Potentially Virulent Multi-Drug Resistant Escherichia fergusonii Isolated from Inanimate Surface in a Medical University: Omphisa fuscidentalis as an Alternative for Bacterial Virulence Determination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Antimicrobial Susceptibility Testing
2.2. Molecular Identification of Isolate KS-1 and Phylogenetic Tree Construction
2.3. Culture Preparation of Escherichia fergusonii for Omphisa fuscidentalis Injection
2.4. Escherichia fergusonii Injection in Omphisa fuscidentalis Larvae as an Infection Model
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Aknowledgements:
Conflicts of Interest
References
- Al-Ghamdi, A.K.; Abdelmalek, S.M.A.; Ashshi, A.M.; Faidah, H.; Shukri, H. Bacterial Contamination of Computer Keyboards and Mice, Elevator Buttons and Shopping Carts. Afr. J. Microbiol. Res. 2011, 5, 3998–4003. [Google Scholar]
- Koroglu, M.; Gunal, S.; Yildiz, F.; Savas, M.; Ozer, A.; Altindis, M. Comparison of Keypads and Touch-Screen Mobile Phones/Devices as Potential Risk for Microbial Contamination. J. Infect. Dev. Ctries. 2015, 9, 1308–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antunes, P.; Novais, C.; Peixe, L. Food-to-Humans Bacterial Transmission. Microb. Transm. 2019, 8, 161–193. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Nimer, N.A. Nosocomial Infection and Antibiotic-Resistant Threat in the Middle East. Infect. Drug Resist. 2022, 15, 631–639. [Google Scholar] [CrossRef]
- Liu, S.; Wang, M.; Zheng, L.; Guan, W. Antimicrobial Resistance Profiles of Nosocomial Pathogens in Regional China: A Brief Report from Two Tertiary Hospitals in China. Med. Sci. Monit. 2018, 24, 8602–8607. [Google Scholar] [CrossRef]
- Sarowska, J.; Futoma-Koloch, B.; Jama-Kmiecik, A.; Frej-Madrzak, M.; Ksiazczyk, M.; Bugla-Ploskonska, G.; Choroszy-Krol, I. Virulence Factors, Prevalence and Potential Transmission of Extraintestinal Pathogenic Escherichia Coli Isolated from Different Sources: Recent Reports. Gut Pathog. 2019, 11, 10. [Google Scholar] [CrossRef] [Green Version]
- Kaito, C.; Murakami, K.; Imai, L.; Furuta, K. Animal Infection Models Using Non-Mammals. Microbiol. Immunol. 2020, 64, 585–592. [Google Scholar] [CrossRef]
- Tsai, C.J.Y.; Loh, J.M.S.; Proft, T. Galleria Mellonella Infection Models for the Study of Bacterial Diseases and for Antimicrobial Drug Testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [Green Version]
- Ilsan, N.A.; Lee, Y.J.; Kuo, S.C.; Lee, I.H.; Huang, T.W. Antimicrobial Resistance Mechanisms and Virulence of Colistin-and Carbapenem-Resistant Acinetobacter Baumannii Isolated from a Teaching Hospital in Taiwan. Microorganisms 2021, 9, 1295. [Google Scholar] [CrossRef]
