossRef]
- Gopal, A.; Yan, L.; Kashif, S.; Munshi, T.; Roy, V.A.; Voelcker, N.H.; Chen, X. Biosensors and Point-of-Care Devices for Bacterial Detection: Rapid Diagnostics Informing Antibiotic Therapy. Adv. Healthc. Mater. 2022, 11, 2101546. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Köse, Ş.; Dao, S.; Ganbarov, K.; Tanomand, A.; Dal, T.; Kafil, H.S. How CRISPR-Cas system could be used to combat antimicrobial resistance. Infect. Drug Resist. 2020, 13, 1111. [Google Scholar] [CrossRef] [Green Version]
- Legenza, L.; Barnett, S.; Lacy, J.P.; See, C.; Desotell, N.; Eibergen, A.; Piccirillo, J.F.; Rose, W.E. Geographic mapping of Escherichia coli susceptibility to develop a novel clinical decision support tool. Antimicrob. Agents Chemother. 2019, 63, 19. [Google Scholar] [CrossRef]



| Fluorescent Molecule | Working | Volume of the Reaction Mixture | Cost-Effective | Sensitivity | Specificity | Sample to Result Time | Detection Limit | Refs. |
|---|---|---|---|---|---|---|---|---|
| SYBR Green | Intercalates between the DNA bases to bind to ds-DNA molecules. | 10–20 µL | ★★★ | ★ | ★ | 2–3 h | 60 pg DNA | [63,64] |
| TaqMan Probes | Taq polymerase performs 5–3 exonuclease activity during hybridization of fluorophore-based detection, cleaving a dual-labeled probe to the corresponding target sequence. | 5–10 µL | ★ | ★★ | ★★★ | 1–2 h | 0.3 pg DNA | [65,66] |
| Molecular Beacon Probes | The hairpin ring formed by the DNA sequences on the probes ends is designed to be complimentary to one another. The intervening loop portion of the probe is intended to complement the target DNA sequence of interest. | 20–25 µL | ★ | ★★ | ★★★ | 5–9 h | 3–5 pg DNA | [67,68,69] |
| Scorpion Probes | On the 5′ and 3′ sides of the probe, complementary stem sequences hold a distinct probe sequence in a hairpin loop shape. After the primer is extended during PCR amplification, the identical probe sequence will attach to its compliment inside the same strand of DNA. | 20–25 µL | ★ | ★★ | ★★★ | 3–5 h | 10pg DNA | [70,71,72] |
| Antibiotics Class | Genes | Bacteria | Reference |
|---|---|---|---|
| β-lactamases and Cephalosporins | ctxM | Klebsiella pneumonia | [106] |
| Tem | K. pneumonia, Pseudomonas aeruginosa, Escherichia coli, Acinetobacter baumannii | [106] [107] [104] [108,109] | |
| Shv | K. pneumonia | [106] | |
| Fluoroquinolones | parC | Streptococcus sp. | [109] |
| gyrA | Streptococcus sp. | [109] | |
| Vancomycin | vanA | Staphylococcus aureus | [110] |
| vanB | Enterococcus faecalis | [111] | |
| vanC | E. faecalis | [111] | |
| Methicillin | mecA | S.aureus | [112] |
| mecC | S.aureus | [113] |
| Technology | Cost-Effective | Sensitivity | Specificity | Turnaround Time | Multiplexing Capability | Refs. |
|---|---|---|---|---|---|---|
| Microbiological Methods | ||||||
| Blood Culture/Gram Staining | ★★★ | ★ | ★ | ★★★ | ★ | [179] |
| BactecFx/VITEK 2 | ★★ | ★★ | ★★ | ★★★ | ★★ | [180] |
| Biochemical Methods | ★★★ | ★ | ★ | ★★★ | ★ | [181] |
| Modern Methods | ||||||
| Molecular Methods | ||||||
| Real-Time PCR | ★★ | ★★★ | ★★ | ★★★ | ★★ | [182] |
| SERS | ★★ | ★★ | ★★ | ★★ | ★★ | [183] |
| MALDI-TOF | ★ | ★ | ★★ | ★ | ★ | [184] |
| AMR Detection Methods | ||||||
| HRM | ★★ | ★★ | ★★ | ★★★ | ★ | [185] |
| Sequencing | ★ | ★★★ | ★★★ | ★ | ★★★ | [173] |
| DNA Microarray | ★ | ★★ | ★★ | ★★ | ★★★ | [186] |
| Advanced Methods | ||||||
| Biosensors | ★ | ★★ | ★★ | ★ | ★★★ | [187] |
| POCT | ★ | ★★ | ★★ | ★ | ★★★ | [186,187] |
| CRISPR/Cas9 | ★ | ★★ | ★★ | ★★ | ★★★ | [188] |
| CRISPR/Cas9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, E.; Saxena, J.; Kumar, S.; Sharma, U.; Rastogi, S.; Srivastava, V.K.; Kaushik, S.; Jyoti, A. Fast Track Diagnostic Tools for Clinical Management of Sepsis: Paradigm Shift from Conventional to Advanced Methods. Diagnostics 2023, 13, 277. https://doi.org/10.3390/diagnostics13020277
Gupta E, Saxena J, Kumar S, Sharma U, Rastogi S, Srivastava VK, Kaushik S, Jyoti A. Fast Track Diagnostic Tools for Clinical Management of Sepsis: Paradigm Shift from Conventional to Advanced Methods. Diagnostics. 2023; 13(2):277. https://doi.org/10.3390/diagnostics13020277
Chicago/Turabian StyleGupta, Ena, Juhi Saxena, Sanni Kumar, Umang Sharma, Saundarya Rastogi, Vijay Kumar Srivastava, Sanket Kaushik, and Anupam Jyoti. 2023. "Fast Track Diagnostic Tools for Clinical Management of Sepsis: Paradigm Shift from Conventional to Advanced Methods" Diagnostics 13, no. 2: 277. https://doi.org/10.3390/diagnostics13020277
APA StyleGupta, E., Saxena, J., Kumar, S., Sharma, U., Rastogi, S., Srivastava, V. K., Kaushik, S., & Jyoti, A. (2023). Fast Track Diagnostic Tools for Clinical Management of Sepsis: Paradigm Shift from Conventional to Advanced Methods. Diagnostics, 13(2), 277. https://doi.org/10.3390/diagnostics13020277








