Estimation of the Differential Pathlength Factor for Human Skin Using Monte Carlo Simulations
Abstract
1. Introduction
2. Methods
2.1. Human Skin Model
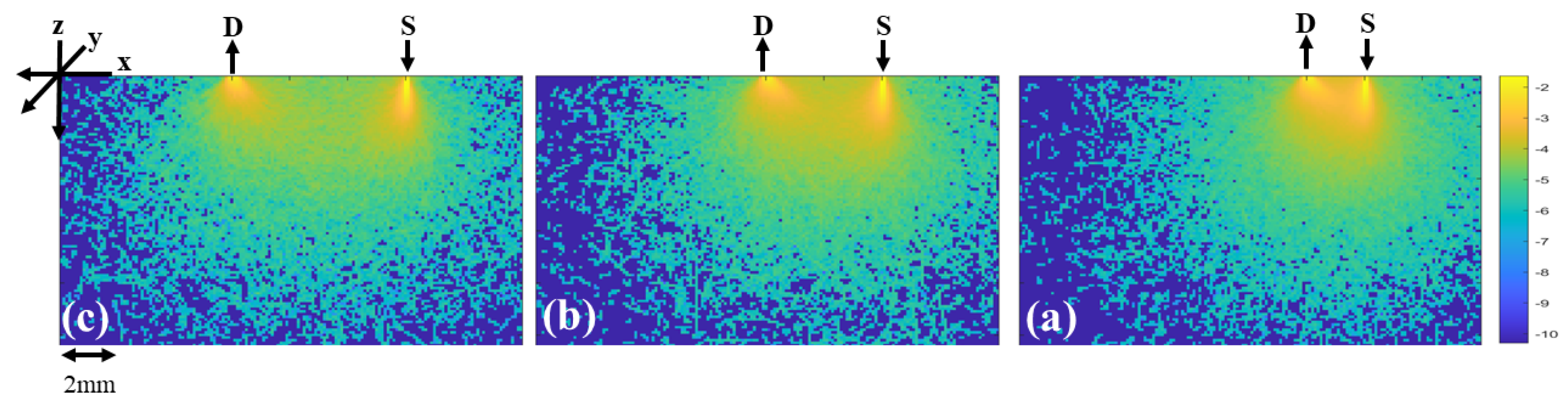
2.2. Monte Carlo Simulation
3. Results and Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boas, D.; Brooks, D.; Miller, E.; DiMarzio, C.; Kilmer, M.; Gaudette, R.; Zhang, Q. Imaging the body with diffuse optical tomography. IEEE Signal Process. Mag. 2001, 18, 57–75. [Google Scholar] [CrossRef]
- Guven, M.; Yazici, B.; Intes, X.; Chance, B. Diffuse optical tomography with a priori anatomical information. Phys. Med. Biol. 2005, 50, 2837–2858. [Google Scholar] [CrossRef] [PubMed]
- Üncü, Y.A.; Sevim, G.; Canpolat, M. Approaches to preclinical studies with heterogeneous breast phantom using reconstruction and three-dimensional image processing algorithms for diffuse optical imaging. Int. J. Imaging Syst. Technol. 2022, 32, 343–353. [Google Scholar] [CrossRef]
- Üncü, Y.A.; Sevim, G.; Mercan, T.; Vural, V.; Durmaz, E.; Canpolat, M. Differentiation of tumoral and non-tumoral breast lesions using back reflection diffuse optical tomography: A pilot clinical study. Int. J. Imaging Syst. Technol. 2021, 31, 2023–2031. [Google Scholar] [CrossRef]
- Uddin, K.M.S.; Zhang, M.; Anastasio, M.; Zhu, Q. Optimal breast cancer diagnostic strategy using combined ultrasound and diffuse optical tomography. Biomed. Opt. Express 2020, 11, 2722–2737. [Google Scholar] [CrossRef]
- Chae, E.Y.; Kim, H.H.; Sabir, S.; Kim, Y.; Kim, H.; Yoon, S.; Ye, J.C.; Cho, S.; Heo, D.; Kim, K.H.; et al. Development of digital breast tomosynthesis and diffuse optical tomography fusion imaging for breast cancer detection. Sci. Rep. 2020, 10, 13127. [Google Scholar] [CrossRef]
