Approach to Imaging of Mediastinal Masses
Abstract
:1. Introduction
2. Imaging Modalities
Prevascular (Anterior) Compartment
3. Cystic Lesions
4. Thymic Hyperplasia
5. Thymic Epithelial Neoplasms
6. Lymphoma
7. Germ Cell Tumors
Visceral (Middle) Compartment
8. Cystic Lesions
9. Hypervascular Lesions
10. Esophageal Lesions
Paravertebral (Posterior) Compartment
11. Conclusions
Funding
Conflicts of Interest
References
- Carter, B.W.; Tomiyama, N.; Bhora, F.Y.; de Christenson, M.L.R.; Nakajima, J.; Boiselle, P.M.; Detterbeck, F.C.; Marom, E.M. A modern definition of mediastinal compartments. J. Thorac. Oncol. 2014, 9, S97–S101. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.W.; Benveniste, M.F.; Madan, R.; Godoy, M.C.; De Groot, P.M.; Truong, M.T.; Rosado-De-Christenson, M.L.; Marom, E.M. ITMIG Classification of Mediastinal Compartments and Multidisciplinary Approach to Mediastinal Masses. Radiographics 2017, 37, 413–436. [Google Scholar] [CrossRef] [PubMed]
- Bhalla, S.; Marom, E. Approach to Imaging of Mediastinal Conditions in the Adult. In Diseases of the Chest, Breast, Heart and Vessels 2019–2022: Diagnostic and Interventional Imaging; Hodler, J., Kubik-Huch, R.A., von Schulthess, G.K., Eds.; Springer: Cham, Switzerland, 2019; pp. 27–35. [Google Scholar]
- Tomiyama, N. Approach to the prevascular mass. Mediastinum 2019, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Kalra, K.; Rastogi, S.; Sidhu, H.S. Clinical approach to childhood mediastinal tumors and management. Mediastinum 2020, 4, 21. [Google Scholar] [CrossRef] [PubMed]
- D’Cruz, I.A.; Feghali, N.; Gross, C.M. Echocardiographic manifestations of mediastinal masses compressing or encroaching on the heart. Echocardiography 1994, 11, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Hovgaard, H.L.; Nielsen, R.R.; Laursen, C.B.; Frederiksen, C.A.; Juhl-Olsen, P. When appearances deceive: Echocardiographic changes due to common chest pathology. Echocardiography 2018, 35, 1847–1859. [Google Scholar] [CrossRef] [PubMed]
- Garrana, S.H.; Rosado-de-Christenson, M.L. Imaging of the Anterior/Prevascular Mediastinum. Radiol. Clin. N. Am. 2021, 59, 155–168. [Google Scholar] [CrossRef]
- Expert Panel on Thoracic Imaging; Ackman, J.B.; Chung, J.H.; Walker, C.M.; Bang, T.J.; Carter, B.W.; Hobbs, S.B.; Kandathil, A.; Lanuti, M.; Madan, R.; et al. ACR Appropriateness Criteria(R) Imaging of Mediastinal Masses. J. Am. Coll. Radiol. 2021, 18, S37–S51. [Google Scholar] [CrossRef]
