Fructose Metabolism and Its Effect on Glucose-Galactose Malabsorption Patients: A Literature Review
Abstract
1. Introduction
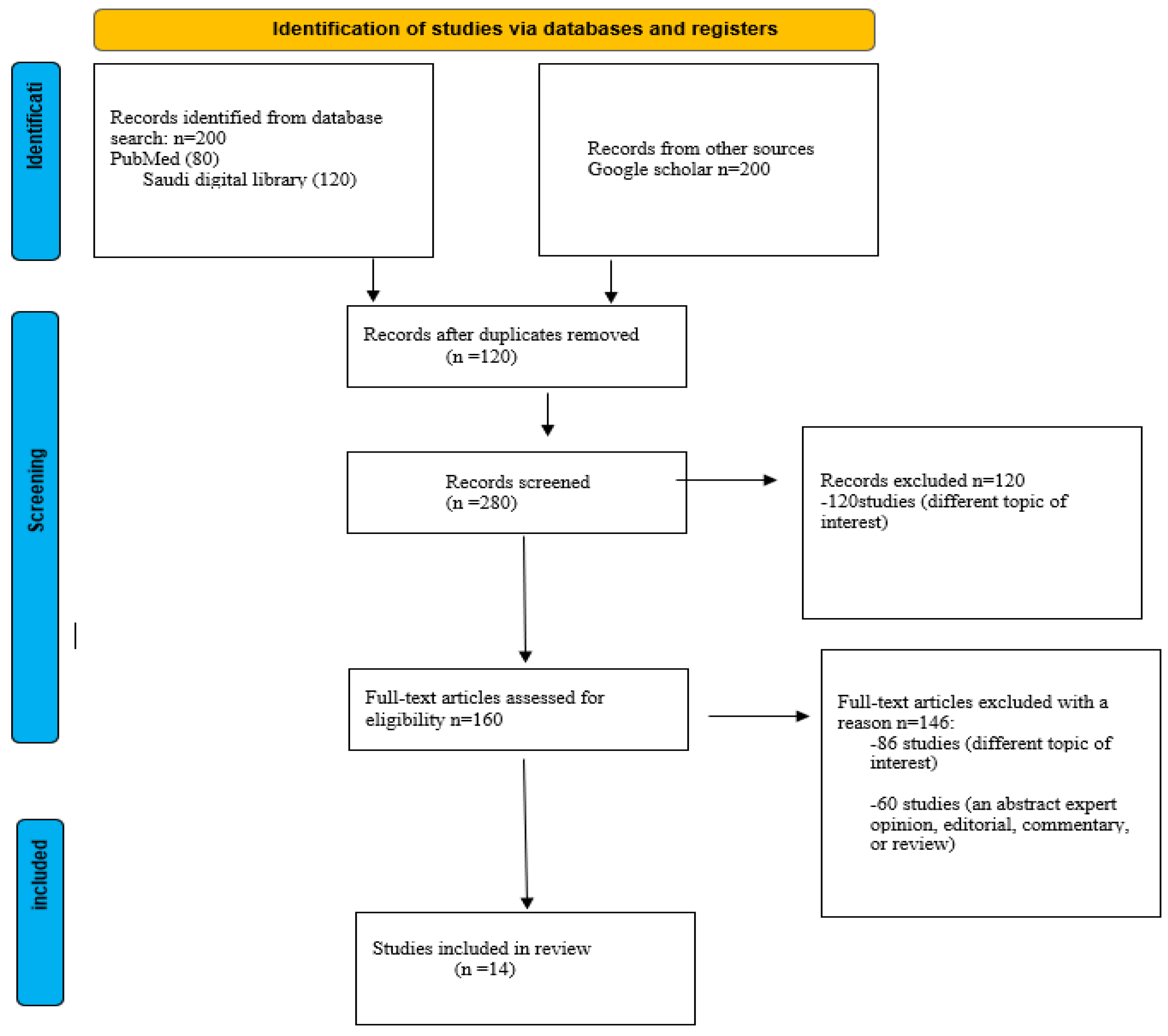
2. Materials and Methods
3. Results and Discussion
| Study | Results | Conclusion |
|---|---|---|
| [15] | Cases were from Kingdom of Saudi Arabia (55 cases) and Turkey (43 patients) (Total 78.2%) and they were consanguineous marriage. Positive responds when using fructose-based formula. SLC5A1 gene mutations were found in 73 cases (68.2%) after gene testing. | Two major mechanisms in detecting and diagnosing CGGM is fasting and gene testing. The use of fructose-based formulas is the best management for CGGM cases. |
| [7] | GLUT5 is regulated by LXRα in both mice and humans. | The treatment of metabolic disease and cancer as well LXRα might provide novel pharmacologic strategies for the selective modulation of GLUT5 activity. |
| [10] | Reduce the intake of SBBS several strategies and policies are needed. | Increased risk of CVD, weight gain, and NIDDM (type 2 diabetes mellitus) with the consumption of SBBS. |
| [4] | Non-immune- mediated food intolerance has a cumulative prevalence of 30% to 40%, while true (immune-mediated) food allergies affect only 2% to 5% of the German population. | Eliminate the intake of the responsible carbohydrate substance or reduce it to a fair amount might improve the malabsorption. |
| [13] | Mutant protein is inserted into the plasma. In a duodenal biopsy from a GGM patient, the mutant protein is in the brush border membrane of enterocytes. | Autosomal recessive mode of inheritance was confirmed by the studies of this isolated pedigree of GGM patients, and this provides unique insights into the molecular mechanism of glucose transport by SGLT-1. |
| [16] | Absorption rate of glucose and galactose is 13% and 22%, respectively. The control group was 85% for glucose and galactose. | There was no difference in the rate absorption of fructose in the control group (healthy individuals) and the study group (three CGGM patients). the rate was 66%. |
| [45] | The role of excessive intake of fructose and SSBS in developing cardiometabolic diseases was supported by epidemiological data and mechanistic data. | Holistic approaches to bear on present metabolic epidemics to better understand the underlying pathways through which sugar and fructose can cause disease. |
| [12] | Five patients reported one or more symptoms of bloating (n = 3), diarrhea (n = 3) and abdominal pain (n = 1) during follow up. All had normal development, and none had neurological complications secondary to dehydration. | Early detection and management are crucial might prevent the consequences of dehydration and death resulting from this condition. |