- Vavadi, H.; Mostafa, A.; Zhou, F.; Uddin, K.M.S.; Althobaiti, M.; Xu, C.; Bansal, R.; Ademuyiwa, F.; Poplack, S.; Zhu, Q. Compact ultrasound-guided diffuse optical tomography system for breast cancer imaging. J. Biomed. Opt. 2019, 24, 021203. [Google Scholar] [CrossRef]
- Althobaiti, M.; Vavadi, H.; Zhu, Q. An Automated Preprocessing Method for Diffuse Optical Tomography to Improve Breast Cancer Diagnosis. Technol. Cancer Res. Treat. 2018, 17, 1533033818802791. [Google Scholar] [CrossRef]
- Zhang, Q.; Brukilacchio, T.J.; Li, A.; Stott, J.; Chaves, T.; Hillman, E.M.C.; Wu, T.; Chorlton, M.; Rafferty, E.; Moore, R.H.; et al. Coregistered tomographic X-ray and optical breast imaging: Initial results. J. Biomed. Opt. 2005, 10, 24033–240339. [Google Scholar] [CrossRef]
- Brooksby, B.; Jiang, S.; Dehghani, H.; Pogue, B.; Paulsen, K.D.; Weaver, J.; Kogel, C.; Poplack, S.P. Combining near-infrared tomography and magnetic resonance imaging to study in vivo breast tissue: Implementation of a Laplacian-type regularization to incorporate magnetic resonance structure. J. Biomed. Opt. 2005, 10, 51504–51510. [Google Scholar] [CrossRef]
- Gibson, A.; Hebden, J.C.; Arridge, S. Recent advances in diffuse optical imaging. Phys. Med. Biol. 2005, 50, R1–R43. [Google Scholar] [CrossRef]
- Althobaiti, M.; Al-Naib, I. Recent Developments in Instrumentation of Functional Near-Infrared Spectroscopy Systems. Appl. Sci. 2020, 10, 6522. [Google Scholar] [CrossRef]
- Ortega, P.; Faisal, A.A. Deep learning multimodal fNIRS and EEG signals for bimanual grip force decoding. J. Neural Eng. 2021, 18, 0460e6. [Google Scholar] [CrossRef]
- Khan, R.A.; Naseer, N.; Qureshi, N.K.; Noori, F.M.; Nazeer, H.; Khan, M.U. FNIRS-based Neurorobotic Interface for gait rehabilitation. J. Neuroeng. Rehabil. 2018, 15, 7. [Google Scholar] [CrossRef]