- Carter, B.W.; Betancourt, S.L.; Benveniste, M.F. MR Imaging of Mediastinal Masses. Top. Magn. Reson. Imaging 2017, 26, 153–165. [Google Scholar] [CrossRef]
- Ackman, J.B. MR Imaging of Mediastinal Masses. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 141–164. [Google Scholar] [CrossRef]
- Carter, B.W.; Lichtenberger, J.P., 3rd; Benveniste, M.F. MR Imaging of Thymic Epithelial Neoplasms. Top. Magn. Reson. Imaging 2018, 27, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zeman, M.N.; Akin, E.A.; Merryman, R.W.; Jacene, H.A. Interim FDG-PET/CT for Response Assessment of Lymphoma. Semin. Nucl. Med. 2023, 53, 371–388. [Google Scholar] [CrossRef] [PubMed]
- Zanoni, L.; Bezzi, D.; Nanni, C.; Paccagnella, A.; Farina, A.; Broccoli, A.; Casadei, B.; Zinzani, P.L.; Fanti, S. PET/CT in Non-Hodgkin Lymphoma: An Update. Semin. Nucl. Med. 2023, 53, 320–351. [Google Scholar] [CrossRef] [PubMed]
- Taka, M.; Kobayashi, S.; Mizutomi, K.; Inoue, D.; Takamatsu, S.; Gabata, T.; Matsumoto, I.; Ikeda, H.; Kobayashi, T.; Minato, H.; et al. Diagnostic approach for mediastinal masses with radiopathological correlation. Eur. J. Radiol. 2023, 162, 110767. [Google Scholar] [CrossRef] [PubMed]
- Prosch, H.; Rohrich, S.; Tekin, Z.N.; Ebner, L. The role of radiological imaging for masses in the prevascular mediastinum in clinical practice. J. Thorac. Dis. 2020, 12, 7591–7597. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.W.; Benveniste, M.F.; Marom, E.M. Diagnostic approach to the anterior/prevascular mediastinum for radiologists. Mediastinum 2019, 3, 18. [Google Scholar] [CrossRef] [PubMed]
- Nakazono, T.; Yamaguchi, K.; Egashira, R.; Mizuguchi, M.; Irie, H. Anterior mediastinal lesions: CT and MRI features and differential diagnosis. Jpn. J. Radiol. 2021, 39, 101–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, K.; Li, X.; Li, Y.; Yang, F.; Li, J.; Jiang, G.; Liu, J.; Wang, J. Clinical features, diagnosis and thoracoscopic surgical treatment of thymic cysts. J. Thorac. Dis. 2017, 9, 5203–5211. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Rojas, C.A. Imaging modalities (MRI, CT, PET/CT), indications, differential diagnosis and imaging characteristics of cystic mediastinal masses: A review. Mediastinum 2023, 7, 3. [Google Scholar] [CrossRef]
- Oramas, D.M.; Moran, C.A. Multilocular thymic cyst (MTC) and other tumors with MTC features: Pitfalls in diagnosis. Semin. Diagn. Pathol. 2022, 39, 105–112. [Google Scholar] [CrossRef]