| [8] | Describes how dietary fructose can affect the expression and activity of fructolytic enzymes and transporters by affecting the cellular response to fructose in the gut. | Dietary fructose may have a potential relation to the gastrointestinal and gut microbe. |
| [56] | Children, with their preference for sweet foods and drinks, are prone to excessive sugar consumption. Toddlers under age two are especially vulnerable. | The effects that have been observed with the consumption of large amounts of fructose cannot be reliably distinguished from the effects of a generally excessive caloric intake. |
| [55] | Logistic regression analysis demonstrated that a higher level of Hs-CRP, fructose consumption, and smoking were independently associated with SCF. | The SCF group demonstrated a higher level of fructose consumption. Excessive fructose consumption may play a role in SCF pathophysiology. |
| [58] | An energy-matched (isocaloric) exchange of dietary carbohydrates by fructose promoted hepatic insulin resistance but had no effect on fasting plasma insulin concentrations. Hypercaloric fructose raised fasting plasma insulin concentrations. | Short-term fructose consumption, in isocaloric exchange or in hypercaloric supplementation, promotes the development of hepatic insulin resistance in nondiabetic adults without affecting peripheral or muscle insulin sensitivity. |
| [14] | In 22 out of the 23 missense mutations tested in the oocyte expression system, the proteins were translated and were stable in the cell but did not reach the plasma membrane. In four of these mutants, an alanine residue was replaced by a valine, and in two, the trafficking defect was rescued by changing the valine to cysteine. One mutant protein (Q457R) did reach the plasma membrane, but it was unable to transport the sugar across the cell membrane. | Mutations in the SGLT-1 gene are the cause of glucose-galactose malabsorption, and sugar transport is impaired mainly because the mutant proteins are either truncated or are not targeted properly to the cell membrane. |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hess, J.; Latulippe, M.E.; Ayoob, K.; Slavin, J. The confusing world of dietary sugars: Definitions, intakes, food sources and international dietary recommendations. Food Funct. 2012, 3, 477–486. [Google Scholar] [CrossRef]
- Snetselaar, L.G.; de Jesus, J.M.; Desilva, D.M.; Stoody, E.E. Dietary Guidelines for Americans, 2020-2025: Understanding the Scientific Process, Guidelines, and Key Recommendations. Nutr. Today 2021, 56, 287–295. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Guideline: Sugars Intake for Adults and Children; WHO Library Cataloguing-in-Publication Data; World Health Organization: Geneva, Switzerland, 2015; Volume 26.