- Rea, M.; Rana, M.; Lugato, N.; Terekhin, P.; Gizzi, L.; Brötz, D.; Caria, A. Lower limb movement preparation in chronic stroke: A pilot study toward an fnirs-bci for gait rehabilitation. Neurorehabil. Neural Repair 2014, 28, 564–575. [Google Scholar] [CrossRef]
- Almulla, L.; Al-Naib, I.; Ateeq, I.S.; Althobaiti, M. Observation and motor imagery balance tasks evaluation: An fNIRS feasibility study. PLoS ONE 2022, 17, e0265898. [Google Scholar] [CrossRef]
- Delbeck, S.; Vahlsing, T.; Leonhardt, S.; Steiner, G.; Heise, H.M. Non-invasive monitoring of blood glucose using optical methods for skin spectroscopy—Opportunities and recent advances. Anal. Bioanal. Chem. 2019, 411, 63–77. [Google Scholar] [CrossRef]
- Alsunaidi, B.; Althobaiti, M.; Tamal, M.; Albaker, W.; Al-Naib, I. A Review of Non-Invasive Optical Systems for Continuous Blood Glucose Monitoring. Sensors 2021, 21, 6820. [Google Scholar] [CrossRef]
- Rachim, V.P.; Chung, W.-Y. Wearable-band type visible-near infrared optical biosensor for non-invasive blood glucose monitoring. Sensors Actuators B Chem. 2019, 286, 173–180. [Google Scholar] [CrossRef]
- Haxha, S.; Jhoja, J. Optical Based Noninvasive Glucose Monitoring Sensor Prototype. IEEE Photon. J. 2016, 8, 1–11. [Google Scholar] [CrossRef]
- Althobaiti, M.; Al-Naib, I. Optimization of Dual-Channel Near-Infrared Non-Invasive Glucose Level Measurement Sensors Based On Monte-Carlo Simulations. IEEE Photon. J. 2021, 13, 1–9. [Google Scholar] [CrossRef]
- Shokrekhodaei, M.; Quinones, S. Review of non-invasive glucose sensing techniques: Optical, electrical and breath acetone. Sensors 2020, 20, 1251. [Google Scholar] [CrossRef] [PubMed]
- Fantini, S.; Sassaroli, A. Frequency-Domain Techniques for Cerebral and Functional Near-Infrared Spectroscopy. Front. Neurosci. 2020, 14, 300. [Google Scholar] [CrossRef] [PubMed]