- Zahornacký, O.; Porubčin, Š.; Rovňáková, A.; Jarčuška, P. Gram-Negative Rods on Inanimate Surfaces of Selected Hospital Facilities and Their Nosocomial Significance. Int. J. Environ. Res. Public Health 2022, 19, 6039. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.R.; Sato, T.; Weightman, A.J.; Martin, T.A.; Fry, J.C.; Hiom, S.J.; Wade, W.G. Marchesi JR 1998 Primer Für 16S RRNA.Pdf. Appl. Environ. Microbiol. 1998, 64, 795–799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, K.H.; Chen, L.R.; Wang, Y.K. Contamination of Medical Charts: An Important Source of Potential Infection in Hospitals. PLoS ONE 2014, 9, e78512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russotto, V.; Cortegiani, A.; Raineri, S.M.; Giarratano, A. Bacterial Contamination of Inanimate Surfaces and Equipment in the Intensive Care Unit. J. Intensive Care 2015, 3, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulger, F.; Esen, S.; Dilek, A.; Yanik, K.; Gunaydin, M.; Leblebicioglu, H. Are We Aware How Contaminated Our Mobile Phones with Nosocomial Pathogens? Ann. Clin. Microbiol. Antimicrob. 2009, 8, 4–7. [Google Scholar] [CrossRef] [Green Version]
- Chaoui, L.; Mhand, R.; Mellouki, F.; Rhallabi, N. Contamination of the Surfaces of a Health Care Environment by Multidrug-Resistant (MDR) Bacteria. Int. J. Microbiol. 2019, 2019, 1–7. [Google Scholar] [CrossRef]
- Dancer, S.J. Importance of the Environment in Meticillin-Resistant Staphylococcus Aureus Acquisition: The Case for Hospital Cleaning. Lancet Infect. Dis. 2008, 8, 101–113. [Google Scholar] [CrossRef]
- Pittet, D.; Allegranzi, B.; Sax, H.; Dharan, S.; Pessoa-Silva, C.L.; Donaldson, L.; Boyce, J.M. Evidence-Based Model for Hand Transmission during Patient Care and the Role of Improved Practices. Lancet Infect. Dis. 2006, 6, 641–652. [Google Scholar] [CrossRef]
- Kiros, T.; Workineh, L.; Tiruneh, T.; Eyayu, T.; Damtie, S.; Belete, D. Prevalence of Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Ethiopia: A Systematic Review and Meta-Analysis. Int. J. Microbiol. 2021, 2021, 1–13. [Google Scholar] [CrossRef]
- Nazeri, M.; Salmani Arani, J.; Ziloochi, N.; Delkhah, H.; Hesami Arani, M.; Asgari, E.; Hosseini, M. Microbial Contamination of Keyboards and Electronic Equipment of ICU (Intensive Care Units) in Kashan University of Medical Sciences and Health Service Hospitals. MethodsX 2019, 6, 666–671. [Google Scholar] [CrossRef]
- Couturier, J.; Ginevra, C.; Nesa, D.; Adam, M.; Gouot, C.; Descours, G.; Campèse, C.; Battipaglia, G.; Brissot, E.; Beraud, L.; et al. Transmission of Legionnaires’ Disease through Toilet Flushing. Emerg. Infect. Dis. 2020, 26, 1526–1528. [Google Scholar] [CrossRef] [PubMed]