- Raithel, M.; Weidenhiller, M.; Hagel, A.F.K.; Hetterich, U.; Neurath, M.F.; Konturek, P.C. The malabsorption of commonly occurring mono and disaccharides: Levels of investigation and differential diagnoses. Dtsch. Arztebl. Int. 2013, 110, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, S.; Chen, J.; Su, Z. Unraveling the Regulation of Hepatic Gluconeogenesis. Front. Endocrinol. 2019, 9, 802. [Google Scholar] [CrossRef] [PubMed]
- Wood, I.S.; Trayhurn, P. Glucose transporters (GLUT and SGLT): Expanded families of sugar transport proteins. Br. J. Nutr. 2003, 89, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Zwarts, I.; van Zutphen, T.; Kruit, J.K.; Liu, W.; Oosterveer, M.H.; Verkade, H.J.; Uhlenhaut, N.H.; Jonker, J.W. Identification of the fructose transporter GLUT5 (SLC2A5) as a novel target of nuclear receptor LXR. Sci. Rep. 2019, 9, 9299. [Google Scholar] [CrossRef] [PubMed]
- Ferraris, R.P.; Choe, J.-Y.; Patel, C.R. Intestinal Absorption of Fructose. Annu. Rev. Nutr. 2018, 38, 41–67. [Google Scholar] [CrossRef]
- Stanhope, K.L. Critical Reviews in Clinical Laboratory Sciences. Online Journal, 7 September 2015. [Google Scholar]
- Malik, V.S.; Hu, F.B. Fructose and Cardiometabolic Health What the Evidence from Sugar-Sweetened Beverages Tells Us. J. Am. Coll. Cardiol. 2015, 66, 1615–1624. [Google Scholar] [CrossRef]
- Martín, M.G.; Turk, E.; Lostao, M.P.; Kerner, C.; Wright, E.M. Defects in Na+/glucose cotransporter (SGLT1) trafficking and function cause glucose-galactose malabsorption. Nat. Genet. 1996, 12, 216–220. [Google Scholar] [CrossRef]
- Abdullah, A.M.A.; El-Mouzan, M.I.; El Shiekh, O.K.; Al Mazyad, A. Congenital Glucose-Galactose Malabsorption in Arab Children. J. Craniofacial. Surg. 1996, 23, 561–564. [Google Scholar] [CrossRef]
- Lostao, M.P.; Loo, D.D.; Hernell, O.; Meeuwisse, G.; Martin, M.G.; Wright, E.M. The Molecular Basis of Glucose Galactose Malabsorption in a Large Swedish Pedigree. Function 2021, 2, zqab040. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M.; Turk, E.; Martin, M.G. Molecular Basis for Glucose-Galactose Malabsorption. Cell Biochem. Biophys. 2002, 36, 115–122. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Ma, M. Literature review on congenital glucose–galactose malabsorption from 2001 to 2019. J. Paediatr. Child Health 2020, 56, 1779–1784. [Google Scholar] [CrossRef] [PubMed]
- Beyreiss, K.; Hoepffner, W.; Scheerschmidt, G.; Müller, F. Digestion and absorption rates of lactose, glucose, galactose, and fructose in three infants with congenital glucose-galactose malabsorption: Perfusion studies. J. Pediatr. Gastroenterol. Nutr. 1985, 4, 887–892. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.; Grasset, E.; Heyman, M.; Dumontier, A.M.; Beau, J.-P.; Desjeux, J.-F. Congenital Selective Malabsorption of Glucose and Galactose. J. Craniofacial. Surg. 1985, 4, 878–886. [Google Scholar] [CrossRef]
- Lindquist, B.; Meeuwisse, G.W. Chronic Diarrhoea Caused by Monosaccharide Malabsorption. Acta Paediatr. 1962, 51, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.M. Genetic disorders of membrane transport I. Glucose galactose malabsorption. Am. J. Physiol. 1998, 275, G879–G882. [Google Scholar]
- Desjeux, J.F.; Wright, E.M. Thirty years of research on congenital glucose and galactose malabsorption: From phenotype to genotype. Bull. Acad. Natl. Med. 1993, 177, 125–131. [Google Scholar]