- Althobaiti, M. In Silico Investigation of SNR and Dermis Sensitivity for Optimum Dual-Channel Near-Infrared Glucose Sensor Designs for Different Skin Colors. Biosensors 2022, 12, 805. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.M.; Jain, P.; Mohanty, S.P.; Agrawal, N. iGLU 2.0: A New Wearable for Accurate Non-Invasive Continuous Serum Glucose Measurement in IoMT Framework. IEEE Trans. Consum. Electron. 2020, 66, 327–335. [Google Scholar] [CrossRef]
- Kocsis, L.; Herman, P.; Eke, A. The modified Beer-Lambert law revisited. Phys. Med. Biol. 2006, 51, N91. [Google Scholar] [CrossRef]
- Delpy, D.T.; Cope, M.; Van Der Zee, P.; Arridge, S.; Wray, S.; Wyatt, J. Estimation of optical pathlength through tissue from direct time of flight measurement. Phys. Med. Biol. 1988, 33, 1433–1442. [Google Scholar] [CrossRef]
- Chatterjee, S.; Abay, T.Y.; Phillips, J.P.; Kyriacou, P.A. Investigating optical path and differential pathlength factor in reflectance photoplethysmography for the assessment of perfusion. J. Biomed. Opt. 2018, 23, 075005. [Google Scholar] [CrossRef]
- Duncan, A.; Meek, J.H.; Clemence, M.; E Elwell, C.; Tyszczuk, L.; Cope, M.; Delpy, D. Optical pathlength measurements on adult head, calf and forearm and the head of the newborn infant using phase resolved optical spectroscopy. Phys. Med. Biol. 1995, 40, 295–304. [Google Scholar] [CrossRef]
- Chatterjee, S.; Kyriacou, P.A. Estimating the Dependence of Differential Pathlength Factor on Blood Volume and Oxygen Saturation using Monte Carlo method. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Delpy, D.T.; Cope, M. Quantification in tissue near–infrared spectroscopy. Philos. Trans. R. Soc. B Biol. Sci. 1997, 352, 649–659. [Google Scholar] [CrossRef]
- Scholkmann, F.; Wolf, M. General equation for the differential pathlength factor of the frontal human head depending on wavelength and age. J. Biomed. Opt. 2013, 18, 105004. [Google Scholar] [CrossRef]