- Carter, B.W.; Benveniste, M.F.; Truong, M.T.; Marom, E.M. State of the Art: MR Imaging of Thymoma. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Jeong, W.G.; Lee, J.E.; Lee, H.-J.; Ki, S.Y.; Lee, B.C.; Kim, H.O.; Kim, S.K.; Heo, S.H.; Lim, H.S.; et al. Pictorial Review of Mediastinal Masses with an Emphasis on Magnetic Resonance Imaging. Korean J. Radiol. 2021, 22, 139–154. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choe, J.; Kim, H.K.; Lee, H.Y. MRI-Based Stepwise Approach to Anterior Mediastinal Cystic Lesions for Diagnosis and Further Management. Korean J. Radiol. 2023, 24, 62–78. [Google Scholar] [CrossRef] [PubMed]
- Varma, V.; Alabousi, A.; Burute, N.; Haider, E. Thymic masses and mimics in adults: Review of common and uncommon pathologies. Clin. Imaging 2021, 77, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Gentili, F.; Pelini, V.; Lucii, G.; Luzzi, L.; Mazzei, F.G.; Fausto, A.; Volterrani, L.; Mazzei, M.A. Update in diagnostic imaging of the thymus and anterior mediastinal masses. Gland. Surg. 2019, 8, S188–S207. [Google Scholar] [CrossRef] [PubMed]
- Guleria, P.; Jain, D. Thymic lesions of the paediatric age group: A comprehensive review of non-neoplastic and neoplastic etiologies. Mediastinum 2019, 3, 24. [Google Scholar] [CrossRef]
- Marx, A.; Chan, J.K.; Chalabreysse, L.; Dacic, S.; Detterbeck, F.; French, C.A.; Hornick, J.L.; Inagaki, H.; Jain, D.; Lazar, A.J.; et al. The 2021 WHO Classification of Tumors of the Thymus and Mediastinum: What Is New in Thymic Epithelial, Germ Cell, and Mesenchymal Tumors? J. Thorac. Oncol. 2022, 17, 200–213. [Google Scholar] [CrossRef]
- Lichtenberger, J.P., 3rd; Carter, B.W.; Fisher, D.A.; Parker, R.F.; Peterson, P.G. Thymic Epithelial Neoplasms: Radiologic-Pathologic Correlation. Radiol. Clin. N. Am. 2021, 59, 169–182. [Google Scholar] [CrossRef]
- Benveniste, M.F.K.; Betancourt Cuellar, S.L.; Carter, B.W.; Strange, C.D.; Marom, E.M. Thymic Epithelial Neoplasms: Tumor-Node-Metastasis Staging. Radiol. Clin. N. Am. 2021, 59, 183–192. [Google Scholar] [CrossRef]
- Priola, A.M.; Priola, S.M.; Di Franco, M.; Cataldi, A.; Durando, S.; Fava, C. Computed tomography and thymoma: Distinctive findings in invasive and noninvasive thymoma and predictive features of recurrence. Radiol. Med. 2010, 115, 1–21. [Google Scholar] [CrossRef]
- Strange, C.D.; Ahuja, J.; Shroff, G.S.; Truong, M.T.; Marom, E.M. Imaging Evaluation of Thymoma and Thymic Carcinoma. Front. Oncol. 2021, 11, 810419. [Google Scholar] [CrossRef] [PubMed]