- Xin, B.; Wang, H. Multiple sequence variations in SLC5A1 gene are associated with glucose-galactose malabsorption in a large cohort of Old Order Amish. Clin. Genet. 2010, 79, 86–91. [Google Scholar] [CrossRef]
- Gracey, M.; Burke, V. Sugar-induced diarrhoea in children. Arch. Dis. Child. 1973, 48, 331–336. [Google Scholar] [CrossRef]
- Hediger, M.A.; Budarf, M.L.; Emanuel, B.S.; Mohandas, T.; Wright, E.M. Assignment of the human intestinal Na+/glucose cotransporter gene (SGLT1) to the q11.2 → qter region of chromosome 22. Genomics 1989, 4, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Lam, J.T.; Martín, M.G.; Turk, E.; Hirayama, B.A.; Bosshard, N.U.; Steinmann, B.; Wright, E.M. Missense mutations in SGLT1 cause glucose–galactose malabsorption by trafficking defects. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 1999, 1453, 297–303. [Google Scholar] [CrossRef]
- Wright, E.M.; Loo, D.D.F.; Hirayama, B.A. Biology of Human Sodium Glucose Transporters. Physiol. Rev. 2011, 91, 733–794. [Google Scholar] [CrossRef]
- Lebenthal, E.; Garti, R.; Mathoth, Y.; Cohen, B.E.; Katzenelson, D. Glucose-galactose malabsorption in an Oriental-Iraqui Jewish family. J. Pediatr. 1971, 78, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Saadah, O.I.; Alghamdi, S.A.; Sindi, H.H.; Alhunaitti, H.; Bin-Taleb, Y.Y.; Alhussaini, B.H. Congenital glucose–galactose malabsorption: A descriptive study of clinical characteristics and outcome from Western Saudi Arabia. Arab. J. Gastroenterol. 2014, 15, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Pode-Shakked, B.; Reish, O.; Aktuglu-Zeybek, C.; Kesselman, D.; Dekel, B.; Bujanover, Y.; Anikster, Y. Bitterness of glucose/galactose: Novel mutations in the SLC5A1 gene. J. Pediatr. Gastroenterol. Nutr. 2014, 58, 57–60. [Google Scholar] [CrossRef]
- Turk, E.; Zabel, B.; Mundlos, S.; Dyer, J.; Wright, E.M. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 1991, 350, 354–356. [Google Scholar] [CrossRef]
- Wright, E.M.; Martín, M.G.; Turk, E. Intestinal absorption in health and disease – Sugars. Bailliere’s Best Pract. Res. Clin. Gastroenterol. 2003, 17, 943–956. [Google Scholar] [CrossRef]
- Al-Lawati, T.; Vargees, T. Glucose Galactose Malabsorption complicated with Rickets and Nephrogenic Diabetes Insipidus. Oman Med. J. 2008, 23, 197–198. [Google Scholar]
- Fiscaletti, M.; Lebel, M.-J.; Alos, N.; Benoit, G.; Jantchou, P. Two Cases of Mistaken Polyuria and Nephrocalcinosis in Infants with Glucose-Galactose Malabsorption: A Possible Role of 1,25(OH)2D3. Horm. Res. Paediatr. 2017, 87, 277–282. [Google Scholar] [CrossRef]
- Soylu, B.; Ecevit, .; Altınöz, S.; Öztürk, A.A.; Temizkan, A.K.; Maeda, M.; Kasahara, M. Nephrocalcinosis in glucose-galactose malabsorption: Nephrocalcinosis and proximal tubular dysfunction in a young infant with a novel mutation of SGLT1. Eur. J. Pediatr. 2008, 167, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Pahari, A.; Milla, P.J.; Hoff, W.G.V. Neonatal nephrocalcinosis in association with glucose-galactose malabsorption. Pediatr. Nephrol. 2003, 18, 700–702. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, R.; Fujii, T.; Endo, A.; Kudo, T.; Shimizu, T. Congenital glucose-galactose malabsorption diagnosed from macrohematuria in an infant. Pediatr. Int. 2016, 58, 1365–1366. [Google Scholar] [CrossRef]