- Kohl, M.; Nolte, C.; Heekeren, H.; Horst, S.; Scholz, U.; Obrig, H.; Villringer, A. Determination of the wavelength dependence of the differential pathlength factor from near-infrared pulse signals. Phys. Med. Biol. 1998, 43, 1771–1782. [Google Scholar] [CrossRef]
- Boas, D.A.; Gaudette, T.; Strangman, G.; Cheng, X.; Marota, J.J.A.; Mandeville, J.B. The accuracy of near infrared spectroscopy and imaging during focal changes in cerebral hemodynamics. Neuroimage 2001, 13, 76–90. [Google Scholar] [CrossRef]
- Uludağ, K.; Steinbrink, J.; Villringer, A.; Obrig, H. Separability and cross talk: Optimizing dual wavelength combinations for near-infrared spectroscopy of the adult head. Neuroimage 2004, 22, 583–589. [Google Scholar] [CrossRef]
- Jacques, S.L. Optical properties of biological tissues: A review. Phys. Med. Biol. 2013, 58, R37. [Google Scholar] [CrossRef]
- Meglinski, I.; Matcher, S. Computer simulation of the skin reflectance spectra. Comput. Methods Programs Biomed. 2003, 70, 179–186. [Google Scholar] [CrossRef]
- Nishidate, I.; Aizu, Y.; Mishina, H. Estimation of melanin and hemoglobin in skin tissue using multiple regression analysis aided by Monte Carlo simulation. J. Biomed. Opt. 2004, 9, 700–710. [Google Scholar] [CrossRef]
- Chatterjee, S.; Budidha, K.; A Kyriacou, P. Investigating the origin of photoplethysmography using a multiwavelength Monte Carlo model. Physiol. Meas. 2020, 41, 084001. [Google Scholar]
- Kessoku, S.; Maruo, K.; Okawa, S.; Masamoto, K.; Yamada, Y. Influence of blood glucose level on the scattering coefficient of the skin in near-infrared spectroscopy. In ASME/JSME 2011 8th Thermal Engineering Joint Conference, AJTEC 2011; ASME: Honolulu, HI, USA, 2011. [Google Scholar]