- Barker, J.; Bloomfield, S.F. Survival of Salmonella in Bathrooms and Toilets in Domestic Homes Following Salmonellosis. J. Appl. Microbiol. 2000, 89, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Farmer, J.J.; Fanning, G.R.; Davis, B.R.; O’Hara, C.M.; Riddle, C.; Hickman-Brenner, F.W.; Asbury, M.A.; Lowery, V.A.; Brenner, D.J. Escherichia Fergusonii and Enterobacter Taylorae, Two New Species of Enterobacteriaceae Isolated from Clinical Specimens. J. Clin. Microbiol. 1985, 21, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Glover, B.; Wentzel, J.; Jenkins, A.; Van Vuuren, M. The First Report of Escherichia Fergusonii Isolated from Non-Human Primates, in Africa. One Health 2017, 3, 70–75. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.C.; Cheng, A.; Huang, Y.T.; Chung, K.P.; Lee, M.R.; Liao, C.H.; Hsueh, P.R. Escherichia Fergusonii Bacteremia in a Diabetic Patient with Pancreatic Cancer. J. Clin. Microbiol. 2011, 49, 4001–4002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheux, A.F.; Boudreau, D.K.; Bergeron, M.G.; Rodriguez, M.J. Characterization of Escherichia Fergusonii and Escherichia Albertii Isolated from Water. J. Appl. Microbiol. 2014, 117, 597–609. [Google Scholar] [CrossRef]
- Savini, V.; Catavitello, C.; Talia, M.; Manna, A.; Pompetti, F.; Favaro, M.; Fontana, C.; Febbo, F.; Balbinot, A.; Di Berardino, F.; et al. Multidrug-Resistant Escherichia Fergusonii: A Case of Acute Cystitis. J. Clin. Microbiol. 2008, 46, 1551–1552. [Google Scholar] [CrossRef] [Green Version]
- Lagacé-Wiens, P.R.S.; Baudry, P.J.; Pang, P.; Hammond, G. First Description of an Extended-Spectrum-β-Lactamase-Producing Multidrug-Resistant Escherichia Fergusonii Strain in a Patient with Cystitis. J. Clin. Microbiol. 2010, 48, 2301–2302. [Google Scholar] [CrossRef] [Green Version]
- Adesina, T.; Nwinyi, O.; De, N.; Akinnola, O.; Omonigbehin, E. First Detection of Carbapenem-Resistant Escherichia Fergusonii Strains Harbouring Beta-Lactamase Genes from Clinical Samples. Pathogens 2019, 8, 164. [Google Scholar] [CrossRef] [Green Version]
- Baek, S.D.; Chun, C.; Hong, K.S. Hemolytic Uremic Syndrome Caused by Escherichia Fergusonii Infection. Kidney Res. Clin. Pract. 2019, 38, 253–255. [Google Scholar] [CrossRef] [Green Version]
- Durieux, M.F.; Melloul, É.; Jemel, S.; Roisin, L.; Dardé, M.L.; Guillot, J.; Dannaoui, É.; Botterel, F. Galleria Mellonella as a Screening Tool to Study Virulence Factors of Aspergillus Fumigatus. Virulence 2021, 12, 818–834. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Vilcinskas, A.; Kanost, M.R. Immunity in Lepidopteran Insects. Adv. Exp. Med. Biol. 2010, 708, 181–204. [Google Scholar] [CrossRef] [PubMed]
- Dziedziech, A.; Shivankar, S.; Theopold, U. Drosophilamelanogaster Responses against Entomopathogenic Nematodes: Focus on Hemolymph Clots. Insects 2020, 11, 62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatogawa, S.I.; Oda, Y.; Kamiya, M.; Kamijima, T.; Aizawa, T.; Clark, K.D.; Demura, M.; Kawano, K.; Strand, M.R.; Hayakawa, Y. A Novel Peptide Mediates Aggregation and Migration of Hemocytes from an Insect. Curr. Biol. 2009, 19, 779–785. [Google Scholar] [CrossRef] [Green Version]