- Lee, W.S.; Tay, C.G.; Nazrul, N.; Paed, M.; Chai, P.F. A case of neonatal diarrhoea caused by congenital glucose-galactose malabsorption. Med J. Malays. 2009, 64, 83. [Google Scholar]
- Posovszky, C. Congenital intestinal diarrhoeal diseases: A diagnostic and therapeutic challenge. Best Pr. Res. Clin. Gastroenterol. 2016, 30, 187–211. [Google Scholar] [CrossRef] [PubMed]
- Tasic, V.; Slaveska, N.; Blau, N. Nephrolithiasis in a child with glucose-galactose malabsorption. Pediatr. Nephrol. 2004, 19, 244–246. [Google Scholar] [CrossRef]
- El-Naggar, W.; Balfe, J.W.; Barbar, M.; Taha, D. Nephrocalcinosis in glucose-galactose malabsorption, association with renal tubular acidosis. Pediatr. Nephrol. 2005, 20, 1336–1339. [Google Scholar] [CrossRef]
- Gok, F.; Aydin, H.I.; Kurt, I.; Gokcay, E.; Maeda, M.; Kasahara, M. A Novel Mutation of Na+/Glucose Cotransporter in a Turkish Newborn with Congenital Glucose-Galactose Malabsorption. J. Craniofacial. Surg. 2005, 40, 508–511. [Google Scholar] [CrossRef]
- Atay, F.Y.; Derme, T.; Sari, F.N.; Ceylaner, S.; Uras, N.; Ceylaner, G.; Oguz, S.S. Congenital Glucose–Galactose Malabsorption in a Turkish Newborn: A Novel Mutation of Na+/Glucose Cotransporter Gene. Dig. Dis. Sci. 2017, 62, 280–281. [Google Scholar] [CrossRef]
- Kianifar, H.-R.; Talebi, S.; Tavakkol-Afshari, J.; Esmaili, M.; Davachi, B.; Brook, A. D28G mutation in congenital glucose-galactose malabsorption. Arch. Iran. Med. 2007, 10. [Google Scholar]
- Wang, C.-W.; Su, S.-C.; Huang, S.-F.; Huang, Y.-C.; Chan, F.-N.; Kuo, Y.-H.; Hung, M.-W.; Lin, H.-C.; Chang, W.-L.; Chang, T.-C. An Essential Role of cAMP Response Element Binding Protein in Ginsenoside Rg1-Mediated Inhibition of Na+/Glucose Cotransporter 1 Gene Expression. Mol. Pharmacol. 2015, 88, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.G.; Wang, J.; Solorzano-Vargas, R.S.; Lam, J.T.; Turk, E.; Wright, E.M. Regulation of the human Na+-glucose cotransporter gene, SGLT1, by HNF-1 and Sp1. Am. J. Physiol. Liver Physiol. 2000, 278, G591–G603. [Google Scholar] [CrossRef] [PubMed]
- Hannou, S.A.; Haslam, D.E.; McKeown, N.M.; Herman, M.A. Fructose metabolism and metabolic disease. J. Clin. Investig. 2018, 128, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Dushay, J.; Chui, P.C.; Gopalakrishnan, G.S.; Varela–Rey, M.; Crawley, M.; Fisher, F.M.; Badman, M.K.; Chantar, M.L.M.; Maratos–Flier, E. Increased Fibroblast Growth Factor 21 in Obesity and Nonalcoholic Fatty Liver Disease. Gastroenterology 2010, 139, 456–463. [Google Scholar] [CrossRef] [PubMed]
- Dushay, J.R.; Toschi, E.; Mitten, E.K.; Fisher, F.M.; Herman, M.A.; Maratos-Flier, E. Fructose ingestion acutely stimulates circulating FGF21 levels in humans. Mol. Metab. 2015, 4, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Fisher, F.M.; Chui, P.C.; Antonellis, P.J.; Bina, H.A.; Kharitonenkov, A.; Flier, J.S.; Maratos-Flier, E. Obesity Is a Fibroblast Growth Factor 21 (FGF21)-Resistant State. Diabetes 2010, 59, 2781–2789. [Google Scholar] [CrossRef]
- Iizuka, K.; Takeda, J.; Horikawa, Y. Glucose induces FGF21 mRNA expression through ChREBP activation in rat hepatocytes. FEBS Lett. 2009, 583, 2882–2886. [Google Scholar] [CrossRef]
- Neuschwander-Tetri, B.A.; Caldwell, S.H. Nonalcoholic steatohepatitis: Summary of an AASLD Single Topic Conference. Hepatology 2003, 37, 1202–1219. [Google Scholar] [CrossRef]