- Calbiyik, M.; Zehir, S. Teratomas from past to the present: A scientometric analysis with global productivity and research trends between 1980 and 2022. Medicine 2023, 102, e34208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Sohani, A.R. Lymphomas of the Mediastinum and Their Differential Diagnosis. Semin. Diagn. Pathol. 2020, 37, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Dong, X.; Tu, M.; Wang, H. Primary mediastinal large B cell lymphoma. Thorac. Cancer 2021, 12, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Priola, A.M.; Galetto, G.; Priola, S.M. Diagnostic and functional imaging of thymic and mediastinal involvement in lymphoproliferative disorders. Clin. Imaging 2014, 38, 771–784. [Google Scholar] [CrossRef] [PubMed]
- Pina-Oviedo, S.; Moran, C.A. Primary Mediastinal Nodal and Extranodal Non-Hodgkin Lymphomas: Current Concepts, Historical Evolution, and Useful Diagnostic Approach: Part 1. Adv. Anat. Pathol. 2019, 26, 346–370. [Google Scholar] [CrossRef] [PubMed]
- Pfau, D.; Smith, D.A.; Beck, R.; Gilani, K.A.; Gupta, A.; Caimi, P.; Ramaiya, N.H. Primary Mediastinal Large B-Cell Lymphoma: A Review for Radiologists. AJR Am. J. Roentgenol. 2019, 213, W194–W210. [Google Scholar] [CrossRef] [PubMed]
- Mallick, S.; Jain, S.; Ramteke, P. Pediatric mediastinal lymphoma. Mediastinum 2020, 4, 22. [Google Scholar] [CrossRef]
- McCarten, K.M.; Nadel, H.R.; Shulkin, B.L.; Cho, S.Y. Imaging for diagnosis, staging and response assessment of Hodgkin lymphoma and non-Hodgkin lymphoma. Pediatr. Radiol. 2019, 49, 1545–1564. [Google Scholar] [CrossRef]
- Rosti, G.; Secondino, S.; Necchi, A.; Fornarini, G.; Pedrazzoli, P. Primary mediastinal germ cell tumors. Semin. Oncol. 2019, 46, 107–111. [Google Scholar] [CrossRef]
- El-Zaatari, Z.M.; Ro, J.Y. Mediastinal Germ Cell Tumors: A Review and Update on Pathologic, Clinical, and Molecular Features. Adv. Anat. Pathol. 2021, 28, 335–350. [Google Scholar] [CrossRef]
- Pini, G.M.; Colecchia, M. Mediastinal germ cell tumors: A narrative review of their traits and aggressiveness features. Mediastinum 2022, 6, 5. [Google Scholar] [CrossRef]
- Sohn, A.; Moran, C.A. Primary mediastinal germ cell tumors. Semin. Diagn. Pathol. 2023, 40, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, A.; Kaur, K.; Verma, A. Pediatric mediastinal germ cell tumors. Mediastinum 2019, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Patnaik, S.; Malempati, A.R.; Uppin, M.; Susarla, R. Rare mediastinal masses-imaging review. J. Cancer Res. Ther. 2021, 17, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ataya, J.; Nahle, A.A.; Hamdar, H.; Sikaria, A.; Souleiman, Y. Mediastinal liposarcoma: A case report and review of the literature. J. Med. Case Rep. 2023, 17, 372. [Google Scholar] [CrossRef] [PubMed]