- Fang, Q.; Boas, D.A. Monte Carlo simulation of photon migration in 3D turbid media accelerated by graphics processing units. Opt. Express 2009, 17, 20178–20190. [Google Scholar] [CrossRef]
- Yan, S.; Fang, Q. Hybrid mesh and voxel based Monte Carlo algorithm for accurate and efficient photon transport modeling in complex bio-tissues. Biomed. Opt. Express 2020, 11, 6262–6270. [Google Scholar]
- Brigadoi, S.; Cooper, R. How short is short? Optimum source–detector distance for short-separation channels in functional near-infrared spectroscopy. Neurophotonics 2015, 2, 025005. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, L.; Doğan, Z.; Fang, Q. Graphics processing units-accelerated adaptive nonlocal means filter for denoising three-dimensional Monte Carlo photon transport simulations. J. Biomed. Opt. 2018, 23, 121618. [Google Scholar] [CrossRef]
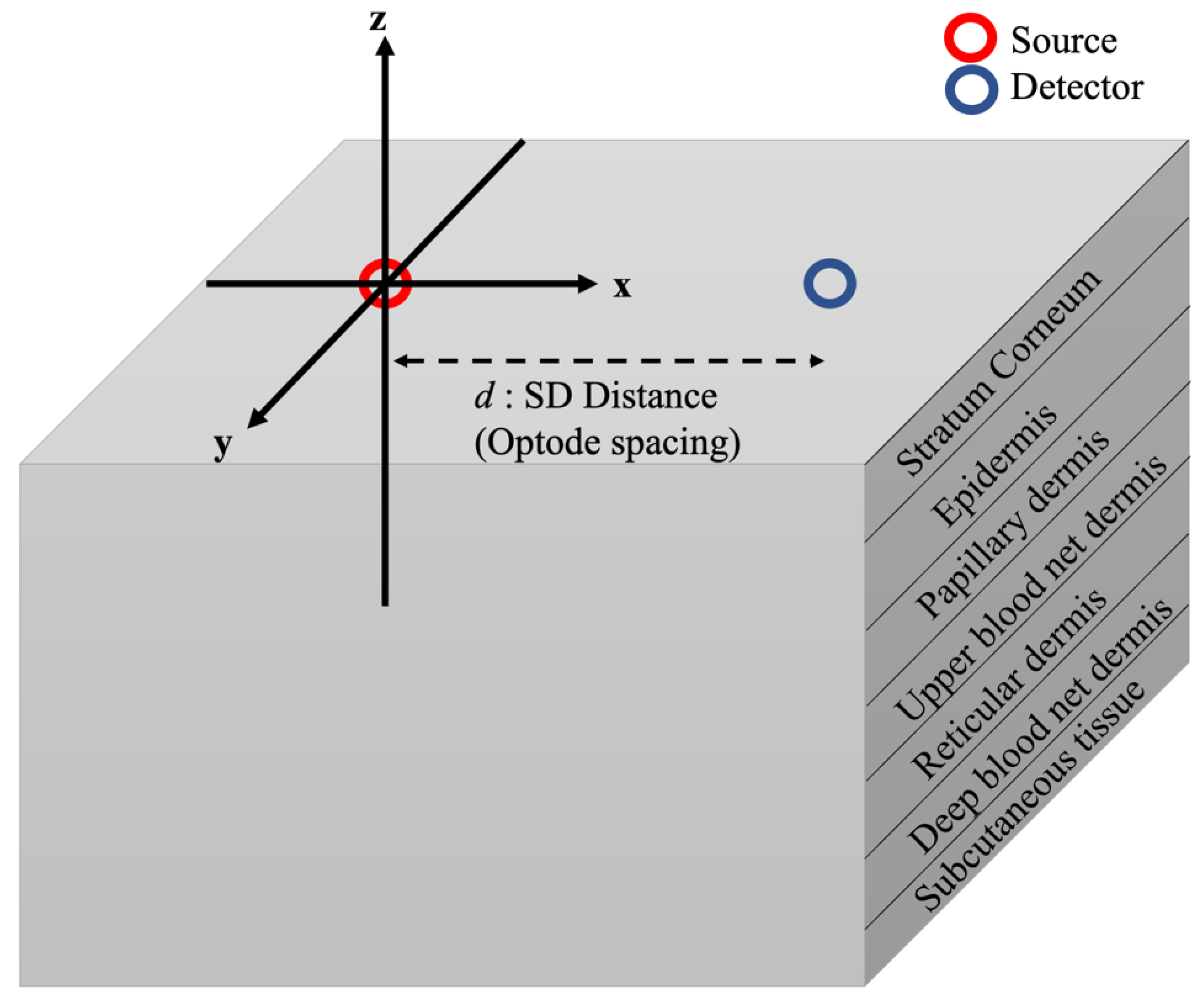
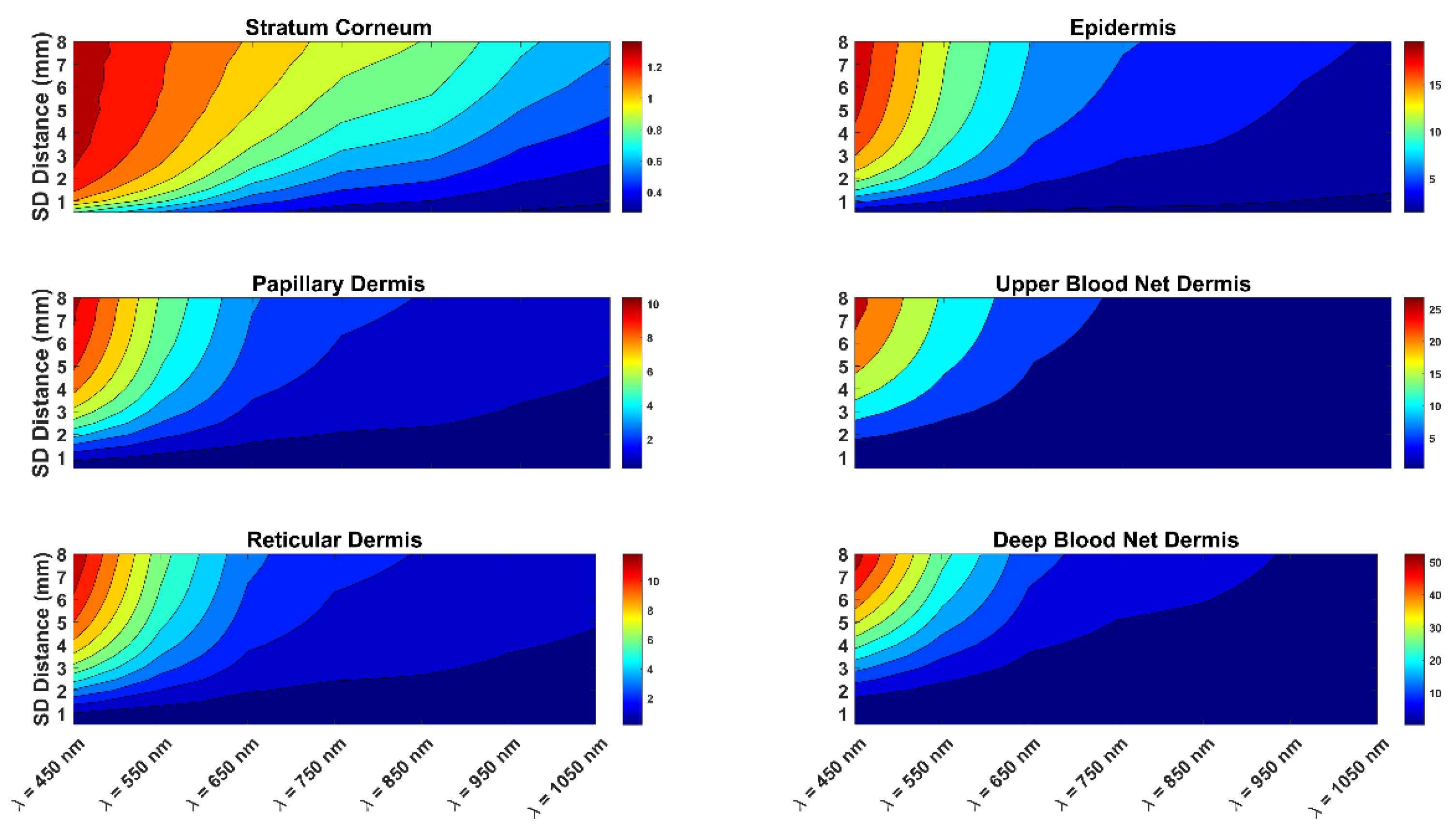

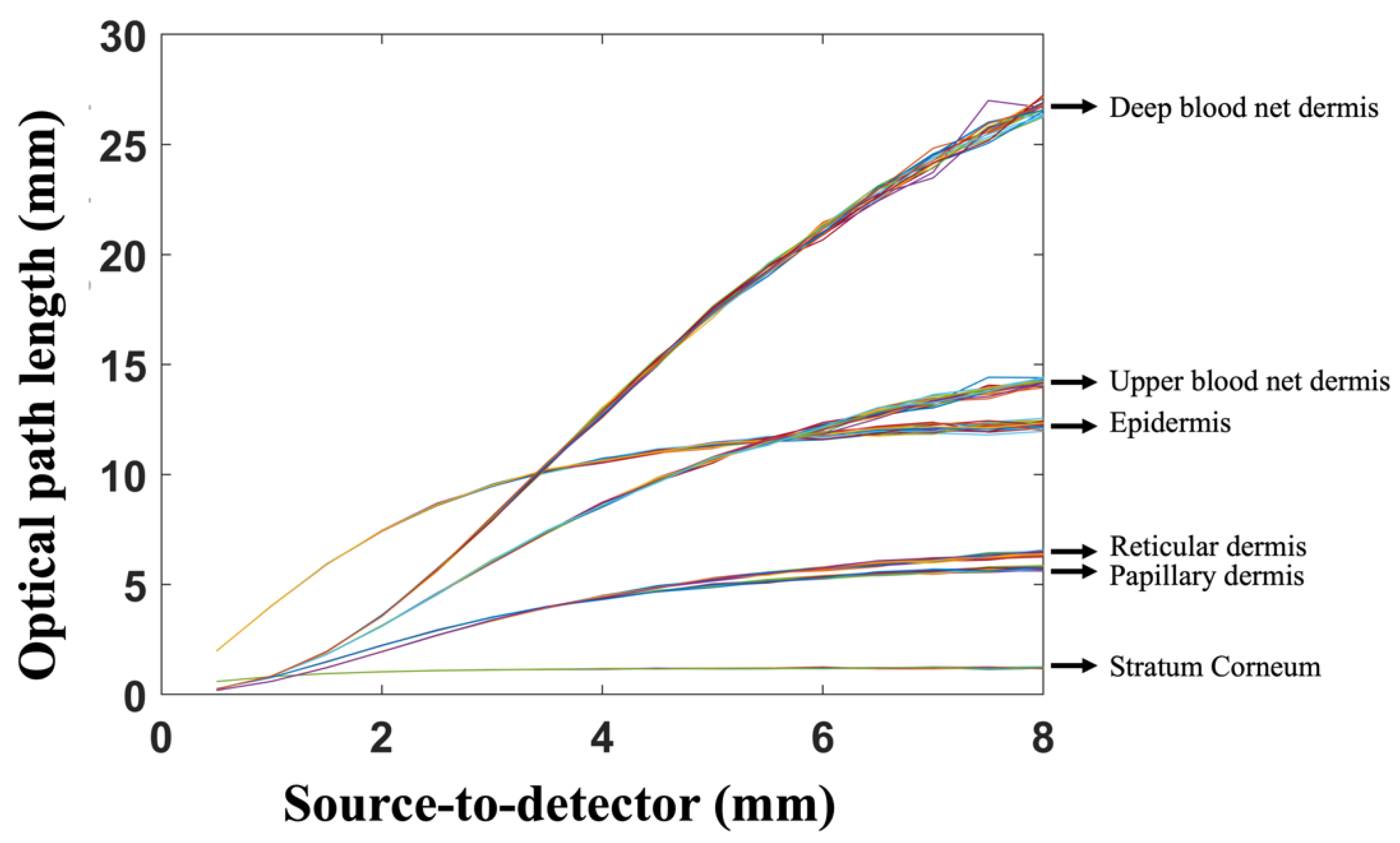
| Skin Layer | Thickness (mm) | Scattering Coefficient (1/mm) | Vblood | VH2O | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stratum Corneum | 0.02 | 60.61 | 41.74 | 22.33 | 15.05 | 13.32 | 9.5 | 7.52 | 0 | 0.05 |
| Epidermis | 0.25 | 0 | 0.2 | |||||||
| Papillary dermis | 0.1 | 0.04 | 0.5 | |||||||
| Upper blood net dermis | 0.08 | 0.3 | 0.6 | |||||||
| Reticular dermis | 0.2 | 0.04 | 0.7 | |||||||
| Deep blood net dermis | 0.3 | 0.1 | 0.7 | |||||||
| Subcutaneous tissue | 2 | 0.05 | 0.7 | |||||||
| SDS (mm) | |||||||
|---|---|---|---|---|---|---|---|
| 0.5 | 9.327229 | 7.613623 | 7.734551 | 8.445864 | 8.731699 | 9.321385 | 9.629079 |
| 1 | 14.26636 | 9.017028 | 6.849151 | 6.742956 | 6.827569 | 7.24332 | 7.628147 |
| 1.5 | 18.92219 | 10.79773 | 6.926007 | 6.171503 | 6.097595 | 6.192436 | 6.473498 |
| 2 | 23.20217 | 12.52319 | 7.222662 | 5.991458 | 5.791009 | 5.613065 | 5.772583 |