- Lazo, M.; Clark, J.M. The Epidemiology of Nonalcoholic Fatty Liver Disease: A Global Perspective. Semin. Liver Dis. 2008, 28, 339–350. [Google Scholar] [CrossRef]
- Tran, T.T.; Changsri, C.; Shackleton, C.R.; Poordad, F.F.; Nissen, N.N.; Colquhoun, S.; A Geller, S.; Vierling, J.M.; Martin, P. Living donor liver transplantation: Histological abnormalities found on liver biopsies of apparently healthy potential donors. J. Gastroenterol. Hepatol. 2006, 21, 381–383. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the United States: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, A.; Mu, W.; Roncal, C.; Cheng, K.-Y.; Johnson, R.J.; Scarpace, P.J. Fructose-induced leptin resistance exacerbates weight gain in response to subsequent high-fat feeding. Am. J. Physiol. Integr. Comp. Physiol. 2008, 295, R1370–R1375. [Google Scholar] [CrossRef] [PubMed]
- Kuyumcu, M.S.; Kuyumcu, M.; Kuyumcu, A. Increased fructose consumption may be associated with slow coronary flow. Turk Kardiyol. Dernegi Arsivi-Arch. Turk. Soc. Cardiol. 2020, 48, 2–697. [Google Scholar] [CrossRef] [PubMed]
- Stricker, S.; Rudloff, S.; Geier, A.; Steveling, A.; Roeb, E.; Zimmer, K.-P. Fructose Consumption—Free Sugars and Their Health Effects. Dtsch. Arztebl. Int. 2021, 118, 71–80. [Google Scholar] [CrossRef]
- ter Horst, K.W.; Gilijamse, P.W.; Ackermans, M.T.; Soeters, M.R.; Nieuwdorp, M.; Romijn, J.A.; Serlie, M.J. Impaired insulin action in the liver, but not in adipose tissue or muscle, is a distinct metabolic feature of impaired fasting glucose in obese humans. Metabolism 2016, 65, 757–763. [Google Scholar] [CrossRef]
- ter Horst, K.W.; Schene, M.R.; Holman, R.; A Romijn, J.; Serlie, M.J. Effect of fructose consumption on insulin sensitivity in nondiabetic subjects: A systematic review and meta-analysis of diet-intervention trials. Am. J. Clin. Nutr. 2016, 104, 1562–1576. [Google Scholar] [CrossRef]
- Ledochowski, M.; Widner, B.; Murr, C.; Sperner-Unterweger, B.; Fuchs, D. Fructose malabsorption is associated with decreased plasma tryptophan. Scand. J. Gastroenterol. 2001, 36, 367–371. [Google Scholar] [CrossRef]
- DeChristopher, L.R.; Auerbach, B.J.; Tucker, K.L. High fructose corn syrup, excess-free-fructose, and risk of coronary heart disease among African Americans– the Jackson Heart Study. BMC Nutr. 2020, 6, 70. [Google Scholar] [CrossRef]
- Melchior, C.; Douard, V.; Coëffier, M.; Gourcerol, G. Fructose and irritable bowel syndrome. Nutr. Res. Rev. 2020, 33, 235–243. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alruwaili, N.W.; Alshdayed, F. Fructose Metabolism and Its Effect on Glucose-Galactose Malabsorption Patients: A Literature Review. Diagnostics 2023, 13, 294. https://doi.org/10.3390/diagnostics13020294
Alruwaili NW, Alshdayed F. Fructose Metabolism and Its Effect on Glucose-Galactose Malabsorption Patients: A Literature Review. Diagnostics. 2023; 13(2):294. https://doi.org/10.3390/diagnostics13020294
Chicago/Turabian StyleAlruwaili, Nawaf W., and Fahad Alshdayed. 2023. "Fructose Metabolism and Its Effect on Glucose-Galactose Malabsorption Patients: A Literature Review" Diagnostics 13, no. 2: 294. https://doi.org/10.3390/diagnostics13020294
APA StyleAlruwaili, N. W., & Alshdayed, F. (2023). Fructose Metabolism and Its Effect on Glucose-Galactose Malabsorption Patients: A Literature Review. Diagnostics, 13(2), 294. https://doi.org/10.3390/diagnostics13020294