- Bourgouin, P.P.; Madan, R. Imaging of the Middle and Visceral Mediastinum. Radiol. Clin. N. Am. 2021, 59, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, Y.; Hiroshima, M.; Maki, H.; Hara, M.; Shibamoto, Y. Imaging findings of lesions in the middle and posterior mediastinum. Jpn. J. Radiol. 2021, 39, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Wahi, J.E.; Safdie, F.M. Esophageal duplication cysts: A clinical practice review. Mediastinum 2023, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.L.; Zayas, G.E.; Abel, M.D.; Young, W.F., Jr.; Schaff, H.V. Mediastinal paragangliomas: The mayo clinic experience. Ann. Thorac. Surg. 2008, 86, 946–951. [Google Scholar] [CrossRef]
- Balcombe, J.; Torigian, D.A.; Kim, W.; Miller, W.T., Jr. Cross-sectional imaging of paragangliomas of the aortic body and other thoracic branchiomeric paraganglia. AJR Am. J. Roentgenol. 2007, 188, 1054–1058. [Google Scholar] [CrossRef] [PubMed]
- McAdams, H.P.; Rosado-de-Christenson, M.; Fishback, N.F.; Templeton, P.A. Castleman disease of the thorax: Radiologic features with clinical and histopathologic correlation. Radiology 1998, 209, 221–228. [Google Scholar] [CrossRef] [PubMed]
- Dispenzieri, A.; Fajgenbaum, D.C. Overview of Castleman disease. Blood 2020, 135, 1353–1364. [Google Scholar] [CrossRef] [PubMed]
- Haap, M.; Wiefels, J.; Horger, M.; Hoyer, A.; Mussig, K. Clinical, laboratory and imaging findings in Castleman’s disease-The subtype decides. Blood Rev. 2018, 32, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bonekamp, D.; Horton, K.M.; Hruban, R.H.; Fishman, E.K. Castleman disease: The great mimic. Radiographics 2011, 31, 1793–1807. [Google Scholar] [CrossRef] [PubMed]
- Din, F.; Mellor, F.; Millard, T.; Pace, E.; Khan, N.; Attygalle, A.; Cunningham, D.; Zafar, S.; Sharma, B. Radiology of Castleman disease: The pivotal role of imaging in diagnosis, staging, and response assessment of this rare entity. Clin. Radiol. 2022, 77, 399–408. [Google Scholar] [CrossRef] [PubMed]