- Schmid, M.R.; Dziedziech, A.; Arefin, B.; Kienzle, T.; Wang, Z.; Akhter, M.; Berka, J.; Theopold, U. Insect Hemolymph Coagulation: Kinetics of Classically and Non-Classically Secreted Clotting Factors. Insect Biochem. Mol. Biol. 2019, 109, 63–71. [Google Scholar] [CrossRef]
- Li, D.; Scherfer, C.; Korayem, A.M.; Zhao, Z.; Schmidt, O.; Theopold, U. Insect Hemolymph Clotting: Evidence for Interaction between the Coagulation System and the Prophenoloxidase Activating Cascade. Insect Biochem. Mol. Biol. 2002, 32, 919–928. [Google Scholar] [CrossRef]
- Eleftherianos, I.; Heryanto, C.; Bassal, T.; Zhang, W.; Tettamanti, G.; Mohamed, A. Haemocyte-Mediated Immunity in Insects: Cells, Processes and Associated Components in the Fight against Pathogens and Parasites. Immunology 2021, 164, 401–432. [Google Scholar] [CrossRef]
- Ma, H.; Abbas, M.N.; Zhang, K.; Hu, X.; Xu, M.; Liang, H.; Kausar, S.; Yang, L.; Cui, H. 20-Hydroxyecdysone Regulates the Transcription of the Lysozyme via Broad-Complex Z2 Gene in Silkworm, Bombyx Mori. Dev. Comp. Immunol. 2019, 94, 66–72. [Google Scholar] [CrossRef]
- Dutta, A.; Dandapat, J.; Mohanty, N. First Report on Transferrin in the Silkworm, Antheraea Mylitta, with a Putative Role in Antioxidant Defense: Insights from Proteomic Analysis and Immunodetection. Comp. Biochem. Physiol. Part—B Biochem. Mol. Biol. 2019, 233, 23–34. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, J.L.; Cheng, Y.; Wang, J.X.; Zou, Z. Pattern Recognition Receptors from Lepidopteran Insects and Their Biological Functions. Dev. Comp. Immunol. 2020, 108, 103688. [Google Scholar] [CrossRef]
- Zhang, W.; Tettamanti, G.; Bassal, T.; Heryanto, C.; Eleftherianos, I.; Mohamed, A. Regulators and Signalling in Insect Antimicrobial Innate Immunity: Functional Molecules and Cellular Pathways. Cell. Signal. 2021, 83, 110003. [Google Scholar] [CrossRef] [PubMed]
- Arteaga Blanco, L.A.; Crispim, J.S.; Fernandes, K.M.; de Oliveira, L.L.; Pereira, M.F.; Bazzolli, D.M.S.; Martins, G.F. Differential Cellular Immune Response of Galleria Mellonella to Actinobacillus Pleuropneumoniae. Cell Tissue Res. 2017, 370, 153–168. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.Y. Galleria Mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell. Infect. Microbiol. 2021, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.Y.; Andrew Keddie, B. The Galleria Mellonella-Enteropathogenic Escherichia Coli Model System: Characterization of Pathogen Virulence and Insect Immune Responses. J. Insect Sci. 2021, 21, 7. [Google Scholar] [CrossRef] [PubMed]

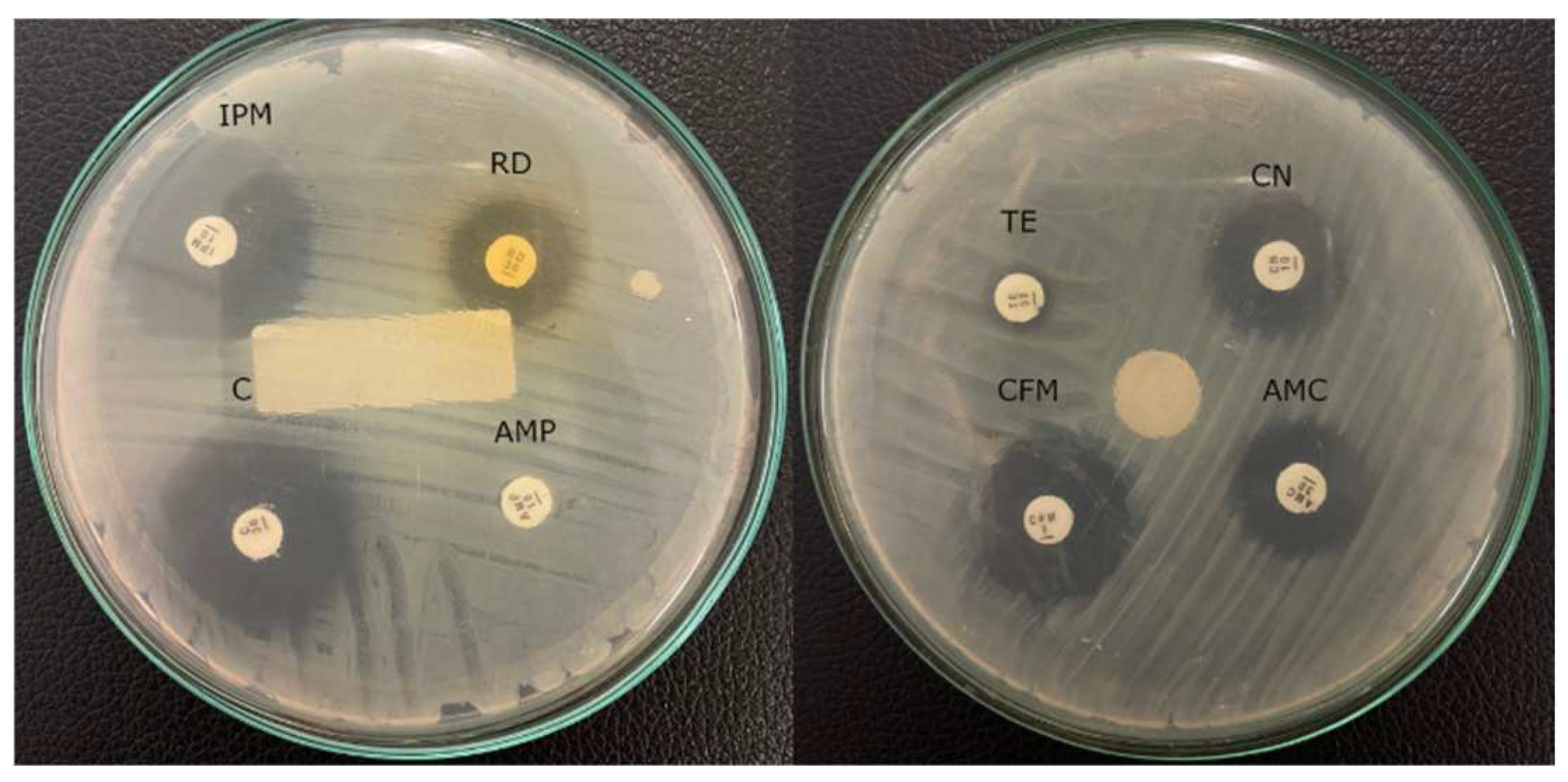


| Surface of Samples (Isolate Code) | Diameter of Inhibition Zone (mm) | |||||||
|---|---|---|---|---|---|---|---|---|
| AMP 10 | IPM 10 | C 30 | AMC 30 | CN 10 | TE 30 | RD 30 | CFM 5 | |
| Mobile phone of security officer (HSO) | 20 (S) | 45 (S) | 0 (R) | 29 (S) | 10 (R) | 10 (R) | 30 | 0 (R) |
| Mobile phone of parking officer (HPO) | 30 (S) | 30 (S) | 44 (S) | 42 (S) | 44 (S) | 30 (S) | 30 | 40 (S) |