| 2.5 | 26.85853 | 14.18709 | 7.579191 | 5.985923 | 5.645795 | 5.265959 | 5.288481 |
| 3 | 30.09627 | 15.64019 | 7.971814 | 6.028839 | 5.61251 | 5.041451 | 4.947292 |
| 3.5 | 32.83371 | 16.98709 | 8.331424 | 6.102681 | 5.596167 | 4.896995 | 4.705665 |
| 4 | 35.20655 | 18.15731 | 8.667086 | 6.193406 | 5.623161 | 4.793538 | 4.519836 |
| 4.5 | 37.30747 | 19.13173 | 9.021267 | 6.297507 | 5.662255 | 4.73143 | 4.384798 |
| 5 | 39.06324 | 20.0042 | 9.277562 | 6.376903 | 5.692662 | 4.65514 | 4.272771 |
| 5.5 | 40.29488 | 20.77222 | 9.541198 | 6.439553 | 5.731603 | 4.625515 | 4.171025 |
| 6 | 41.39538 | 21.38013 | 9.748879 | 6.525236 | 5.773241 | 4.581958 | 4.096541 |
| 6.5 | 42.31183 | 22.13103 | 9.885177 | 6.558909 | 5.777782 | 4.535969 | 4.004704 |
| 7 | 43.00748 | 22.39849 | 10.01952 | 6.623501 | 5.797535 | 4.50906 | 3.953183 |
| 7.5 | 43.06239 | 22.64816 | 10.16962 | 6.62868 | 5.828102 | 4.474714 | 3.897391 |
| 8 | 43.21896 | 22.80911 | 10.2432 | 6.636324 | 5.813229 | 4.445111 | 3.844256 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Althobaiti, M. Estimation of the Differential Pathlength Factor for Human Skin Using Monte Carlo Simulations. Diagnostics 2023, 13, 309. https://doi.org/10.3390/diagnostics13020309
Althobaiti M. Estimation of the Differential Pathlength Factor for Human Skin Using Monte Carlo Simulations. Diagnostics. 2023; 13(2):309. https://doi.org/10.3390/diagnostics13020309
Chicago/Turabian StyleAlthobaiti, Murad. 2023. "Estimation of the Differential Pathlength Factor for Human Skin Using Monte Carlo Simulations" Diagnostics 13, no. 2: 309. https://doi.org/10.3390/diagnostics13020309
APA StyleAlthobaiti, M. (2023). Estimation of the Differential Pathlength Factor for Human Skin Using Monte Carlo Simulations. Diagnostics, 13(2), 309. https://doi.org/10.3390/diagnostics13020309