- Lichtenberger, J.P., 3rd; Zeman, M.N.; Dulberger, A.R.; Alqutub, S.; Carter, B.W.; Manning, M.A. Esophageal Neoplasms: Radiologic-Pathologic Correlation. Radiol. Clin. N. Am. 2021, 59, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.; Wong, J.; Palm, R.; Hoffe, S.; Almhanna, K.; Vignesh, S. Epidemiology, Diagnosis, Staging and Multimodal Therapy of Esophageal and Gastric Tumors. Cancers 2021, 13, 582. [Google Scholar] [CrossRef]
- Carter, B.W.; Lichtenberger, J.P., 3rd. Imaging of the Posterior/Paravertebral Mediastinum. Radiol. Clin. N. Am. 2021, 59, 243–249. [Google Scholar] [CrossRef]
- Rodriguez, E.F.; Jones, R.; Miller, D.; Rodriguez, F.J. Neurogenic Tumors of the Mediastinum. Semin. Diagn. Pathol. 2020, 37, 179–186. [Google Scholar] [CrossRef]
- Fouladi, D.F.; Fishman, E.K.; Kawamoto, S. Extramedullary Hematopoiesis: A Forgotten Diagnosis and a Great Mimicker of Malignancy. J. Comput. Assist. Tomogr. 2023, 47, 445–452. [Google Scholar] [CrossRef]



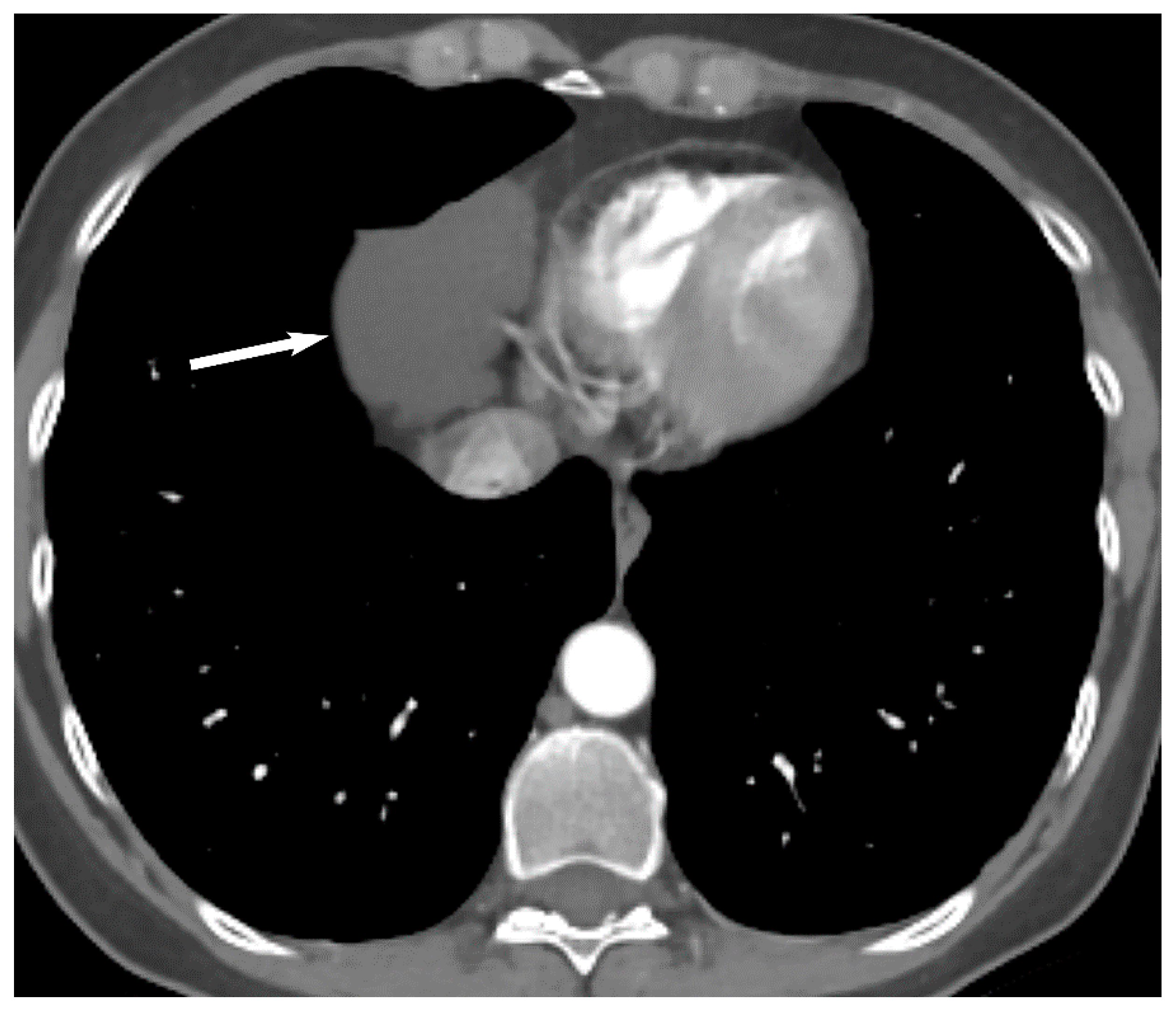

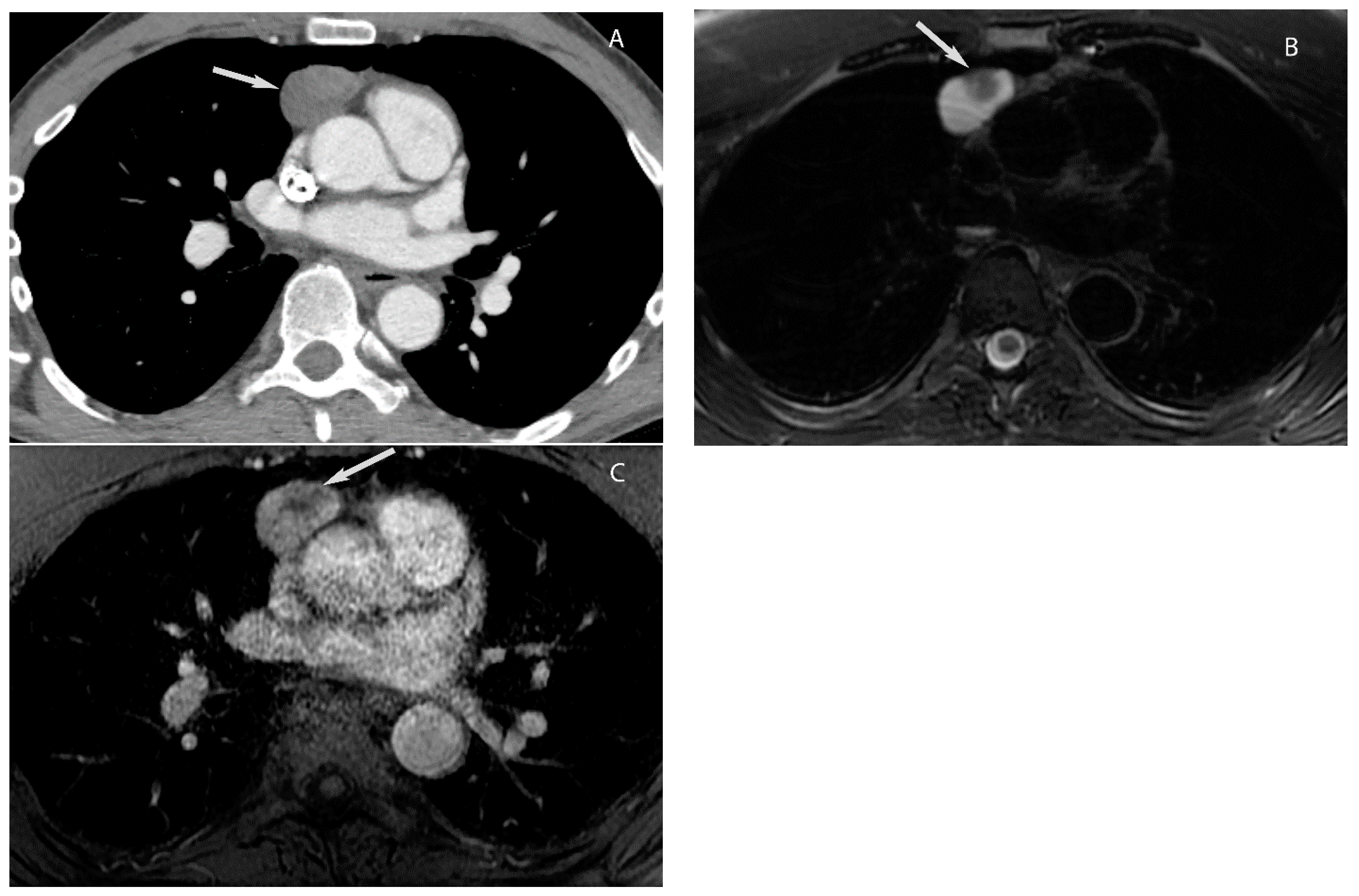















| Compartment | Boundaries |
|---|---|
| Prevascular | Anterior: sternum |
| Posterior: anterior surface of pericardium as it wraps around the heart | |
| Superior: thoracic inlet | |
| Inferior: diaphragm | |
| Lateral: mediastinal pleura | |
| Visceral | Anterior: posterior boundaries of prevascular compartment |
| Posterior: vertical line connecting a point on each thoracic vertebral body 1 cm posterior to its anterior margin | |
| Superior: thoracic inlet | |
| Inferior: diaphragm | |
| Paravertebral | Anterior: posterior boundaries of the visceral compartment |
| Posterolateral: vertical line against the posterior margin of the chest wall at the lateral margin of the transverse process of the thoracic spine | |
| Superior: thoracic inlet | |
| Inferior: diaphragm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahuja, J.; Strange, C.D.; Agrawal, R.; Erasmus, L.T.; Truong, M.T. Approach to Imaging of Mediastinal Masses. Diagnostics 2023, 13, 3171. https://doi.org/10.3390/diagnostics13203171
Ahuja J, Strange CD, Agrawal R, Erasmus LT, Truong MT. Approach to Imaging of Mediastinal Masses. Diagnostics. 2023; 13(20):3171. https://doi.org/10.3390/diagnostics13203171
Chicago/Turabian StyleAhuja, Jitesh, Chad D. Strange, Rishi Agrawal, Lauren T. Erasmus, and Mylene T. Truong. 2023. "Approach to Imaging of Mediastinal Masses" Diagnostics 13, no. 20: 3171. https://doi.org/10.3390/diagnostics13203171
APA StyleAhuja, J., Strange, C. D., Agrawal, R., Erasmus, L. T., & Truong, M. T. (2023). Approach to Imaging of Mediastinal Masses. Diagnostics, 13(20), 3171. https://doi.org/10.3390/diagnostics13203171