| Toilet bowl 1 (TB1) | 0 (R) | 17 (R) | 16 (I) | 16 (I) | 12 (R) | 8 (R) | 15 | 20 (S) |
| Toilet bowl 2 (TB2) | 16 (I) | 32 (S) | 26 (S) | 12 (R) | 27 (S) | 33 (S) | 35 | 0 (R) |
| Motorcycle handlebar 1 (MH1) | 36 (S) | 50 (S) | 30 (S) | 38 (S) | 26 (S) | 30 (S) | 44 | 8 (S) |
| Motorcycle handlebar 2 (MH2) | 35 (S) | 40 (S) | 24 (S) | 40 (S) | 27 (S) | 30 (S) | 40 | 8 (S) |
| Sauce bottle in canteen 1 (SBIC1) | 25 (S) | 52 (S) | 34 (S) | 34 (S) | 25 (S) | 30 (S) | 40 | 26 (S) |
| Sauce bottle in canteen 2 (SBIC2) | 27 (S) | 50 (S) | 30 (S) | 32 (S) | 25 (S) | 33 (S) | 40 | 24 (S) |
| Storefront canteen 1 (SC1) | 23 (S) | 56 (S) | 23 (S) | 36 (S) | 26 (S) | 31 (S) | 58 | 26 (S) |
| Storefront canteen 2 (SC2) | 23 (S) | 0 (R) | 32 (S) | 34 (S) | 30 (S) | 31 (S) | 0 | 17 (I) |
| Parking keyboard 1 (PK1) | 23 (S) | 50 (S) | 25 (S) | 34 (S) | 28 (S) | 30 (S) | 44 | 20 (S) |
| Parking keyboard 2 (PK2) | 22 (S) | 42 (S) | 24 (S) | 32 (S) | 28 (S) | 34 (S) | 30 | 0 (R) |
| Parking computer mouse (PCM) | 15 (S) | 32 (S) | 30 (S) | 26 (S) | 22 (S) | 23 (S) | 0 | 0 (R) |
| Parking ticket button (PTB) | 18 (S) | 47 (S) | 26 (S) | 23 (S) | 23 (S) | 20 (S) | 28 | 0 (R) |
| Description | Max Score | Query Cover | E Value | Identity | Accession Length (bp) | Accession |
|---|---|---|---|---|---|---|
| Escherichia fergusonii strain 2611 16S ribosomal RNA gene | 2361 | 99% | 0.0 | 99.84% | 1438 | MT611634.1 |
| Escherichia fergusonii strain 389 16S ribosomal RNA gene | 2361 | 99% | 0.0 | 99.84% | 1384 | MT573069.1 |
| Escherichia fergusonii strain 346 16S ribosomal RNA gene | 2361 | 99% | 0.0 | 99.92% | 1376 | MT573049.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilsan, N.A.; Yunita, M.; Dewi, N.K.; Irham, L.M.; Sipriyadi; Nurfajriah, S.; Inggraini, M. Potentially Virulent Multi-Drug Resistant Escherichia fergusonii Isolated from Inanimate Surface in a Medical University: Omphisa fuscidentalis as an Alternative for Bacterial Virulence Determination. Diagnostics 2023, 13, 279. https://doi.org/10.3390/diagnostics13020279
Ilsan NA, Yunita M, Dewi NK, Irham LM, Sipriyadi, Nurfajriah S, Inggraini M. Potentially Virulent Multi-Drug Resistant Escherichia fergusonii Isolated from Inanimate Surface in a Medical University: Omphisa fuscidentalis as an Alternative for Bacterial Virulence Determination. Diagnostics. 2023; 13(2):279. https://doi.org/10.3390/diagnostics13020279
Chicago/Turabian StyleIlsan, Noor Andryan, Melda Yunita, Nurul Kusuma Dewi, Lalu Muhammad Irham, Sipriyadi, Siti Nurfajriah, and Maulin Inggraini. 2023. "Potentially Virulent Multi-Drug Resistant Escherichia fergusonii Isolated from Inanimate Surface in a Medical University: Omphisa fuscidentalis as an Alternative for Bacterial Virulence Determination" Diagnostics 13, no. 2: 279. https://doi.org/10.3390/diagnostics13020279






